Abstract
Identification of the select agent Burkholderia pseudomallei in macaques imported into the United States is rare. A purpose-bred, 4.5-y-old pigtail macaque (Macaca nemestrina) imported from Southeast Asia was received from a commercial vendor at our facility in March 2012. After the initial acclimation period of 5 to 7 d, physical examination of the macaque revealed a subcutaneous abscess that surrounded the right stifle joint. The wound was treated and resolved over 3 mo. In August 2012, 2 mo after the stifle joint wound resolved, the macaque exhibited neurologic clinical signs. Postmortem microbiologic analysis revealed that the macaque was infected with B. pseudomallei. This case report describes the clinical evaluation of a B. pseudomallei-infected macaque, management and care of the potentially exposed colony of animals, and protocols established for the animal care staff that worked with the infected macaque and potentially exposed colony. This article also provides relevant information on addressing matters related to regulatory issues and risk management of potentially exposed animals and animal care staff.
Abbreviations: CDC, Centers for Disease Control and Prevention; IHA, indirect hemagglutination assay; PEP, postexposure prophylactic
Burkholderia pseudomallei, formerly known as Pseudomonas pseudomallei, is a gram-negative, aerobic, bipolar, motile, rod-shaped bacterium. B. pseudomallei infections (melioidosis) can be severe and even fatal in both humans and animals. This environmental saprophyte is endemic to Southeast Asia and northern Australia, but it has also been found in other tropical and subtropical areas of the world.7,22,32,42 The bacterium is usually found in soil and water in endemic areas and is transmitted to humans and animals primarily through percutaneous inoculation, ingestion, or inhalation of a contaminated source.8, 22,28,32,42 Human-to-human, animal-to-animal, and animal-to-human spread are rare.8,32 In December 2012, the National Select Agent Registry designated B. pseudomallei as a Tier 1 overlap select agent.39 Organisms classified as Tier 1 agents present the highest risk of deliberate misuse, with the most significant potential for mass casualties or devastating effects to the economy, critical infrastructure, or public confidence. Select agents with this status have the potential to pose a severe threat to human and animal health or safety or the ability to be used as a biologic weapon.39
Melioidosis in humans can be challenging to diagnose and treat because the organism can remain latent for years and is resistant to many antibiotics.12,37,41 B. pseudomallei can survive in phagocytic cells, a phenomenon that may be associated with latent infections.19,38 The incubation period in naturally infected animals ranges from 1 d to many years, but symptoms typically appear 2 to 4 wk after exposure.13,17,35,38 Disease generally presents in 1 of 2 forms: localized infection or septicemia.22 Multiple methods are used to diagnose melioidosis, including immunofluorescence, serology, and PCR analysis, but isolation of the bacteria from blood, urine, sputum, throat swabs, abscesses, skin, or tissue lesions remains the ‘gold standard.’9,22,40,42 The prognosis varies based on presentation, time to diagnosis, initiation of appropriate antimicrobial treatment, and underlying comorbidities.7,28,42 Currently, there is no licensed vaccine to prevent melioidosis.
There are several published reports of naturally occurring melioidosis in a variety of nonhuman primates (NHP; Table 1). 2,10,13,17,25,30,31,35 The first reported case of melioidosis in monkeys was recorded in 1932, and the first published case in a macaque species was in 1966.30 In the United States, there have only been 7 documented cases of NHP with B. pseudomallei infection.2,13,17 All of these cases occurred prior to the classification of B. pseudomallei as a select agent. Clinical signs in NHP range from subclinical or subacute illness to acute septicemia, localized infection, and chronic infection. NHP with melioidosis can be asymptomatic or exhibit clinical signs such as anorexia, wasting, purulent drainage, subcutaneous abscesses, and other soft tissue lesions. Lymphadenitis, lameness, osteomyelitis, paralysis and other CNS signs have also been reported.2,7,10,22,28,32 In comparison, human's clinical signs range from abscesses, skin ulceration, fever, headache, joint pain, and muscle tenderness to abdominal pain, anorexia, respiratory distress, seizures, and septicemia.7,9,21,22
Table 1.
Summary of reported cases of naturally occurring Burkholderia pseudomalleiinfections in nonhuman primates
| Countrya | Imported from | Date reported | Species | Reference |
| Australia | Borneo | 1963 | Pongo sp. | 36 |
| Brunei | Unknown | 1982 | Orangutan (Pongo pygmaeus) | 33 |
| France | 1976 | Hamlyn monkey (Cercopithecus hamlyni) Patas monkey (Erythrocebus patas) | 11 | |
| Great Britain | Philippines and Indonesia | 1992 | Cynomolgus monkey (Macaca fascicularis) | 10 |
| 38 | ||||
| Malaysia | Unknown | 1966 | Macaca spp. | 30 |
| Unknown | 1968 | Spider monkey (Brachytelis arachnoides) Lar gibbon (Hylobates lar) | 20 | |
| Unknown | 1969 | Pig-tailed macaque (Macaca nemestrina) | 35 | |
| Unknown | 1984 | Banded leaf monkey (Presbytis melalophos) | 25 | |
| Singapore | Unknown | 1995 | Gorillas, gibbon, mandrill, chimpanzee | 43 |
| Thailand | Unknown | 2012 | Monkey | 19 |
| United States | Thailand | 1970 | Stump-tailed macaque (Macaca arctoides) | 17 |
| India | Pig-tailed macaque (Macaca nemestrina) | |||
| Africa | Rhesus macaque (Macaca mulatta) Chimpanzee (Pan troglodytes) | |||
| Unknown | 1971 | Chimpanzee (Pan troglodytes) | 3 | |
| Malaysia | 1981 | Pig-tailed macaque (Macaca nemestrina) | 2 | |
| Wild-caught, unknown | 1986 | Rhesus macaque (Macaca mulatta) | 13 | |
| Indonesia | 2013 | Pig-tailed macaque (Macaca nemestrina) | Current article |
Country reflects the location where the animal was housed at the time of diagosis.
