Abstract
An adult male chinchilla (Chinchilla lanigera) presented with severe lethargy and tachypnea; the physical examination was otherwise unremarkable. Due to the animal's clinical condition, it was submitted for necropsy but died immediately prior to euthanasia. Clinicopathologic findings included leukocytosis with a left-shift neutrophilia and lymphopenia, azotemia, hyperphosphatemia, hyperglycemia, hyperlipemia, electrolyte imbalance, cholestasis, and hepatocellular damage. Neutrophilic enteritis with gram-negative bacterial colonization, hepatic lipidosis, interstitial pneumonia, suppurative tubulonephritis, erosive gastritis, cerebral edema, and lymphoid depletion were present microscopically. Attaching and effacing, eae-positive, Escherichia coli characterized by the presence of the intimin virulence factor was isolated from both the kidney and spleen. The cause of death was attributed to acute E. coli septicemia and subsequent disseminated intravascular coagulation.
Abbreviations: AEEC, attaching and effacing E. coli.
A black-gray, adult (age, unknown) male, experimentally naïve chinchilla (Chinchilla lanigera) purchased (Moulton Chinchilla Ranch, Rochester, MN) approximately 1 y previously, presented with extreme lethargy and tachypnea during daily health rounds. The chinchilla was group housed with 2 cage mates in accordance with the Guide for the Care and Use of Laboratory Animals9 in an AAALAC-accredited facility. A total of 12 nonSPF chinchillas were acquired together from the same vendor. The chinchillas were housed in a self-contained isolation cubicle within a diverse-species room, which included mice, rats, guinea pigs, and gerbils. The cubicle was maintained at negative pressure with regard to the room. Housing consisted of a modified stainless steel chicken brooder (Alternative Design Manufacturing and Supply, Siloam Springs, AR). The chinchilla was fed a daily ration of pelleted diet (Mazuri Chinchilla Diet 5M01, PMI Nutrition International, Richmond, IN) and maintained on filtered chlorinated municipal water via a glass bottle with a rubber stopper and stainless steel sipper. Caging, feeding bowls, and water bottles were sent to the cage washing area for sanitation. Cage components were sanitized in a rack washer (Better Built Rack Washer, Northwest Systems, Delta, Canada) that provided a 180 °F final water rinse. A blend of organic and inorganic acid detergent (Acidulate 28, Quip Labs, Wilmington, DE) was used during the wash cycle to remove urine deposits and proteinaceous debris. Environmental enrichment included the provision of hide boxes, biweekly dust baths (Critter Bath Powder, Central Garden and Pet, Walnut Creek, CA), certified wood gnawing blocks (Bio-Serv, Frenchtown, NJ), and western timothy hay (Oxbow Animal Health, Murdock, NE). Room conditions included 18.3 to 20.0 °C (65 to 68 °F), 35% to 40% relative humidity, and a 12:12-h light:dark cycle. Routine health monitoring consisted of daily observations by the husbandry and veterinary technical staff for clinical signs of illness. No deviations in appetite or other gross abnormalities were noticed prior to the animal's illness.
On physical examination, the chinchilla was moribund and responsive only when prodded. Copious fecal pellets were palpated on abdominal examination. The animal weighed 560 g and was in good body condition. The chinchilla was submitted immediately for necropsy but died prior to euthanasia. Subsequently, approximately 2 mL of blood was withdrawn by cardiocentesis for hematology and chemistry. Chinchilla-specific reference intervals were not available from the laboratory; therefore, published reference intervals were used as a guide for interpretation of the hematology and clinical chemistry results.24
Case report
A CBC count and manual differential revealed a moderate leukocytosis (22.3 × 103 cells/µL). The leukocytosis resulted from a neutrophilia (64%, 14.2 × 103 cells/µL) with a left shift and relative lymphopenia (32%; 7.1 × 103 cells/µL). Many of the neutrophils and monocytes contained numerous intracytoplasmic clear, round vacuoles, which were presumed to be lipid (Figure 1). Occasional neutrophils had a basophilic cytoplasm, consistent with toxic change. Neutrophils were often hyposegmented, which is common in this species.15 These findings were consistent with inflammatory demand and a concurrent stress response.
Figure 1.
