Abstract
A 5.5-y-old Chinese-origin female rhesus macaque (Macaca mulatta) presented for bilateral hindlimb lameness. The primate had been group-reared in an SPF breeding colony and was seronegative for Macacine herpesvirus 1, SIV, simian retrovirus type D, and simian T-lymphotropic virus. The macaque's previous medical history included multiple occasions of swelling in the left tarsus, and trauma to the right arm and bilateral hands. In addition, the macaque had experienced osteomyelitis of the left distal tibia and rupture of the right cranial cruciate ligament that had been surgically repaired. Abnormal physical examination findings on presentation included a thin body condition, mild dehydration, and bilaterally swollen stifles that were warm to the touch, with the right stifle more severely affected. Mild instability in the left stifle was noted, and decreased range of motion and muscle atrophy were present bilaterally. Hematologic findings included marked neutrophilia and lymphopenia and moderate anemia. Arthrocentesis and culture of joint fluid revealed Moraxella-like organisms. Treatment with enrofloxacin was initiated empirically and subsequently switched to cephalexin, which over time alleviated the joint swelling and inflammation. Definitive diagnosis of Moraxella osloensis septic arthritis was made through isolation of the organism and sequencing of the 16S rDNA region. To our knowledge, this report is the first description of Moraxella osloensis septic arthritis in a rhesus macaque.
Septic arthritis is a condition of joint inflammation that is secondary to bacterial infection5 and is an uncommon diagnosis in nonhuman primates, which instead are more likely to present with rheumatoid arthritis or a reactive arthritis from enteric pathogens such as Shigella flexneri.1 Reported cases of septic arthritis in nonhuman primates include Streptococcus aureus in a male orangutan (Pongo pygmaeus),11 Salmonella spp. osteomyelitis in a rhesus macaque,13 polyarthritis suggestive of Mycoplasma in a rhesus macaque,17 and Streptobacillus moniliformis in a titi monkey (Callicebussp.).20 Osteoarthritis is the most common type of arthritis in humans.5
Case Report
Presentation.
A 5.5-y-old Chinese-origin female rhesus macaque (Macaca mulatta) presented for lameness. The macaque was seronegative for Macacine herpesvirus 1, SIV, simian retrovirus type D, and simian T-lymphotropic virus and had been group-reared in an SPF breeding colony at the Tulane National Primate Research Center. The facility is licensed by the US Department of Agriculture and has had continuous AAALAC accreditation since 1983. All animals in the breeding colony are on IACUC-approved protocols, and their management is consistent with all applicable regulations as prescribed in the Animal Welfare Regulations2 and in accordance with the Guide for the Care and Use of Laboratory Animals.12 Animals in the SPF breeding colony are socially housed outdoors in compatible groups and supplied twice daily with a commercial diet (LabDiet no. 5K63, PMI Nutrition International, Brentwood, MO), which is supplemented with fresh produce and forage materials. Swings, platforms, climbing structures, and toys are provided for enrichment.
On physical examination, the macaque had a body condition score of 1 (maximum, 5) and was mildly dehydrated. Mucous membranes were pink, and the capillary refill time was less than 2 s. The animal was normothermic, with a temperature of 100.0 °F. Pregnancy was detected on abdominal palpation. A rectal swab was taken, but no enteric pathogens were isolated. Both stifles were swollen and warm to the touch, with the right affected more severely than the left and with the majority of swelling proximal to the patella. Both stifles exhibited reduced range of motion, the left stifle had mild instability, and there was bilateral hindlimb muscle atrophy.
Arthrocentesis was performed aseptically on the right stifle, which produced 3 mL of thick, viscous, opaque yellow exudate that was sent for analysis and bacterial culture. Hematologic evaluation revealed mild leukocytosis (15.62 × 103 U/L; reference interval, 6.6 to 15.5 × 103 U/L) with marked neutrophilia (13.04 × 103 U/L; reference interval, 1.4 to 7.3 × 103 U/L), moderate anemia (Hgb, 8.0 g/dL [reference interval, 10.1 to 15.9 g/dL]; Hct, 26.7% [reference interval, 34.8 to 55.2%]), mild lymphopenia (1.47 × 103 U/L; reference interval, 2.3 to 13 × 103 U/L), and thrombocytosis (867 × 103 U/L; reference interval, 193.1 to 676.2 × 103 U/L). Serum biochemistry revealed mild hyponatremia (139 mEq/L; reference interval, 144 to 166 mEq/L), hypochloremia (105 mEq/L; reference interval, 106 to 117 mEq/L), hypoalbuminemia (2.2 g/dL; reference interval, 3.0 to 5.9 g/dL), hyperglobulinemia (5.5 g/dL; reference interval, 1.9 to 3.9 g/dL), and moderate hypoglycemia (27 mg/dL; reference interval, 48 to 119 mg/dL). A stool sample was collected for parasitology and revealed Entamoeba coli, Iodamoebasp., Strongyloides fulleborni, and Trichuris trichiura. An empirical course of enrofloxacin (5 mg/kg IM once daily; Baytril, Bayer Healthcare, Shawnee Mission, KS) was initiated, and buprenorphine (0.009 mg/kg IM twice daily; Buprenex, Reckitt Benckiser Healthcare, Hull, England) was provided for pain management. Supplemental nutrition was administered via orogastric tube, and 250 mL Lactated Ringer solution (Hospira, Lake Forest, IL) was administered subcutaneously. In addition, supplemental fresh fruit and sports beverage (G Series, Gatorade, Chicago, IL) were provided once the macaque had recovered from anesthesia.