Here we describe a case of melioidosis diagnosed in a pigtail macaque (Macaca nemestrina) imported into the United States from Indonesia and the implications of the detection of a select agent identified in a laboratory research colony. We also discuss the management and care of the exposed colony, zoonotic concerns regarding the animal care staff that worked with the shipment of macaques, effects on research studies, and the procedures involved in reporting a select agent incident.
Case Report
Background.
The affected pigtail macaque (Macaca nemestrina) was singly housed in accordance with the Guide for the Care and Use of Laboratory Animals16 in an AAALAC-accredited facility at the Centers for Disease Control and Prevention (CDC; Atlanta, GA). Relative humidity and temperature in the animal room were maintained at 30% to 70% and 64 to 84 °F (18 to 29 °C), respectively, under a 12:12-h light:dark cycle. Water was provided ad libitum through an automatic water delivery system. The diet consisted of a mixture of high-protein chow (Lab Diet High-Protein Monkey Diet 5045, PMI Nutrition International, Saint Louis, MO) and high-fiber chow (Fiber-Plus Monkey Diet 5049, PMI Nutrition International), fruits, and treats (Bio-serv, Frenchtown, NJ). The macaque was assigned to a research protocol but had not been accessed for research. All animal procedures and protocols were approved by the IACUC at CDC and in accordance with the Guide for the Care and Use of Laboratory Animals.16
Case presentation.
In March 2012, the 4.1-kg, 4.5-y-old purpose bred female pigtail macaque (Macaca nemestrina) was received at the CDC after completing quarantine at a CDC-registered import facility. The animal was imported into the United States from Indonesia in January 2012. The pigtail macaque was singly housed at CDC in an indoor facility. After the initial acclimation period of 5 to 7 d, the macaque underwent physical examination, which revealed a subcutaneous abscess overlying the right tibial crest with associated right inguinal lymphadenomegaly. The stifle lesion was approximately 3.0 cm by 5.0 cm. The area surrounding the stifle lesion was soft, swollen, and warm, with purulent fluid draining from a small ulcerated area. Radiographs revealed minimal soft tissue swelling, extending from the stifle joint to the middiaphyseal region of the tibia. No bone or joint involvement was noted. However, the stifle joint exhibited a decrease of approximately 50% in range of motion. A bacterial swab of the lesion, whole blood, and serum were submitted for culture, hematology, and serum chemistry analyses. The hematology and serum chemistry were within normal limits and revealed no significant findings. On culture, growth of coagulase-negative Staphylococcus and a gram-negative bacillus was noted. The gram-negative bacillus could not be furthered identified with the standard biochemical assays used by the diagnostic laboratory. Based on the results of the culture and antimicrobial susceptibility testing, treatment with amoxicillin trihydrate–clavulanate potassium (10 mg/kg) and meloxicam (0.1 mg/kg) was initiated, and the wound was flushed by using a 10% povidone–iodine solution.
The initial abscess gradually resolved over a course of 3 mo, with intermittent clinical episodes of inflammation. During this time, the macaque was continued on treatment with enrofloxacin (5 mg/kg IM), meloxicam (0.1 mg/kg PO), and amoxicillin trihydrate–clavulanate potassium (10 mg/ kg PO) until clinical signs were no longer present. The wound was flushed as needed by using 10% povidone–iodine solution. Due to a sudden return of swelling after 2 mo of antibiotic therapy, an aspirate of the joint lesion was obtained. The aspirated fluid consisted of erythrocytes and was characterized by a veterinary pathologist as consistent with a hematoma. No pathogenic organisms were seen. The lesion was lanced, drained, and flushed with povidone–iodine solution, and the swelling resolved; no further diagnostic samples were obtained. Another unspecified lesion developed surrounding the left stifle but was attributed to trauma and not thought to be a pathologic lesion.
In August 2012 (that is, 2 mo after completing treatment for the wounds surrounding the stifle joints), the macaque presented with new-onset clinical manifestations, including left head tilt, full-body tremors, and vertical nystagmus. Animal care staff reported the macaque as moving around the cage erratically with bruxism and vocalization. On physical examination, a lack of pupillary light reflex was noted, along with complete stiffness and muscle rigidity of the entire body. In light of the macaque's clinical presentation, differential diagnoses included tetanus, Streptococcus pneumonia meningitis, otitis interna, and Guillain–Barré syndrome. Complete stiffness and muscle rigidity with neurologic signs placed tetanus and meningitis as the top differential diagnoses.
The stifle was radiographed, and diagnostic testing, including hematology, serum chemistry, and urinalysis, was performed. Blood culture analysis was not performed. There were no significant findings seen on the hematology report or radiographs. Collection of CSF at the cisterna magna was attempted but was unsuccessful due to the dense composition of the CSF fluid. Hyperglycemia, glycosuria, and ketonuria were noted on the serum chemistry and urinalysis results. Over the course of 2 d, despite medical treatment, the macaque progressed to inability to ambulate, vertical nystagmus with anisocoria, and hypothermia. The animal underwent cardiac and respiratory arrest while receiving supportive care including sodium chloride fluids (50 mL/kg), buprenorphine (0.1 mg/kg), regular insulin (0.25 U/kg), penicillin (20,000 U/kg), and dexamethasone (1 mg/kg) and therefore was submitted to the Infectious Disease Pathology Branch at CDC for necropsy.