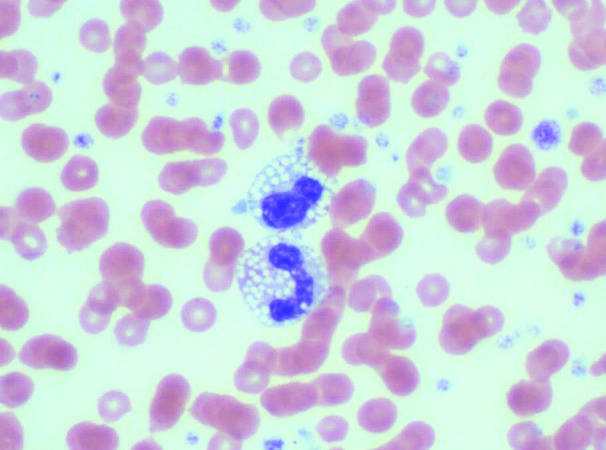
Peripheral blood. Two leukocytes with large numbers of intracytoplasmic clear, round vacuoles. Hematoxylin and eosin stain; magnification, 1000×.
Marked elevations in AST (3010 U/L), LDH (9987 U/L), and creatinine kinase (1844 U/L), and a mild elevation in ALT (661 U/L) were noted on clinical chemistry, consistent with muscle damage from prolonged recumbency and perimortem cardiocentesis. Severe hypertriglyceridemia (1913 mg/dL) and mild hypercholesterolemia (305 mg/dL) and mild increases in ALT (661 U/L), GGT (22 U/L), and ALP (273 U/L), were present, indicative of hepatocellular damage and cholestasis. The marked azotemia (BUN, 127 mg/dL; creatinine, 5.1 mg/dL) and hyperphosphatemia (28.5 mg/dL) indicated decreased glomerular filtration, which may have resulted from both prerenal (dehydration) and renal causes. In addition, sodium (121 mEq/L) and chloride (83 mEq/L) were decreased in the face of probable dehydration, suggestive of loss due to gastrointestinal or renal tubular damage. Glucose (294 mg/dL) was elevated, likely due to stress.
On necropsy, the liver was diffusely yellow to tan and mildly enlarged (5.4% of body weight). The lungs were mottled red and had a normal consistency. Approximately 36 g of soft green feed was present in the stomach.
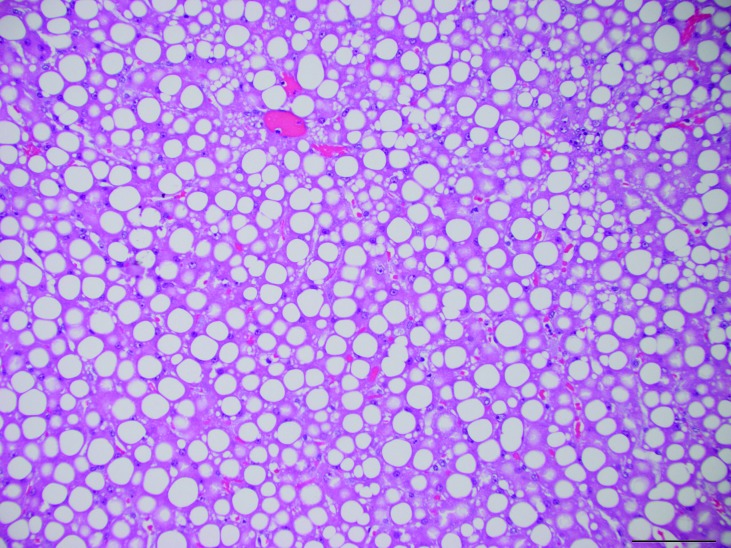
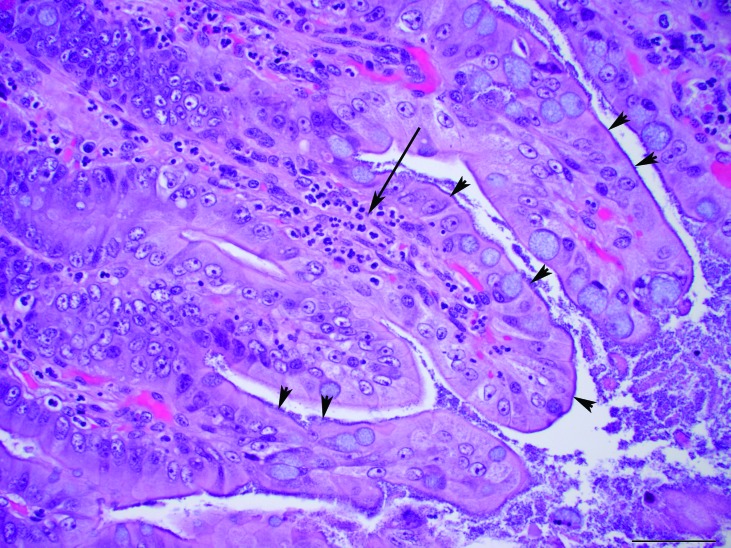
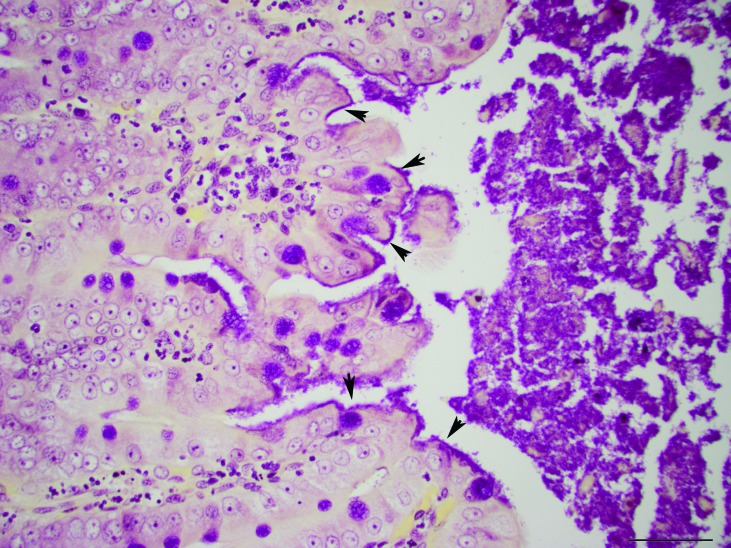
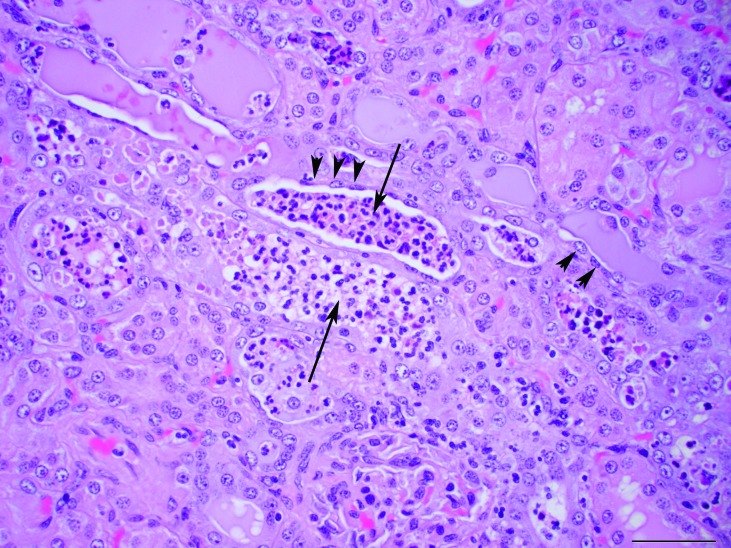
Microscopic examination of the liver showed diffusely enlarged hepatocytes containing macrovesicular and microvesicular, round, clear, intracytoplasmic vacuoles, suggestive of lipid (Figure 2). The pulmonary interstitium was diffusely infiltrated with moderate to large numbers of neutrophils, and the capillary lumens within the alveolar walls often contained brightly eosinophilic material (fibrin thrombi). The ileal lamina propria contained moderate