Medical history.
At the age of 3.2 y, the macaque presented to the clinic for left leg lameness. At that time, left tarsal swelling was observed, and radiographs revealed luxation of the talocalcaneal joint and fracture of the tarsal bone. The animal was treated with analgesics and NSAID for multimodal pain management, and a cast was applied to the leg for 6 wk, followed by cage rest for an additional 2 wk. A normal physical examination and healing of the tarsal injury was verified radiographically prior to return to the social group.
Approximately 2 mo later, the macaque presented for social wounding and dehydration. The left tarsus was swollen again. There was mildly limited range of motion bilaterally in the stifles. Radiographs of the left tarsus were inconclusive. Treatment included fluids, antibiotics, and analgesics. The wounds healed without complications, but left tarsal swelling persisted.
Follow-up radiographs revealed osteomyelitis of the left distal tibia. Curettage was performed under anesthesia by using aseptic technique, and samples were submitted for culture and sensitivity. Enrofloxacin was discontinued, and long-term clindamycin therapy (22.5 mg/kg IM twice daily; APP Pharmaceuticals, Schaumburg, IL) was instituted. The macaque was found to be lactating and therefore was introduced as a foster dam to twin infants. After clinical improvement and completion of therapy, the macaque was deemed healthy on physical examination and was returned to its social group.
After 1.5 mo, the macaque presented for hemorrhagic diarrhea and dehydration, at which time severe instability of the right stifle was noted. Treatment included fluids, antibiotics, analgesics, and supplemental nutrition. Right cranial cruciate ligament repair was performed by using the modified retinacular imbrication technique. The macaque healed without complications and was returned to the social group.
Six months after the cruciate ligament repair, the macaque presented to the clinic for social trauma and diarrhea, at which time bilateral tarsal swelling was present, with purulent exudate from a wound in the right tarsus. Treatment included antibiotics, analgesics, fluid therapy, and iron dextran (Butler Animal Health, Dublin, OH) for microcytic, normochromic anemia. The macaque's wounds and anemia were resolved at follow-up physical examination, and the macaque was allowed to rejoin the social group. Approximately 9 mo passed, during which the animal was routinely monitored in the social group and had no other medical problems until the current presentation.
Bacteriology.
The fluid collected via arthrocentesis of the right stifle was submitted to the inhouse clinical pathology laboratory on a swab with Stuarts's media (BBL Collection and Transportation System, Becton Dickenson, Franklin Lakes, NJ). The swab was inoculated onto trypticase soy agar with 5% sheep blood, MacConkey II agar, thioglycollate broth, and phenylethyl alcohol agar with 5% sheep blood. The blood agar, MacConkey, and phenylethyl alcohol plates were incubated at 37° in 5% CO2 for 48 h; the thioglycollate broth culture was incubated under the same conditions for 5 d.
There was no growth on any of the original plates after 48 h; the thioglycollate broth culture showed evidence of growth at day 5. Gram staining revealed small, weakly gram-negative rods; the isolate was replated onto blood agar, MacConkey, and chocolate agar. After 24 h, the blood agar plate had a clear, tiny colony; MacConkey agar showed no growth, and chocolate agar had multiple clear, medium-sized colonies.
The following tests were performed on the colonies picked from the chocolate agar plate: Gram staining, which showed small gram-negative rods; oxidase, positive; urea, negative; Moraxella catarrhalis Test Disk (Remel, Lenexa, KS), positive; API NH panel (for identification of Neisseriaspp., Haemophilusspp., and M. catarrhalis; bioMérieux, Marcy I'Etoile, France) , inconclusive; RapID NH panel (for the detection of Neisseria spp. and Haemophilus spp.; Remel), inconclusive; and β-lactamase, negative. At this point, Moraxellasp. was strongly suspected, but available tests failed to confirm the diagnosis. The final report after culturing described the isolate as a gram-negative bacterium, possible Moraxellasp., so it was submitted to the Division of Bacteriology and Parasitology for molecular diagnostics.
Molecular diagnostics.
Isolation and characterization.
An isolate from synovial fluid was grown in thioglycollate media by the inhouse clinical pathology laboratory. Then bacteria was restreaked onto Mueller–Hinton plates and grown at 37 °C, 5% CO2 for 48 h for isolation of single colonies. Several colonies were gram-stained and photographed. In addition, the Moraxella catarrhalis disc test (Remel) for detection of butyrate esterase was performed and isolates were grown in brain–heart infusion media for testing by using the API NH strip kit (bioMérieux) for biochemical identification.
DNA sequencing.
Two isolated colonies were grown in 5 mL brain–heart infusion media for 48 h; 1.5 mL of each culture was pelleted (17,000 × g, 10 min) and the supernatant decanted. DNA was isolated by using the DNeasy kit (Qiagen, Hilden, Germany) for bacterial DNA isolation, including treatment with lysozyme. Genomic regions of these 2 different clones were PCR-amplified (Taq DNA Polymerase PCR Kit, Qiagen) by using degenerate primers (5′ AGA GTT TGA TCM TGG CTC AG 3′ and 5′ GWA TTA CCG CGG CKG CTG 3′, where M is A or C and W is A or T) that target 16S rDNA sequences.10 PCR products were purified by using the Qiaquick PCR Purification Kit (Qiagen, Hilden, Germany) and then sequenced (Tulane University Sequencing Core) by using the same primers as for amplification. Sequences obtained from the clones were entered into nucleotide BLAST analyses at the National Center for Biotechnology Information website (http://blast.ncbi.nlm.nih.gov/).