Results
Pathology.
The necropsy was conducted on a down-draft table, with all participants wearing either an N95 respirator or a powered air-purifying respirator and additional appropriate personal protective equipment. At necropsy, the macaque was thin, with a body condition score of 2.5 on a 5.0-point scale. There was scar tissue on the skin of the right stifle, but all underlying tissues, including the joint space, were grossly normal. Pertinent gross findings included a focus of suppurative pneumonia, multiple small areas of necrosis and abscessation with hemorrhage in the brainstem (Figure 1 A), and diffuse suppurative meningitis. These findings were confirmed by histopathology (Figure 1 B). Gram-negative bacilli were identified within lesions in the brainstem, spinal meninges, lung, and liver. These bacilli stained positively for B. pseudomallei during immunohistochemistry (Figure 1 C). A confirmatory diagnosis of B. pseudomallei was obtained via bacterial culture and PCR analysis. PCR testing in liver and lung tissues was positive for B. pseudomallei also. More details on the pathology of this case can be found as previously described.31
Figure 1.
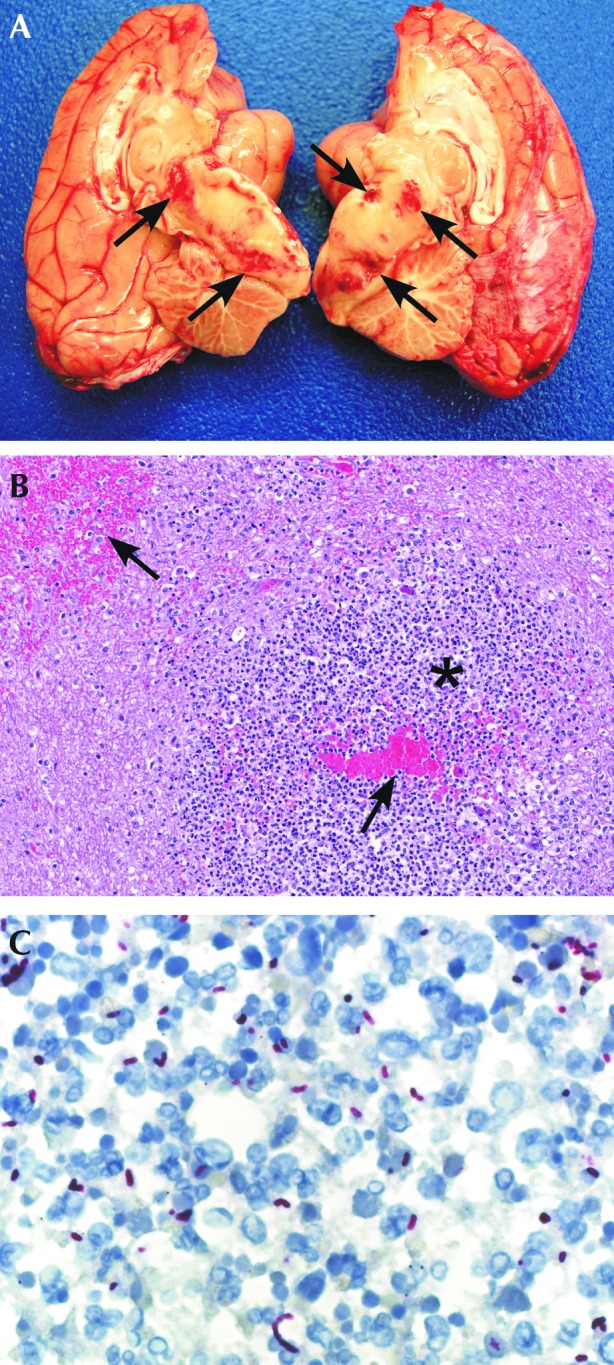
Pathology of Burkholderia pseudomallei in the brain of a pigtail macaque (Macaca nemestrina). (A) Areas of necrosis with hemorrhage (arrows) throughout the brainstem. (B) Microabscess (*) with hemorrhage (arrow) in the brainstem. Hematoxylin-eosin, 100 × . (C) Bacilli show positive immunoreactivity by immunohistochemistry using an antiBurkholderia pseudomallei antibody, Fast Red chromogen, and Mayer modified hematoxylin counterstaining; magnification, 400×. Reprinted and adapted from reference 31 with permission.
B. pseudomallei identification and characterization. Preliminary and confirmatory identification.
Culture of B. pseudomallei from any specimen in a suspected case of melioidosis remains the gold standard for diagnosis. Swabs taken at necropsy from the brainstem abscess and spinal meningeal exudates were submitted to the Georgia Laboratory Animal Diagnostic Service (Athens, GA) for culture. After a gram-negative rod was isolated on MacConkey agar, testing included the Gen III system (Biolog, Haward, CA) and 16S rRNA sequencing, which indicated B. pseudomallei.31 The isolate then was submitted to the Bacterial Special Pathogens Branch at CDC, where confirmation of B. pseudomallei followed the guidelines for identification prescribed by the Laboratory Response Network, including biochemical testing and PCR analysis.
Multilocus sequence typing.