numbers of neutrophils, and the apical surface of the enterocytes along the villus tips were carpeted with gram-negative rod-shaped bacteria, which occasionally infiltrated the lamina propria (Figures 3 and 4). Multiple small (less than 0.5 mm2) areas of necrosis, admixed with small numbers of degenerative neutrophils, occurred within the superficial gastric mucosa, whereas the colonic glands were dilated, lined by attenuated epithelium, and contained increased amounts of foamy amphophilic material (mucin), small to moderate numbers of neutrophils, and cellular debris. Numerous gram-negative rod-shaped bacteria were adherent to the apical surface of the colonic mucosal epithelial cells, similar to those observed in the ileum. The renal tubules were often dilated, and their lumen contained a mix of degenerative neutrophils, cellular debris, brightly eosinophilic amorphous material (suggestive of protein), and mucin (Figure 5). The tubular epithelial lining was attenuated, and the epithelial cytoplasm was basophilic, consistent with tubular degeneration and regeneration. The glomeruli contained occasional neutrophils within their capillary loops, and occasional neutrophils were scattered within the interstitium of the cortex and medulla. Neural gray and white matter vessels were prominent, with frequent capillary branching. Many of these vessels had moderate expansion of the surrounding Virchow–Robins space, indicative of edema. The cerebral cortical astrocytes frequently were enlarged with vesicular nuclear chromatin and clear cytoplasm, consistent with reactive astrocytosis (Figure 6). The thymus had mild lymphocytolysis and lymphoid depletion. The spleen was diffusely contracted, and the lymphoid follicles were moderately depleted of lymphocytes. Within the lumen of a few seminiferous tubules of the testes were small numbers of multinucleated spermatid giant cells, indicative of degeneration.