Results.
Morphology and biochemistry.
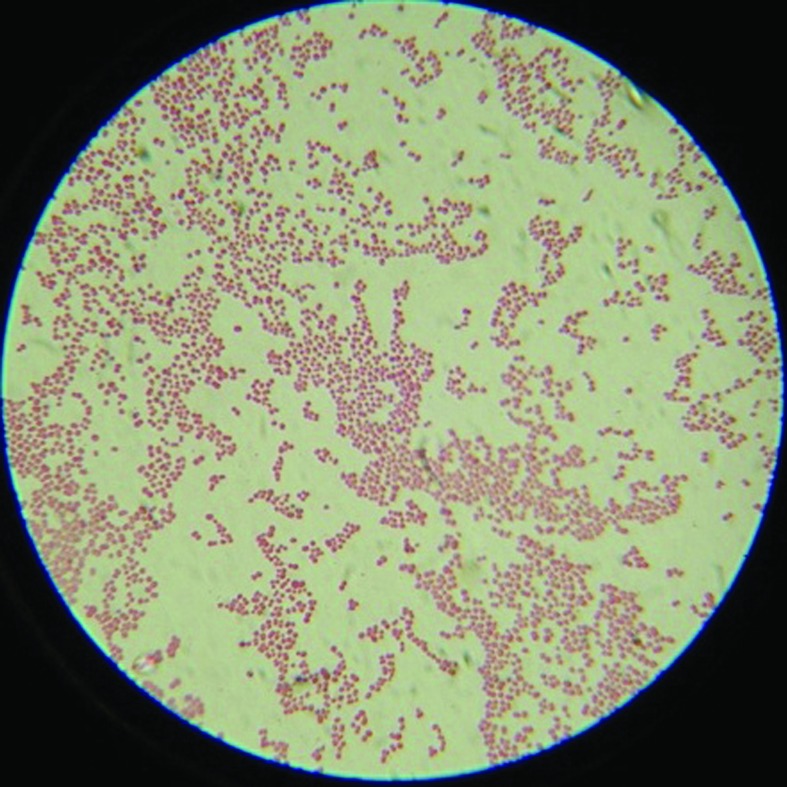
Gram staining of the clinical isolate revealed gram-negative coccobacilli that were greater than 1.5 µm in diameter (Figure 1). The isolate was positive by the Moraxella catarrhalis disc test. The biochemical tests showed that the isolate utilized glucose, fructose, saccharose, and maltose. The isolate was positive for penicillinase, lipase, and GGT, providing a pattern inconsistent with M. catarrhalis but possibly consistent with other Moraxellaspp.
Figure 1.
Gram staining of the clinical isolate showed gram-negative cocci greater than 1.5 µm in diameter. Magnification, 1000×.
DNA sequence.
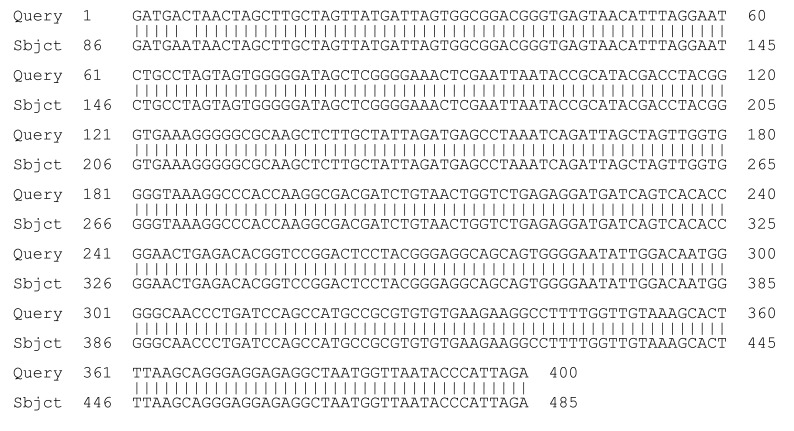
Sequencing of a region within the 16S rDNA is the primary genetic test for identification of a species.6 We sequenced the 16s rDNA region from 2 different clones of the isolate. The sequences from both of these clones showed complete homology with M. osloensis (Figure 2).
Figure 2.
Sequences of the 16S rDNA region from a representative clone of the isolate shows complete homology to Moraxella osloensis.
Follow-up.