The isolate was genotyped by using multilocus sequence typing as previously described;14,15 this assay uses segments of sequences of 7 housekeeping genes to assess levels of relatedness. Each gene sequence is assigned a designation or allele. The more alleles that match of the 7, the more related the strains are considered to be. The isolate was found to be sequence type 46 (ST 46). ST 46 has been reported in B. pseudomallei strains from Indonesia, Bangladesh, Malaysia, Thailand, Vietnam, and Cambodia. The isolate from Indonesia was recovered from a long-tailed macaque (Macaca fascicularis synonym M. cynomolgus) imported into Great Britain.15,38 The other isolates were obtained from human clinical cases or from environmental samples.
Antimicrobial susceptibility.
Because B. pseudomallei can be resistant to multiple antimicrobials, including first-, second-, and third-generation cephalosporins, aminoglycosides, penicillins, and polymixin, the isolate was submitted for antimicrobial susceptibility testing using the broth microdilution method. Susceptibility testing included carbapenems (meropenem and imipenem), ceftazidime, amoxicillin–clavulanic acid, doxycycline, tetracycline, and trimethoprim–sulfamethoxazole. The isolate was susceptible to all antimicrobials except for tetracycline, for which it was intermediate in susceptibility.
Melioidosis serology.
The indirect hemagglutination assay (IHA) is the most widely used and accepted serologic assay for detecting antibodies to B. pseudomallei, the cause of melioidosis.5,22 The IHA detects the agglutination of serum antibodies incubated alongside sheep RBC to polysaccharide and lipopolysaccharide antigens derived from strains of B. pseudomallei. The IHA is an inhouse assay and is not available commercially, so the strains used for antigen preparation can vary by source and lot number. At the CDC, we use B. pseudomallei strains from the 2 major geographic regions endemic for melioidosis, southeast Asia and northern Australia. Although the IHA is only validated for use with human specimens, testing with animal sera has been reported previously.23 Cut-off titers for determining positivity vary; CDC uses 1:40 for human cases, but for animals, specifically NHP, cutoff titers of 1:160 or 1:320 have been used previously.17,23 For this case, we used a cutoff of 1:320 for NHP.
Immunohistochemistry was performed as previously described.1 A total of 3 serum samples from the pigtail macaque were available for testing. These included a baseline serum sample, taken when the macaque arrived at CDC on 15 March 2012; a sample taken 10 May 2012; and a sample taken 20 August 2012 just prior to death. Immunohistochemistry was performed, and titers of 1:1280, 1:2560, and 1:1280, respectively, showed antibodies reactive with B. pseudomallei.
Colony management.
Animals were considered potentially exposed if they were a part of the same shipment of animals originating in Indonesia or housed in the same room with the pigtail macaque infected with B. pseudomallei. All macaques from this shipment were returned to quarantine status. Fortunately, the NHP that arrived with this shipment were not introduced or cohoused with other animals already housed at our facility. We restricted the handling and care of the potentially exposed colony of pigtail macaques to designated personnel, established a foot pattern for entrance into the animal-housing area, and placed appropriate signage in the suite to alert personnel to the restrictions. Biosafety in Microbiologic and Biomedical Laboratories recommends that work with clinical specimens obtained from an animal suspected of having melioidosis be performed by using BSL2 procedures and containment practices and that a respirator should be worn when handling known Burkholderia-infected animals.4 Because the National Institute of Occupational Health and Safety recommends the use of particulate respirators to reduce exposures to animal allergens and prevent animal-induced asthma and allergies,27 it is standard practice for all CDC animal care staff to wear N95 respirators as a part of their personal protective equipment for ABSL2 facilities. Given the potential for animal care staff to have exposure to B. pseudomallei via aerosolized water droplets, the requirements regarding personal protective equipment for designated personnel assigned to the care of this potentially exposed group of animals were elevated to wearing powered air-purifying respirators during animal room wash-down and cage-change–out procedures. The disinfectant (Environ Vesphene II, Steris, Mentor, OH) typically used throughout our facility was continued. No added measures for sanitizing were taken before removal of equipment from the potentially exposed animal rooms.
Although none of the other 40 pigtail macaques that arrived with this shipment displayed clinical signs similar to those of the infected animal and although the efficacy of postexposure prophylaxis after 48 h is not documented, the decision was made to provide the macaques postexposure prophylaxis.29 Doxycycline (50 mg/mL, 2.5 mg/kg daily) and trimethoprim–sulfamethoxazole (280 mg/5 mL, 25 mg/kg twice daily) were administered for 3 wk. Macaques also were given vanilla-flavored yogurt supplemented with 250 mg Saccharomyces boulardii lyo (Florastor, Biocodex USA, San Bruno, CA) daily in a disposable cup to provide probiotics for the intestinal microbiota. Blood samples were collected from the 40 pigtail macaques that arrived in the same shipment as the animal infected with B. pseudomallei to measure antibody titers for B. pseudomallei by IHA. Samples included baseline sera and specimens obtained 4 and 6 wk after the discovery of the sick animal. Five pigtail macaques demonstrated titers at or above the 1:320 cutoff (Table 2). All titers were stable, and follow-up testing 6 mo later showed no rise in antibody titer in any of the colony.
Table 2.