Figure 2.
Liver. Hepatocytes are diffusely vacuolated with both macrovesicular and microvesicular, intracytoplasmic vacuoles. Hematoxylin and eosin stain; magnification, 20×; bar, 100 µm.
Figure 3.
Ileum. The apical surface of the enterocytes of the villi (arrowheads) are lined extensively with rod-shaped bacteria. Moderate numbers of neutrophils are present within the lamina propria of the villi (arrow). Hematoxylin and eosin stain; magnification, 40×; bar, 50 µm.
Figure 4.
Ileum. The bacteria lining the apical surface of the enterocytes are gram-negative (arrowheads). Gram stain; magnification, 40×; bar, 50 µm.
Figure 5.
Kidneys. Renal tubules contain neutrophils and cellular debris within their lumen (arrow). The epithelial cells lining the tubules are often attenuated (arrowheads). Hematoxylin and eosin stain; magnification, 40×; bar, 50 µm.
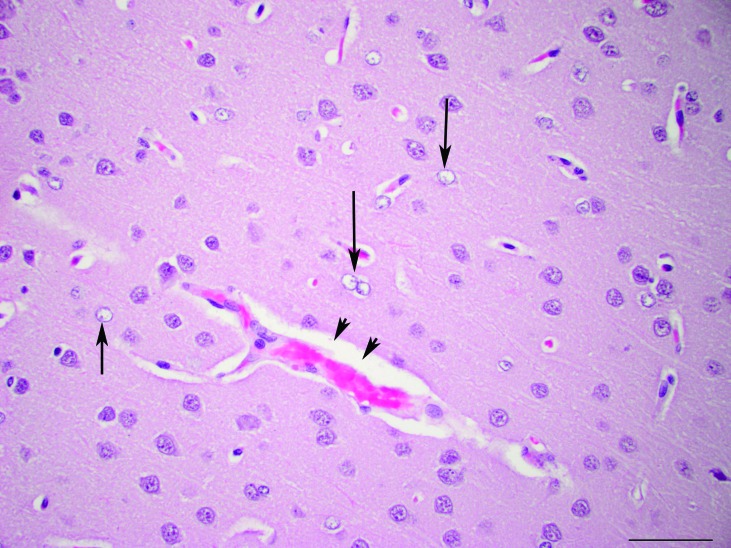
Figure 6.
Brain, cerebral cortex. Branching capillaries with expansion of the Virchow–Robins space (arrowheads). Frequent astrocytes have a large, round, vesicular nucleus and clear cytoplasm (arrow). Hematoxylin and eosin stain; magnification, 40×; bar, 50 µm.
The final diagnoses included neutrophilic enterocolitis with surface bacterial colonization, suppurative tubulonephritis, cerebral edema and congestion with reactive astrocytosis, hepatic lipidosis, neutrophilic interstitial pneumonia with fibrin thrombi, neutrophilic and erosive gastritis, lymphoid depletion, and testicular degeneration.
Kidney and spleen samples were collected aseptically at necropsy and routinely cultured aerobically and anaerobically overnight at 37 °C. Both kidney and spleen were culture-positive for Escherichia coli (Laboratory of Comparative Pathology, Memorial Sloan Kettering Cancer Center and the Weill Cornell Medical College, New York, NY). Adhesins, heat-stable enterotoxins, and shiga toxins of the E. coli isolate were genotyped through PCR analysis (Animal Health Diagnostic Center, Cornell University, Ithaca, NY). The isolate was positive for virulence factor adhesin Eae (E. coli attaching and effacing) and negative for adhesins K99, F17, and F41, heat-stable enterotoxins StaP and Stb, and shiga toxins Stx1 and Stx2. Genotyping and serotyping also were performed by the Pennsylvania State E. coli Reference Center (University Park, PA). The serotype was O13:H30 and was confirmed to be positive for Eae (intimin) and negative for heat-labile toxin (LT), heat stable toxin a, b (STa and STb), shiga toxin types 1 and 2 (Stx1, Stx2), and cytotoxic necrotizing factors 1 and 2 (CNF1, CNF2). The cecal contents were negative for Clostridium difficile toxins A and B according to ELISA (Animal Health Diagnostic Center). The combination of the microscopic findings and bacterial culture and PCR results are consistent with enteric infection and subsequent septicemia due to attaching and effacing E. coli.