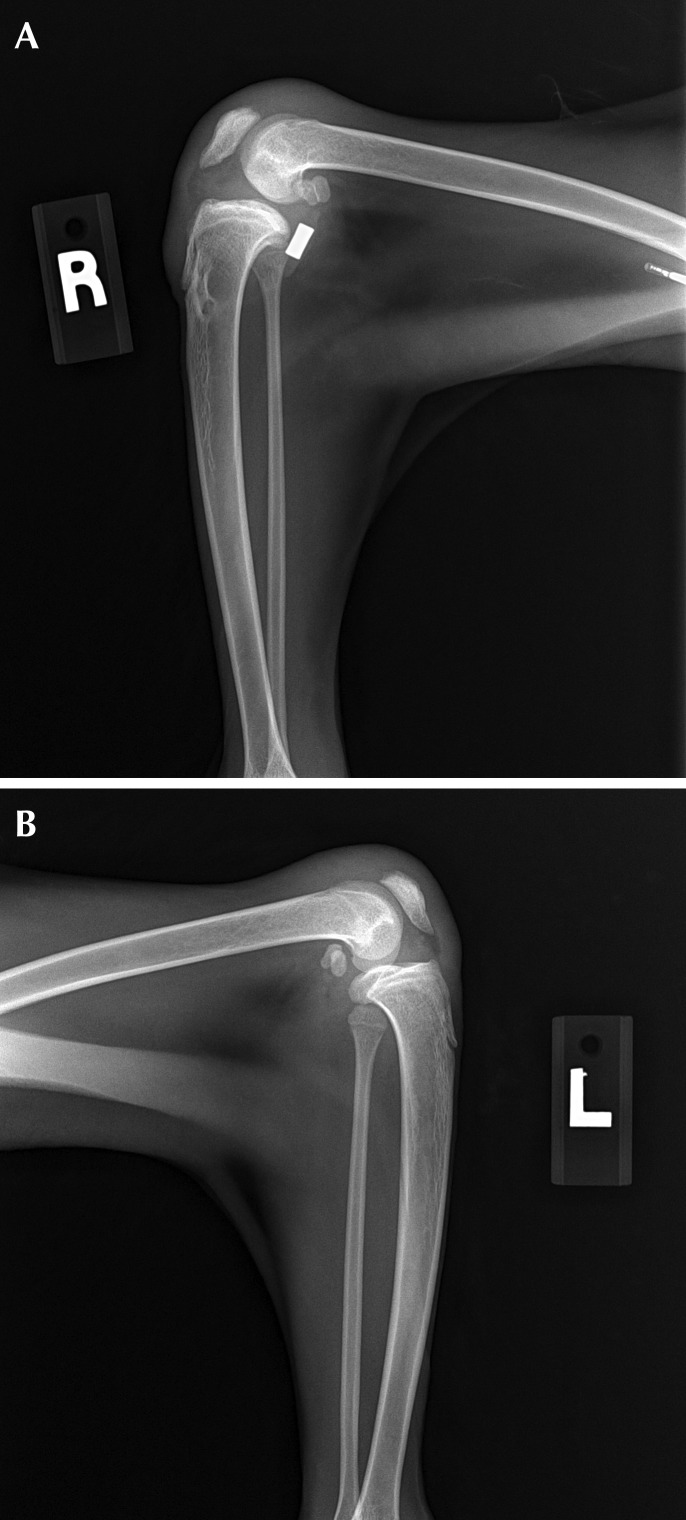
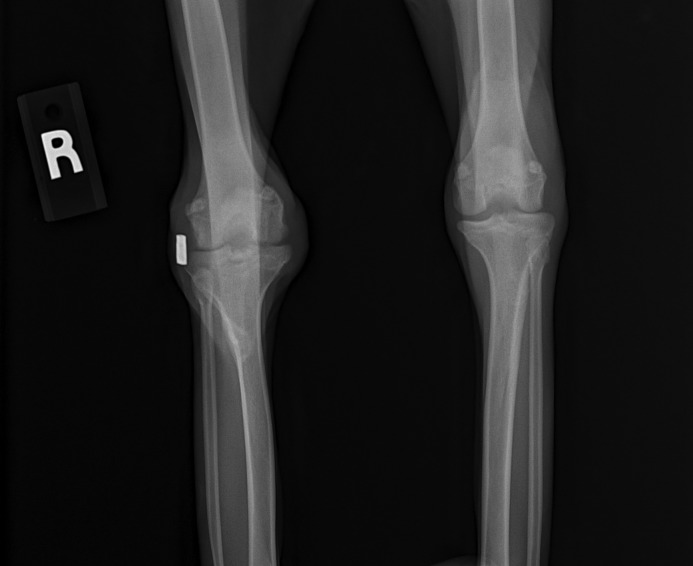
Spontaneous abortion occurred 13 d after presentation. At the macaques first recheck examination, warm joints and moderate swelling of the stifles were apparent bilaterally. Radiographs revealed subchondral bone destruction on the articular surfaces (Figures 3 and 4). Antibiotic therapy subsequently was switched to an extended course of cephalexin (25 mg/kg orally twice daily; Alkem Laboratories, Mumbai, India), and carprofen (Rimadyl, Pfizer Animal Health, New York, NY) was added to the regimen for chronic pain management. Complete resolution of swelling eventually occurred, but the cruciate implant was removed under the suspicion that it might be serving as a nidus of chronic infection. Despite removal of the implant, the range of motion in the stifles continued to decrease, and the macaque began to exhibit weight loss and severe hindlimb lameness. Because of concern regarding the macaque's quality of life in the breeding colony, euthanasia was elected.
Figure 3.
Lateral radiographs of the left and right stifles.
Figure 4.
Craniocaudal radiographs of the stifles.
Postmortem examination.
The macaque was thin (weight, 3.9 kg) and had minimal body fat. The thymus was moderately atrophic, and the heart was round and firm. There was myocardial hypertrophy of the wall of the left ventricle, which was 4 times thicker than normal (Figure 5). The lumen of the left ventricle was significantly narrowed to invisible. The spleen was mildly atrophic. The liver (weight, 0.34 kg) was pale yellow, firm, and enlarged to 3 times normal size. There was a small amount of black digesta in the stomach. Diffusely the mucosa of the jejunum was moderately thickened. Fragmentally, the jejunum and ileum were extended by yellow, undigested food material. The colon was normal. The mesenteric and inguinal lymph nodes were enlarged to 3 times normal size.
Figure 5.