B. pseudomalleiIHA titers in pigtail macaques lacking clinical signs
| Animal | Date of sample | IHA titer |
| PT1 | 3/15/2012 | 1:5120 |
| 9/20/2012 | 1:5120 | |
| 10/16/2012 | 1:2560 | |
| 3/11/2013 | 1:5120 | |
| PT2 | 3/15/2012 | 1:320 |
| 9/20/2012 | 1:320 | |
| 10/16/2012 | 1:320 | |
| 3/11/2013 | 1:320 | |
| PT3 | 3/15/2012 | 1:320 |
| 9/20/2012 | 1:320 | |
| 10/16/2012 | 1:320 | |
| 3/11/2013 | 1:320 | |
| PT4 | 3/15/2012 | 1:10240 |
| 9/20/2012 | 1:10240 | |
| 10/16/2012 | 1:10240 | |
| 3/11/2013 | 1:10240 | |
| PT5 | 3/15/2012 | 1:40 |
| 9/20/2012 | 1:640 | |
| 10/16/2012 | 1:320 | |
| 3/11/2013 | 1:40 |
Antibody titers to B. pseudomallei were evaluated from blood samples collected from potentially exposed macaques on arrival to the CDC facility and at 4 wk, 6 wk, and 6 mo after diagnosis of B. pseudomallei in the index macaque. The titers of the animals listed in the table were all positive according to a cut-off value of ≥ 1:320. None of these macaques exhibited clinical signs of infection.
Management protocol for animal care staff.
Animal care personnel were assembled for an informational staff meeting to discuss possible exposure risks associated with handling infected and potentially infected animals. B. pseudomallei subject-matter experts from CDC's Bacterial Special Pathogens Branch, occupational health and safety personnel (a physician and microbiologist), veterinary staff, and senior management officials were present to address questions and concerns. A list was compiled of all personnel (technicians, veterinarians, husbandry staff, laboratorians, and pathology staff) that may have had contact with the Burkholderia-infected animal or the animals potentially exposed to the infected animal.
Criteria for potential exposure were developed to perform a risk assessment. Subsequently, a medical risk assessment questionnaire (Figure 2) was devised and disseminated to animal care staff and laboratory personnel with potential occupational exposure. The risk assessment included queries about underlying conditions likely to increase the risk of melioidosis, including diabetes and chronic liver and kidney diseases. The staff were instructed to complete the medical risk assessment questionnaire and report to the CDC Occupational Health and Safety Clinic for medical and environmental risk evaluation. In addition, animal care staff received additional training, with topics including appropriate cleaning and husbandry procedures to minimize potential exposure, clinical signs of melioidosis in humans and animals, and the appropriate use of powered air-purifying respirators for staff that were assigned to the room. Those staff identified during the risk assessment who had potential exposure to the infected macaque were given thermometers and daily fever logs to record their temperatures and were advised to provide serial blood samples for serologic testing. Samples submitted included baseline sera (taken at employment), serum taken at discovery of infected animal in August, and samples taken after 2 wk. All samples were negative and showed no rise in titers between baseline, acute, and convalescent sera.
Figure 2.
This questionnaire was given by occupational health and safety to all employees that had contact with the infected melioidosis animal or potentially exposed animals housed at CDC. According to the answers provided by each employee, the potential risk of exposure and level of risk were determined, and postexposure monitoring was recommended.
Reporting and notification procedures.
The APHIS–CDC Select Agent Regulations (Title 42 Code of Federal Regulations Part 73, Title 9 Code of Federal Regulations Part 121, and Title 7 Code of Federal Regulations Part 121) were devised and implemented to establish requirements for possession, use, and transfer of select agents and toxins. There is a possibility for NHP to harbor or acquire naturally occurring pathogens that are classified as select agents according to the National Select Agent Registry Program. APHIS–CDC Select Agent Regulations and guidance documents state the appropriate timeframe for reporting by telephone, fax, or email the theft, loss, and release of a select agent based on the pathogen or toxin.39 Because B. pseudomallei is classified as a Tier 1 select agent, diagnostic identification requires immediate notification to the Division of Select Agents and Toxins. However, during the time that this organism was identified, B. pseudomallei had not yet been classified as a Tier 1 select agent.
We contacted the CDC Responsible Official immediately after obtaining all information required by the regulation. According to APHIS–CDC Select Agent Regulations,32 the melioidosis case had to be reported as an identification of a select agent and the release of a select agent because of the following: (1) B. pseudomallei was newly identified in an animal and its clinical samples; (2) the incident was considered a possible occupational exposure to a select agent; and (3) the incident required postexposure medical surveillance. The forms for identification of a select agent (CDC–APHIS Form 4A) and for its theft, loss, or release (CDC–APHIS Form 3) were completed in accordance with the immediate regulatory reporting requirements of the Division of Select Agents and Toxins. CDC was notified within 24 h after receiving the report of identification of a select agent from the B. pseudomallei-infected animal. In addition, the disposition of the infected carcass and clinical specimens containing B. pseudomallei was reported to the Division of Select Agents and Toxins.
Because the isolate was obtained from a NHP imported into the United States, the Division of Global Migration and Quarantine at CDC was contacted. Information reported to Division of Global Migration and Quarantine included the commercial animal vendor, number of recently imported animals in the potentially exposed shipment, compiled medical history and diagnostics on the infected macaque, the laboratories that performed diagnostics on samples obtained from the B. pseudomallei-infected macaque, and the commercial courier that transported the animals. The CDC Bacterial Special Pathogens Branch and Office of Safety, Health, and Environment were consulted for their expertise in conducting occupational health and medical surveillance, epidemiologic investigations, and environmental health assessments. As a professional courtesy, we contacted the commercial vendor from whom we received the recently imported colony with the melioidosis-infected animal and told the vendor of our reporting actions.