Discussion
Attaching and effacing E. coli (AEEC) attach intimately to the brush border of intestinal epithelial cells, causing effacement of microvilli.17 AEEC is characterized by the presence of the Locus of Enterocyte Effacement chromosomal pathogenicity island.16,19 Within this region are genes that encode effector proteins that are delivered into host cells and cause harmful effects, including antiphagocytic activities, disruption of tight junctions, mitochondrial dysfunction, pore formation, and microvilli effacement.5,21 One essential effector is Tir (translocated intimin receptor), which adheres to mammalian cells by inserting its own receptor onto the surface of host cells triggering signaling events.5,12 The pathogenicity island also contains the eae gene, which is required for the creation of attaching and effacing lesions and which was confirmed to be present in the isolate cultured from this chinchilla.11 The eae gene encodes the virulence factor and adhesin intimin.6,7,10 Intimin is not delivered into the host cell but is an important protein that causes many effects through binding to its receptor Tir.5
There is limited information regarding AEEC infections in rodents. The eae gene is present in most highly pathogenic strains of E. coli that have been isolated from rabbits with diarrhea.2,3,19,20,23 E. coli is not considered a component of the normal gastrointestinal flora of chinchillas but rather is thought to be an opportunist.15 This organism can cause an acute fatal disease in immunocompromised patients that is aided by poor nutrition, environmental stress, and age.8,15 Septicemia due to infection with enteropathogenic E. coli has been reported to occur in chinchillas.4,15 However, this report is the first description in this species of an E. coli isolate that carries the eae gene. In reported cases of enteropathogenic E. coli, parenteral treatment with antibiotics helped to alleviate clinical signs.4,15 Because the isolate we cultured did not produce known enterotoxins or shiga toxins in concert with its attaching and effacing properties, it was deemed to be an enteropathogenic strain of E. coli.14,20
The presumptive cause of death in the case we present was acute E. coli septicemia and subsequent disseminated intravascular coagulation. The presence of large numbers of attaching and effacing E. coli in the ileum and large intestine suggest that these regions were the site of bacterial entry to the vascular system. Lesions suggestive of septicemia included the multifocal neutrophilic inflammation in the gastrointestinal tract; neutrophilic inflammation, fibrin thrombi, and edema in the lungs; and neutrophilic tubulonephritis in the kidney. Other noteworthy lesions in this case included hepatic lipidosis, lymphoid depletion in the spleen and thymus, and mild testicular degeneration. Hepatic lipidosis often occurs secondary to inappetence and has been described previously in chinchillas.1,15 Hepatic lipidosis likely led to the marked hypertriglyceridemia we noted. We hypothesize that the presence of the presumed lipid vacuoles within the circulating leukocytes resulted from its uptake from the circulation. There was no evidence suggesting a primary lipid storage disorder, and bone marrow leukocytes did not contain vacuoles.
The large amount of feed within the stomach suggested gastric stasis and ileus. The chinchilla's moribund state, presence of neutrophilic inflammation, and AEEC colonization throughout the gastrointestinal tract likely contributed to the development of stasis. The cerebral edema presumably was caused by a combination of vasculopathy due to disseminated intravascular coagulation as well as to hyponatremia. The reactive astrocytes within the brain were consistent with early Alzheimer type II astrocytes, which likely resulted from the uremic toxins present. Hepatic encephalopathy secondary to hepatic dysfunction could have contributed to the cerebral edema also.