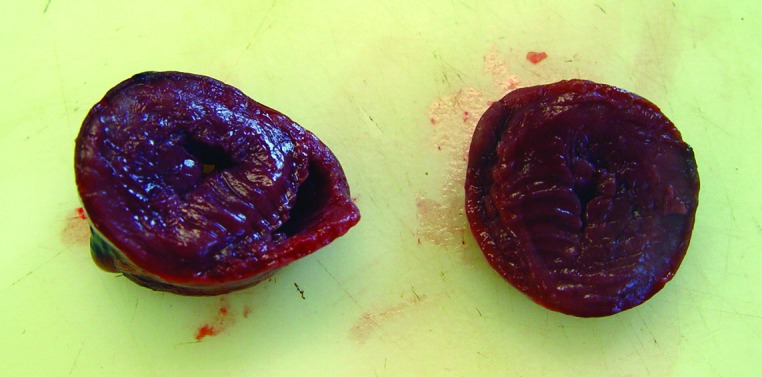
Left ventricular hypertrophy and left ventricular stenosis.
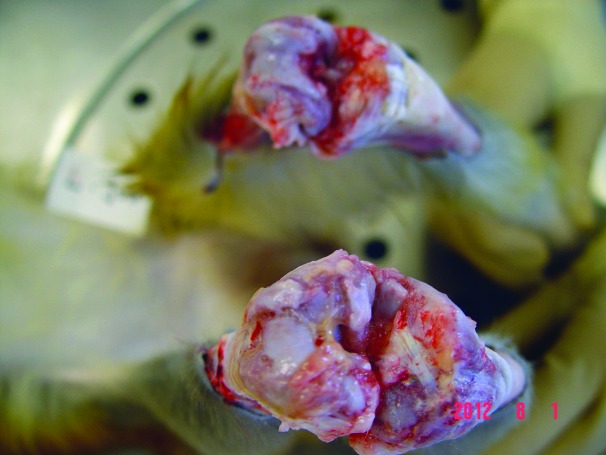
Both stifles appeared enlarged, with the left being more severely affected than the right. A small amount of yellow, hemorrhagic fibrinous fluid was present in both joint cavities. There was erosion of the cartilage in both stifles that was more pronounced on the left side, and the fluid extended dorsally into adjacent tendon and muscle (Figure 6, bottom). A 3-cm hematoma with edema was present in the tissue at the left lateral tarsus. Swab samples of both stifles were culture-negative.
Figure 6.
Bilateral suppurative hemorrhagic arthritis. Top, right stifle; bottom, left stifle.
Histopathology.
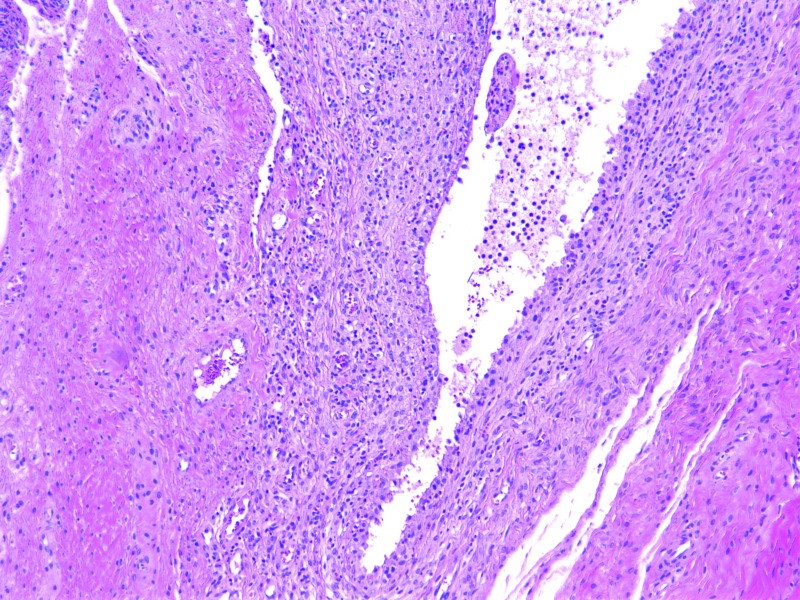
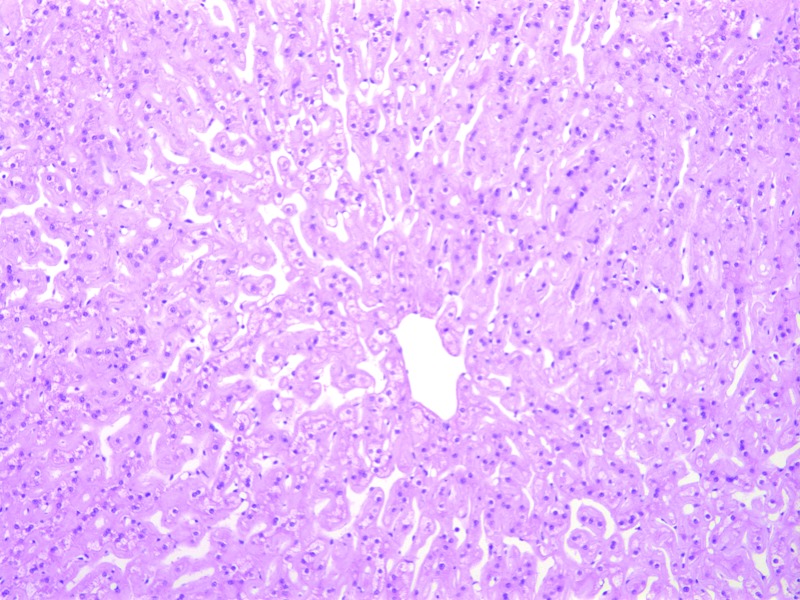
Bilateral, suppurative, hemorrhagic arthritis was present in both stifles (Figure 7). A hematoma on the left tarsus was accompanied by suppurative arthritis and proliferative synovitis. There was severe diffuse hypertrophy of the myocardium and papillary muscles of the left ventricle (Figure 8). The left ventricular wall exhibited myocardial vascular degeneration and necrosis. The liver had severe, diffuse amyloid deposits (Figure 9). There was mild kidney nephrosis. Multiple lymph nodes had mild lymphoid hyperplasia. There was moderate, chronic gastric inflammation and mild hyperplasia of the gastric mucosa. The colon displayed mild, chronic inflammation.