Discussion
The pigtail macaque that is described in this case report was imported from Indonesia and died as a result of B. pseudomallei infection. Our report is unique in that it not only describes clinical manifestations and diagnosis of naturally occurring melioidosis in an Indonesian pigtail macaque imported into the United States, but it also presents the current regulatory requirements, management considerations, and practices that were implemented for both animals and animal care personnel potentially exposed to B. pseudomallei.
There are few reports of NHP infected with B. pseudomallei from Indonesia, and, to our knowledge, none of the NHP previously described were pigtail macaques (Macaca nemestrina). Naturally occurring melioidosis has rarely been encountered and reported in a laboratory animal facility with NHP, so it is not readily considered as a differential diagnosis. However, the low number of reported cases of melioidosis in NHP from southeast Asia in a laboratory setting may be a result of institutions not recognizing, diagnosing, or reporting the disease.
Clinical signs of melioidosis vary considerably in humans and animals. We speculate that the clinical signs that manifested in the B. pseudomallei-infected pigtail macaque in this case report were due to chronic melioidosis, likely attained by percutaneous inoculation in the country of origin, given the initial stifle lesion. The disease later became systemic, affecting the CNS, respiratory system, and abdominal organs and resulting in the macaque's death. Postmortem evaluation of our B. pseudomallei-infected macaque revealed respiratory infection similar to cases reported in humans and NHP.10,17,30,32 The CNS infection with manifestation of prominent neurologic signs is an unusual occurrence, with cranial nerve palsy associated with brainstem encephalitis in humans.6,18,21,26,42 Three previous reports have acknowledged neurologic melioidosis in NHP that exhibited clinical signs of paralysis.10,13,17 We recommend that melioidosis be considered as a differential diagnosis in NHP that come from Burkholderia pseudomallei-endemic areas and that exhibit similar clinical signs as described in this and previous case reports.2,13,17,25,30,32,35,38
We used IHA as a screening test for melioidosis to measure antibody response after exposure to B. pseudomallei. Serologic examination can provide presumptive evidence of infection with B. pseudomallei, but it is not considered to be confirmatory for the diagnosis of melioidosis. NHP in B. pseudomallei-endemic areas can exhibit high titers without evidence of clinical disease or latent infection antemortem or postmortem.5,17,30 Some authors have defined titers of 1:80 to 1:160 in NHP as suspect for infection with B. pseudomallei, with titers of greater than or equal to 1:320 being highly suggestive of active infection.17 Other animal surveys have used a cutoff of 1:40 to estimate prevalence of infection.24,34 A 2012 report of melioidosis in animals from Thailand used an IHA positive cutoff value of greater than or equal to 1:320 as providing evidence of melioidosis as the cause of death, but the authors excluded animals from the study that lacked culture confirmation in light of the high titers that can be seen in apparently healthy animals in melioidosis-endemic areas.24,34
Although we discussed culling all potentially exposed animals or those with high titers because of the risk of latent infection and zoonotic transmission, a unanimous decision was made not to cull any of the animals based on the following factors: (1) there was negligible risk of zoonotic and epizootic transmission of melioidosis in the laboratory setting; (2) the macaques were separately housed away from other NHP previously housed in our indoor vivarium; (3) no other potentially exposed animals displayed clinical signs of active infection; and (4) there was no rise in IHA titers in the series of sera samples tested. To prospectively follow these macaques, we developed a clinical assessment protocol for observation of these NHP for active signs of infection. Steps in the clinical assessment state that when a macaque in the potentially exposed colony shows a significant (that is, at least 4-fold) rise in antibody titer compared with baseline serum samples or active signs of infection (or both), it will be culled from this colony and screened for melioidosis. Moreover, the potentially exposed colony will remain separate from the general colony, and serologic tests for melioidosis will be performed on this cohort of animals at the time of routine semiannual and annual physical examinations. We plan to follow this protocol until the animals are no longer housed in our vivarium or are deceased. Initially, there was concern that the colony of pigtail macaques might not be useful for a specific research protocol as originally planned. CDC subject-matter experts and principal investigators reviewed information and concluded that latent B. pseudomallei infection, if present, likely will not affect or confound proposed research in these macaques.22,28 Therefore, the potential exposure to B. pseudomallei was not considered a confounding factor for the potentially exposed animals, and on release from postexposure prophylaxis quarantine, they were assigned for use in research studies but remained separate from the general colony. However, as another alternative, we suggested that the macaques with high positive titers be used for terminal pharmacokinetic studies.
In view of reports that B. pseudomallei exhibits innate resistance to multiple antimicrobials, a select-agent–registered laboratory at CDC performed susceptibility testing on the postmortem isolate that was obtained prior to administration of postexposure prophylaxis in the rest of the potentially exposed colony. The antibiotic combination (doxycycline and trimethoprim–sulfamethoxazole) chosen for postexposure prophylaxis was based on the susceptibility results, recommendations by CDC subject-matter experts, and published reports.12,29 However, the most current literature recommendation is that trimethoprim–sulfamethoxazole can be used alone rather than in conjunction with doxycycline.42 The duration of postexposure prophylaxis should be at least 14 d, but a 21-d course of therapy has been recommended also.29
There are several management issues that veterinarians should consider regarding B. pseudomallei-infected NHP or potentially infected colonies. At our facility, these issues included occupational health and safety, colony management, biosafety and security, regulatory and reporting procedures, and scientific integrity of the research. Work with clinical specimens from animals suspected of having melioidosis can be performed with ABSL2 practices, containment, equipment, and facilities. However, ABSL3 practices are recommended for the culture or manipulation of B. pseudomallei, or when infectious aerosols or droplets will be produced or generated.4 Veterinarians, animal care staff, and laboratorians should follow specific precautions to avoid exposure, including the use of gloves and protective clothing when working with infected animals or collecting diagnostic samples.