To our knowledge, this report is the first to describe AEEC in a chinchilla. The origin of the AEEC in our case was never discovered. Domestic animals and pets have been shown to be an important natural reservoir of AEEC, with many strains also being pathogenic in humans.13 In humans, the reservoir of EPEC infections is thought primarily to be symptomatic and asymptomatic children and potentially asymptomatic adult carriers.18 EPEC has also been found in asymptomatic rabbits, mice, rats, gerbils, guinea pigs, and other laboratory species, indicating the possibility that these species may be reservoirs and potential zoonotic risks.22,23 Although the chinchillas at our facility had been housed in a separate cubicle, their housing status still presented a possible risk of exposure given the diversity of species within the room and the potential for AEEC to spread via fomites. There were no shared items or instruments between species, and because of the microbial status of the chinchillas, they were the last species to be handled. In addition, at the time of the incident clinically healthy New Zealand white rabbits were housed in the same facility (separate rooms). It is unknown whether rabbits or other species that were housed in the same room as the chinchillas were infected asymptomatically, because their feces were never cultured for E. coli. The remaining 12 chinchillas, including cage mates, remained healthy. After this incident, the chinchillas were isolated into a separate room.
Acknowledgment
We thank Dr Neil Lipman for critical review and editing of the manuscript and Naomi M Seufert and Susan Hinklein for their valuable input.
References
- 1.Association of Avian Veterinarians 2011. Proceedings of 2011 annual meeting of the Association of Avian Veterinarians, August 6–12, 2011. Seattle (WA): Association of Avian Veterinarians [Google Scholar]
- 2.Blanco JE, Blanco M, Blanco J, Mora A, Balaguer L, Cuervo L, Balsalobre C, Munoa F. 1997. Prevalence and characteristics of enteropathogenic Escherichia coli with the eae gene in diarrhoeic rabbits. Microbiol Immunol 41:77–82 [DOI] [PubMed] [Google Scholar]
- 3.Blanco JE, Blanco M, Blanco J, Mora A, Balaguer L, Mourino M, Juarez A, Jansen WH. 1996. O serogroups, biotypes, and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J Clin Microbiol 34:3101–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brem M. 1982. Untersuchungen über Erkrankungen des Magen–Darmkanals beim Chinchilla: orientierende Voruntersuchungen. Munich (Germany): University of Munich [Google Scholar]
- 5.Dean P, Kenny B. 2009. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol 12:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg MS, Whittam TS. 2001. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest 107:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg MS, Yu J, Kaper JB. 1993. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol 175:4670–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkness J, Murray K, Wagner J. 2002. Biology and diseases of guinea pigs. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 10.Jerse AE, Kaper JB. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun 59:4302–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA 87:7839–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520 [DOI] [PubMed] [Google Scholar]
- 13.Krause G, Zimmermann S, Beutin L. 2005. Investigation of domestic animals and pets as a reservoir for intimin (eae) gene-positive Escherichia coli types. Vet Microbiol 106:87–95 [DOI] [PubMed] [Google Scholar]
- 14.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev 47:510–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mans C, Donnelly T. 2011. Disease problems of chinchillas. In: Quesenberry KE, Carpenter JW, editors. Ferrets, rabbits, and rodents: clinical medicine and surgery. St Louis (MO): Elsevier–Saunders [Google Scholar]
- 16.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA 92:1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun 41:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penteado AS, Ugrinovich LA, Blanco J, Blanco M, Blanco JE, Mora A, Andrade JR, Correa SS, Pestana de Castro AF. 2002. Serobiotypes and virulence genes of Escherichia coli strains isolated from diarrheic and healthy rabbits in Brazil. Vet Microbiol 89:41–51 [DOI] [PubMed] [Google Scholar]
- 20.Pohl PH, Peeters JE, Jacquemin ER, Lintermans PF, Mainil JG. 1993. Identification of eae sequences in enteropathogenic Escherichia coli strains from rabbits. Infect Immun 61:2203–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruchaud-Sparagano MH, Muhlen S, Dean P, Kenny B. 2011. The enteropathogenic E. coli (EPEC) Tir effector inhibits NFκB activity by targeting TNFα receptor-associated factors. PLoS Pathog 7:e1002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiff LJ, Barbera PW, Port CD, Yamashiroya HM, Shefner AM, Poiley SM. 1972. Enteropathogenic Escherichia coli infections: increasing awareness of a problem in laboratory animals. Lab Anim Sci 22:705–708 [PubMed] [Google Scholar]
- 23.Swennes AG, Buckley EM, Parry NM, Madden CM, Garcia A, Morgan PB, Astrofsky KM, Fox JG. 2012. Enzootic enteropathogenic Escherichia coli infection in laboratory rabbits. J Clin Microbiol 50:2353–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washington IM, Hoosier GV. 2011. Clinical biochemistry and hematology. In: Suckow MA, Stevens KA, Wilson RP, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. New York (NY): Academic Press [Google Scholar]