Figure 7.
Histopathology. Left stifle, suppurative hemorrhagic arthritis. Hematoxylin and eosin stain; magnification, 20×.
Figure 8.
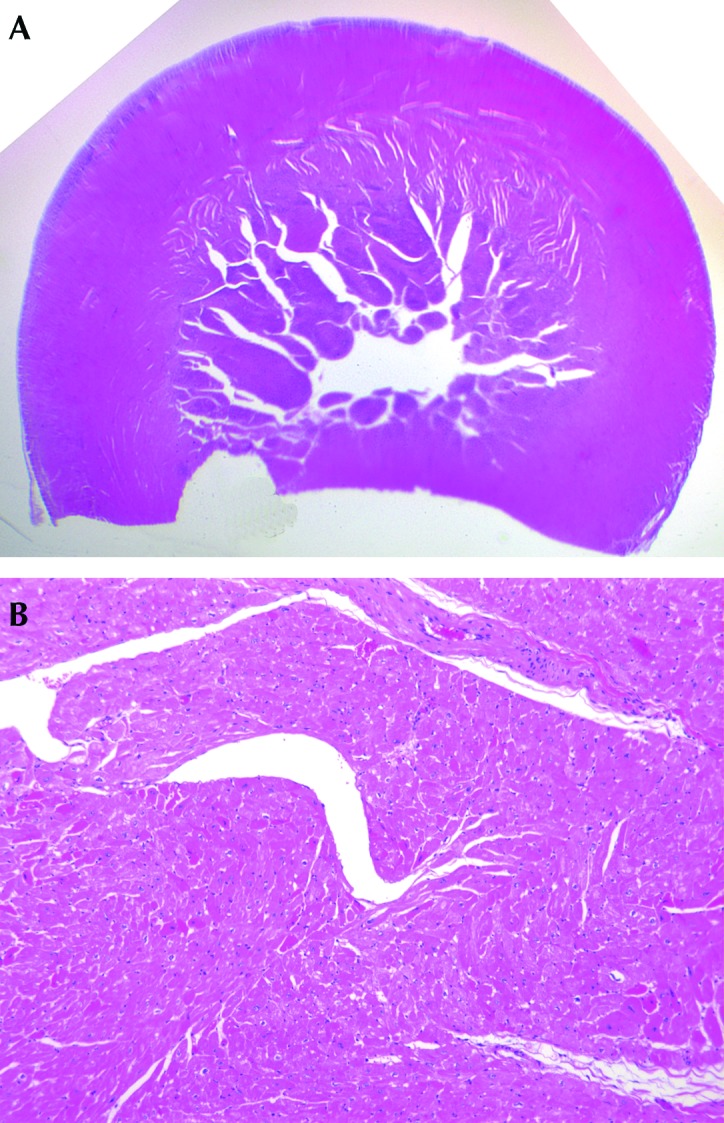
Histopathology. Severe diffuse hypertrophy of the myocardium and papillary muscles of the left ventricle. Hematoxylin and eosin stain; magnification: subgross (upper panel), 50× (lower panel).
Figure 9.
Histopathology. Severe, diffuse amyloid deposits in the liver. Hematoxylin and eosin stain; magnification, 100×.
Discussion
Cases of septic arthritis in nonhuman primates are rare and include a male orangutan (Pongo pygmaeus) who presented with umbilical swelling and necrotic plaques on the tongue and gingiva. Disease involvement progressed to swelling of the hip and elbow, at which time arthrocentesis revealed the causative organism to be Streptococcus aureus.11 In another case, Salmonella osteomyelitis in a rhesus macaque presumably resulted after presentation and treatment for severe watery diarrhea. Swelling of the stifle and fever were reported, and diagnosis was established from culture of the stifle's purulent exudate.13 Polyarthritis suggestive of Mycoplasma was reported in a rhesus macaque who had a history of Shigella-induced diarrhea. More than 1 y later, the macaque presented with multiple swollen joints; Gram stains and cultures were noninformative, but complement fixation titers were suggestive of a Mycoplasma infection.17 Although neither Shigella spp. nor other enteric pathogens were cultured from rectal swabs taken from the current case, this possibility cannot be ruled out. Streptobacillus moniliformis was diagnosed in a titi monkey (Callicebusspp.) who presented for lethargy, low body weight, and stifle swelling; arthrocentesis revealed no organisms on Gram stain. Empirical treatment was unsuccessful, and the monkey developed a hemorrhagic diarrhea and later died. Complications, such as endocarditis and arthritis, were reported, and the route of infection for S. moniliformis was thought to be contamination of feed or water with rodent feces.20
Recently, there has been an increased interest in nonhuman primate arthritides,1 which appear to be naturally occurring and sporadic, resembling those encountered in humans.1 Medical value is gained by excluding or identifying etiologic or pathogenic factors of disease in nonhuman primate arthritis, raising awareness of the diagnostic options and challenges, and developing animal models of disease.1
Moraxella is a genus of fastidious gram-negative bacteria compromising more than 14 species, few of which have been characterized. Species of Moraxella include M. bovis, the causative agent of infectious keratoconjunctivitis in cattle and horses, and M. catarrhalis, which causes otitis media and sinusitis in humans. Species about which less is known include M. nonliquefaciens, M. lacunata, M. atlantae, and M. lincolnii.7
A species of Moraxella that causes epistaxis in nonhuman primates was identified recently at our facility.7 This organism initially was thought to be M. catarrhalis,4,21 because it was positive by the disc test. In addition, the biochemical tests for that isolate were consistent with several known human pathogens in the Moraxella genus. However, when portions of the 16S rDNA and other housekeeping genes were sequenced, they did not align with known pathogens in the database, and the isolate was deemed a novel species.7 In comparison, in our current analysis of the clinical isolate associated with arthritic inflammation in a rhesus macaque stifle, the results derived from the API strip and DNA sequencing were consistent. Moraxella subgenus Moraxella species can have pleomorphic morphology,9 which explains the disparities in Gram staining characteristics after growth on chocolate agar compared with those from Mueller–Hinton agar. Therefore, according to the morphology of the organism and the biochemistry and DNA sequencing results, the isolate appears to be M. osloensis.