In conclusion, we encourage laboratory facilities to increase their awareness of the clinical signs and diagnostic recommendations for melioidosis in NHP and other laboratory animals so that precautionary measures can be implemented when indicated to avoid potential exposures. These precautions can include staff training in appropriate husbandry procedures for a potentially exposed colony of animals; appropriate use of personal protective equipment; storage of baseline serum samples from animal care staff and NHP; enhanced containment procedures, treatment and diagnostic procedures; and safe handling of diagnostic samples. Because infections of melioidosis are confined to specific world regions, we consider that screening for B. pseudomallei need not be added to routine screening of domestic-bred and raised NHP but should be considered as a differential diagnosis when applicable. Specifically, we recommend that any NHP imported into the United States from an endemic area that exhibits clinical signs of melioidosis (for example, abscesses, osteomyelitis, neurologic clinical signs) should be tested via IHA for B. pseudomallei, with confirmation by culture and PCR analysis. Furthermore, we urge laboratory animal facilities to become more aware of melioidosis so that, when present, the disease can be reported in a timely manner to local, state, and national agencies for the purpose of providing additional information for global NHP distribution and surveillance databases. In addition, reporting may enhance the development of suitable animal models, screening assays, and appropriate treatment regimens and the establishment of endpoints for future biomedical research.
Acknowledgments
We thank the managers and technicians in the Animal Resources Branch at CDC for their efforts in supporting the care of the animals housed at our facility. Special acknowledgments are also extended to Dr Jana Ritter and Dr Clifton Drew (Infectious Disease Pathology Branch, CDC) for providing necropsy and histopathology information and David Lonsway (Division of Healthcare Quality Promotion, CDC) for performing antimicrobial susceptibility testing. We also thank Ms Joanne Jones (Responsible Official, CDC); the Division of HIV–AIDS Prevention, Laboratory Branch (CDC); and the numerous people who assisted in the review of this article. Clinical care of the animals discussed in this case report was supported in part by the Laboratory Animal Medicine Residency Program at CDC.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Alexander AD, Huxsoll DL, Warner AR, Jr, Shepler V, Dorsey A. 1970. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol 20:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt JO, Jr, Howard EB, Kean CJ, Jones J. 1981. Melioidotic osteomyelitis in an imported primate. J Am Vet Med Assoc 179: 1303–1305 [PubMed] [Google Scholar]
- 3.Butler TM, Schnidt RE, Wiley GL.1971. Melioidosis in a chimpanzee. Am J Vet Res 32:1109–1117 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention and National Institutes of Health 2009. Biosafety in microbiological and biomedical laboratories, 5th ed Washington (DC): Department of Health and Human Services [Google Scholar]
- 5.Cheng AC, O'Brien M, Freeman K, Lum G, Currie BJ. 2006. Indirect hemagglutination assay in patients with melioidosis in northern Australia. Am J Trop Med Hyg 74:330–334 [PubMed] [Google Scholar]
- 6.Currie BJ, Fisher DA, Howard DM, Burrow JN. 2000. Neurological melioidosis. Acta Trop 74:145–151 [DOI] [PubMed] [Google Scholar]
- 7.Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20-year Darwin prospective study. PLoS Negl Trop Dis 4:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dance DA. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human–animal hosts. Acta Trop 74:159–168 [DOI] [PubMed] [Google Scholar]
- 9.Dance DA. 2002. Melioidosis. Curr Opin Infect Dis 15: 127–132 [DOI] [PubMed] [Google Scholar]
- 10.Dance DA, King C, Aucken H, Knott CD, West PG, Pitt TL. 1992. An outbreak of melioidosis in imported primates in Britain. Vet Rec 130:525–529 [DOI] [PubMed] [Google Scholar]
- 11.Demontoy-Bomsel MC, Chove MA. 1976. [Aspects cliniques de la melioidosis dans un zoo]. Rec Med Vet 152:663–669 [Article in French] [Google Scholar]
- 12.Estes DM, Dow SW, Schweizer HP, Torres AG. 2010. Present and future therapeutic strategies for melioidosis and glanders. Expert Rev Anti Infect Ther 8:325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz PE, Miller JG, Slayter M, Smith TJ. 1986. Naturally occurring melioidosis in a colonized rhesus monkey (Macaca mulatta). Lab Anim 20:281–285 [DOI] [PubMed] [Google Scholar]
- 14.Glass MB, Steigerwalt AG, Jordan JG, Wilkins PP, Gee JE. 2006. Burkholderia oklahomensis sp. nov., a Burkholderia pseudomallei-like species formerly known as the Oklahoma strain of Pseudomonas pseudomallei. Int J Syst Evol Microbiol 56:2171–2176 [DOI] [PubMed] [Google Scholar]
- 15.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol 41:2068–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 17.Kaufmann AF, Alexander AD, Allen MA, Cronin RJ, Dillingham LA, Douglas JD, Moore TD. 1970. Melioidosis in imported nonhuman primates. J Wildl Dis 6:211–219 [DOI] [PubMed] [Google Scholar]
- 18.Kung CT, Li CJ, Ko SF, Lee CH. 2013. A melioidosis patient presenting with brainstem signs in the emergency department. J Emerg Med 44:e9–e12 [DOI] [PubMed] [Google Scholar]
- 19.Lazar Adler NR, Govan B, Cullinane M, Harper M, Adler B, Boyce JD. 2009. The molecular and cellular basis of pathogenesis in melioidosis: how does Burkholderia pseudomallei cause disease? FEMS Microbiol Rev 33:1079–1099 [DOI] [PubMed] [Google Scholar]
- 20.Lim SY, Mukundhan V. 1968. Melioidosis in a spider monkey and a gibbon. Kajian Veterinaire 1:180–183 [Google Scholar]
- 21.Limmathurotsakul D, Chaowagul W, Wongsrikaew P, Narmwong A, Day NP, Peacock SJ. 2007. Variable presentation of neurological melioidosis in northeast Thailand. Am J Trop Med Hyg 77:118–120 [PubMed] [Google Scholar]
- 22.Limmathurotsakul D, Peacock SJ. 2011. Melioidosis: a clinical overview. Br Med Bull 99:125–139 [DOI] [PubMed] [Google Scholar]
- 23.Limmathurotsakul D, Thammasart S, Warrasuth N, Thapanagulsak P, Jatapai A, Pengreungrojanachai V, Anun S, Joraka W, Thongkamkoon P, Saiyen P, Wongratanacheewin S, Day NP, Peacock SJ. 2012. Melioidosis in animals, Thailand, 2006–2010. Emerg Infect Dis 18:325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meekaprateep M, Jiwakanon N, Jongkajornpong L, Wara-assawapite W, Leesirikul N. 1998. Use of indirect hemagglutination (IHA) test for serodiagnosis of melioidosis in dairy cow. J Thai Vet Med Assoc 49:35–44 [Google Scholar]
- 25.Mutalib AR, Sheikh-Omar AR, Zamri M. 1984. Melioidosis in a banded leaf monkey (Presbytis melalophos). Vet Rec 115: 438–439 [DOI] [PubMed] [Google Scholar]
- 26.Muthusamy KA, Waran V, Puthucheary SD. 2007. Spectra of central nervous system melioidosis. J Clin Neurosci 14:1213–1215 [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Occupational Safety and Health 1998. Preventing asthma in animal handlers. NIOSH alert. Publication No. 97–116
- 28.Peacock SJ. 2006. Melioidosis. Curr Opin Infect Dis 19: 421–428 [DOI] [PubMed] [Google Scholar]
- 29.Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V, DeShazer D, Steinmetz I, Tan P, Currie BJ. 2008. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis 14:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retnasabapathy A, Joseph PG. 1966. A case of melioidosis in a macaque monkey. Vet Rec 79:72–73 [DOI] [PubMed] [Google Scholar]
- 31.Ritter JM, Sanchez S, Jones TL, Zaki SR, Drew CP. 2013. Neurologic melioidosis in an imported pigtail macaque (Macaca nemestrina). Vet Pathol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Sprague LD, Neubauer H. 2004. Melioidosis in animals: a review on epizootiology, diagnosis, and clinical presentation. J Vet Med B Infect Dis Vet Public Health 51:305–320 [DOI] [PubMed] [Google Scholar]
- 33.Smith NR, Damit M. 1982. Fatal bronchopneumonia in a young orang utan caused by Pseudomonas pseudomallei. Vet Rec 110:251–252 [DOI] [PubMed] [Google Scholar]
- 34.Srikitjakarn L, Sirimalaisuwan A, Khattiya R, Krueasukhon K, Mekaprateep M. 2002. Seroprevalence of melioidosis in dairy cattle in Chiang Mai Province, northern Thailand. Southeast Asian J Trop Med Public Health 33:739–741 [PubMed] [Google Scholar]
- 35.Strauss JM, Jason S, Lee H, Gan E. 1969. Melioidosis with spontaneous remission of osteomyelitis in a macaque (Macaca nemestrina). J Am Vet Med Assoc 155:1169–1175 [PubMed] [Google Scholar]
- 36.Tammemagi L, Johnston LAY. 1963. Melioidosis in an orang-utan in north Queensland. Aust Vet J 39:241–242 [Google Scholar]
- 37.Thibault FM, Hernandez E, Vidal DR, Girardet M, Cavallo JD. 2004. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J Antimicrob Chemother 54:1134–1138 [DOI] [PubMed] [Google Scholar]
- 38.Trakulsomboon S, Pitt TL, Dance DA. 1994. Molecular typing of Pseudomonas pseudomallei from imported primates in Britain. Vet Rec 135:65–66 [DOI] [PubMed] [Google Scholar]
- 39.United States Department of Health and Human Services, United States Department of Agriculture. [Internet]. National Select Agent Registry. January 7, 2013. [Cited January 2013]. Available at: http://www.selectagents.gov/, http://www.selectagents.gov/resources/list_of_select_agents_and_toxins_2012-12-4-english.pdf.
- 40.White NJ. 2003. Melioidosis. Lancet 361:1715–1722 [DOI] [PubMed] [Google Scholar]
- 41.Wiersinga WJ. 2010. Beyond antibiotics: new horizons in treating Burkholderia species infections. J Infect Dis 201:1786–1787 [DOI] [PubMed] [Google Scholar]
- 42.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N Engl J Med 367:1035–1044 [DOI] [PubMed] [Google Scholar]
- 43.Yap EH, Thong TW, Tan AL, Yeo M, Tan HC, Loh H, Teo TP, Thong KT, Singh M, Chan YC. 1995. Comparison of Pseudomonas pseudomallei from humans, animals, soil and water by restriction endonuclease analysis. Singapore Med J 36:60–62 [PubMed] [Google Scholar]