M. osloensis has rarely been implicated in disease in humans and has not previously been reported as the cause of arthritis in nonhuman primates. The organism typically presents in humans systemically,8 causing endopthalamitis,22 meningitis, osteomyelitis, pneumonia, peritonitis, bacteremia, vaginitis, and arthritis8 as well as synovitis.19 In addition, M. osloensis was cultured from the central venous catheters of 10 human patients undergoing cancer treatment.9 Common clinical findings in all cases were high fever and leukocytosis.
A reported human case18 of septic arthritis due to M. osloensis illustrates the difficulty in correct diagnosis and treatment due to the organism's fastidious growth characteristics. The patient presented with only a 5-d history of joint pain and swelling without injury. As in the nonhuman primate case we present here, there was pyrexia, joint warmth, effusion, and decreased range of motion in the knee. Arthrocentesis, CBC count, and biochemistry were performed. As in the case we describe, no organisms were cultured, and there was difficulty interpreting the Gram stain. An extended course of antibiotics did not resolve the knee pain 39 d after presentation, and subsequent arthroscopy revealed synovitis and osteoporosis. Biopsy and culture of the synovium of the knee during this procedure led to the diagnosis of M. osloensis.18 Like the aforementioned human case, the macaque we describe in this case report exhibited pyrexia on multiple occasions and showed mild leukocytosis. In an attempt to trace the origination of this particular infection, banked serum samples taken on multiple admission dates were tested as described, and all samples were culture-negative.
Treatment of our macaque's arthritis with cell-wall–active antibiotics seemed to us most likely to be effective, and a long-term course of cephalexin indeed appeared to alleviate the animal's clinical signs. A review of clinical manifestations of M. osloensis infections in humans examined comorbid conditions, the anatomic locations from which M. osloensis was cultured, and treatments administered.19 The authors noted that most reported cases were susceptible to cephalosporins, penicillins, or aminoglycosides. However, some isolates showed resistance to penicillin.19
Abortion, death, embryo resorption, and small litter production has been noted in cases of M. bovis strain EPP63 (300) infection in pregnant mice, guinea pigs, rats, and rabbits.16 M. osloensis may also cause abortion, although this effect has not been reported. In addition, enrofloxacin was administered to our macaque on presentation, and an extensive controlled study using a larger population in humans reported no increase in major malformations or musculoskeletal dysfunctions due to fluoroquinolone therapy during pregnancy.15 Furthermore, a more recent study showed that fluoroquinolones for treatment of Brucellosis have no effect on pregnancy in dogs.23 As previously stated, the macaque we present was lactating but not pregnant or carrying an infant during a previous admission, suggesting a possible prior abortion.
The presence of left ventricular hypertrophy and stenosis on necropsy of our macaque were surprising given the animal's young age and lack of clinical signs associated with cardiovascular disease. However, these findings, in conjunction with kidney nephrosis, may help to explain the hypoalbuminemia observed on presentation, given that nephrosis can lead to protein loss followed by cardiomegaly. These findings may be important to our understanding of the pathogenesis of systemic M. osloensis. Conversely, a comorbid state, such as the endocarditis, renal failure, and chronic central venous catheterization and associated chemotherapy reported in human cases,1 may have created an immunocompromised state, which then increased susceptibility to the organism. Our macaque could have been immunocompromised due to underlying renal disease and cardiac manifestations, which were found on necropsy.
Gastritis and colitis also were present on necropsy. Diarrhea resulting from enteritis likely led to the electrolyte perturbations we noted in this case. Hematogenous spread of Moraxella from the intestines to the joints may have occurred, given that the organism seems to be ubiquitous, and could have been the source of infection.14,19 As mentioned earlier, many of the cases of septic arthritis in nonhuman primate that had a bacterial etiology have been associated with bouts of diarrhea and enteritis.1,11,13,17,20
Amyloidosis in macaques often occurs after chronic inflammation, and the liver frequently is affected. Inflammation in both the gastrointestinal tract and joints were present in our macaque and may have contributed to liver amyloidosis. However, the interaction of both enterocolitis and arthritis with amyloidosis has not been fully defined.3 Amyloidosis, arthritis, or any of the other comorbid conditions noted on necropsy could have contributed to anemia of chronic disease, as seen in this case.
We cannot definitively rule out the possibility that the surgical implant procedure introduced the bacterial agent into the joint; several human cases reported the presence of foreign materials such as artificial shunts, aortic valves, and central venous catheters.19 However, the macaque we report did not demonstrate signs of infection postoperatively or during the recovery period before being returned to the breeding colony.
To our knowledge, this report is the first description of M. osloensis septic arthritis in a rhesus macaque.
Acknowledgments
We thank Gail Plauche and the TNPRC lab staff for their technical expertise in culturing our samples and Dr Rajunor Ettarh for histology and case interpretation. I appreciate the inspiration and encouragement from Bob Dauchy, my friend. This work was supported in part by an Institutional Training Grant (no. 1 R25 RR032028).
References
- 1.Abee CR, Mansfield K, Tardiff S, Morris T. 2012. Nonhuman primates in biomedical research: diseases. San Diego (CA): Academic Press [Google Scholar]
- 2.Animal Welfare Regulations 2008. 9 CFR §3.129. [Google Scholar]
- 3.Blanchard JL, Baskin GB, Watson EA. 1986. Generalized amyloidosis in rhesus monkeys. Vet Pathol 23:425–430 [DOI] [PubMed] [Google Scholar]
- 4.Bowers LC, Purcell JE, Plauche GB, Denoel PA, Lobet Y, Philipp MT. 2002. Assessment of the nasopharyngeal bacterial flora of rhesus macaques: Moraxella, Neisseria, Haemophilus, and other genera. J Clin Microbiol 40:4340–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention [Internet]. 2011. Arthritis-related statistics. [Cited 5 August 2012]. Available at: http://www.cdc.gov/arthritis/data_statistics/arthritis_related_stats.htm#2 [Google Scholar]
- 6.Clarridge JE. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17:840–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Embers ME, Doyle LA, Whitehouse CA, Selby EB, Chappell M, Philipp MT. 2011. Characterization of a Moraxella species that causes epistaxis in macaques. Vet Microbiol 147:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigin RD, Joaquin VS, Middlekamp JN. 1969. Septic arthritis due to Moraxella osloensis. J Pediatr 75:116–117 [DOI] [PubMed] [Google Scholar]
- 9.Han XY, Tarrand JJ. 2004. Moraxella osloensis blood and catheter infections during anticancer chemotherapy: clinical and microbiologic studies of 10 cases. Am J Clin Pathol 121:581–587 [DOI] [PubMed] [Google Scholar]
- 10.Harmsen D, Singer C, Rothganger J, Tonjum T, de Hoog GS, Shah H, Albert J, Frosch M. 2001. Diagnostics of Neisseriaceae and Moraxellaceae by ribosomal DNA sequencing: ribosomal differentiation of medical microorganisms. J Clin Microbiol 39:936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoopes PJ, McKay DW, Daisley GW, Jr, Kennedy S, Bush M. 1978. Suppurative arthritis in an infant orangutan. J Am Vet Med Assoc 173:1145–1147 [PubMed] [Google Scholar]
- 12.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 13.Klumpp SA, Weaver DS, Jerome CP, Jokinen MP. 1986. Salmonella osteomyelitis in a rhesus monkey. Vet Pathol 23:190–197 [DOI] [PubMed] [Google Scholar]
- 14.Kubota H, Mitani A, Niwano Y, Takeuchi K, Tanaka A, Yamaguchi N, Kawamura Y, Hitomi J. 2012. Moraxella species are primarily responsible for generating malodor in laundry. Appl Environ Microbiol 78:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loebstein R, Addis A, Ho E, Andreou R, Sage S, Donnenfeld AE, Schick B, Bonati M, Moretti M, Lalkin A, Pastuszak A, Koren G. 1998. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob Agents Chemother 42:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman JO, Elissalde MH. 1979. Abortion in laboratory animals induced by Moraxella bovis. Infect Immun 24:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeck DK, Toft JD, 2nd, Dupuy HJ. 1976. Severe polyarthritis in a rhesus monkey: suggested mycoplasma etiology. Lab Anim Sci 26:613–618 [PubMed] [Google Scholar]
- 18.Schonholtz GJ, Scott WO. 1986. Moraxella septic arthritis of the knee joint: a case report. Arthroscopy 2:96–97 [DOI] [PubMed] [Google Scholar]
- 19.Shah SS, Ruth A, Coffin SE. 2000. Infection due to Moraxella osloensis: case report and review of the literature. Clin Infect Dis 30:179–181 [DOI] [PubMed] [Google Scholar]
- 20.Valverde CR, Lowenstine LJ, Young CE, Tarara RP, Roberts JA. 2002. Spontaneous rat bite fever in nonhuman primates: a review of 2 cases. J Med Primatol 31:345–349 [DOI] [PubMed] [Google Scholar]
- 21.VandeWoude SJ, Luzarraga MB. 1991. The role of Branhamella catarrhalis in the ‘bloody-nose syndrome’ of cynomolgus macaques. Lab Anim Sci 41:401–406 [PubMed] [Google Scholar]
- 22.Walls A, Wald E. 2005. Neonatal Moraxella osloensis ophthalmia. Emerg Infect Dis 11:1803–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanke MM, Delpino MV, Baldi PC. 2006. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial). Theriogenology 66:1573–1578 [DOI] [PubMed] [Google Scholar]