Abstract
Immunocompromised mice were infected intranasally with Aspergillus fumigatus as part of a vaccine efficacy study. Although body temperature was measured throughout the study, a formal evaluation of its usefulness as an endpoint criterion was not performed. We retrospectively evaluated survival data and temperature records to determine whether body temperature can be used as an objective predictor of death and included in the humane endpoint criteria for this mouse model. CF1 mice were immunosuppressed with either cortisone acetate or by treatment with antiGR1 (a neutrophil-depleting antibody) and then intranasally challenged with A. fumigatus. Body temperature was measured by using an infrared noncontact thermometer a maximum of 3 times daily until death or euthanasia. A surface body temperature below 29.0 °C was correlated with a poor chance of survival, and using this cutoff point with signs of morbidity (hunched, ruffled fur, respiratory distress) reliably indicates mice for euthanasia without negatively affecting data collection. Using 2 subsequent readings of less than 31.0 °C as an endpoint would have led to premature euthanasia of only one mouse (2.2%). As a single reading, a body temperature of 28.8 °C had a sensitivity of 92.2% and specificity of 90.9%. Hypothermia proved to be a useful addition to the humane endpoint criteria for this mouse model, and veterinary and research groups should discuss their study needs in relation to animal welfare to best determine the most appropriate means of including this parameter.
Abbreviations: BT, body temperature; IPA, invasive pulmonary aspergillosis; ROC, receiver operator characteristics
Aspergillus species ubiquitously inhabit the environment, and people inhale as many as a few hundred conidia daily.1 The conidia of many Aspergillus species are relatively large and typically are deposited in the paranasal sinuses and upper airways, whereas A. fumigatus conidia are small enough to reach the pulmonary alveoli.1 Although these conidia are essentially benign for healthy persons, those who are immunosuppressed can develop severe disease known as invasive pulmonary aspergillosis (IPA).8 This fungal disease is one of the most severe and often fatal complications associated with patients undergoing immunosuppressive treatment, such as those with hematologic malignancies who have received a hematopoietic cell transplant.3 Given the severe nature of IPA and the high mortality rate in this high-risk population, an animal model that accurately reflects the natural progression of the disease is imperative for vaccination and treatment studies.
One of our investigators has been working on developing an A. fumigatus vaccine by using a murine model of aspergillosis. IPA is induced in immunosuppressed mice after intranasal inoculation. This model closely mimics the bronchopneumonia seen in human patients and therefore has historically been one of the more popular animal models for studying the disease.2 Similar to the infection seen in humans, mice develop severe respiratory distress and have a high mortality rate. The mortality tends to be fairly acute, within 24 to 72 h after inoculation, and it can be very difficult to predict which mice will survive and which will eventually succumb to infection. The humane endpoints that the investigator had being using with this model were somewhat subjective. Given our goal to refine experiments as much as possible to enhance animal welfare and reduce animal suffering and morbidity, the veterinary care staff met with the investigative group in an attempt to further refine the endpoints and to include some objective criteria.
Because hypothermia has been discussed as a quick and reliable indicator of mortality in several animal models,7,12,14-16,19-22 we investigated its use as a potential endpoint in our model. However, when looking through the literature, we were unable to find information on using body temperature (BT) measurements as a predictor of death in an animal model similar to our intranasal inoculation of Aspergillus. The publications we found mostly focused on bacterial12,13,15,19,20 or viral infections,18,19,22 with only one fungal model that involved Candida albicans.19,21 These studies recommended vastly different hypothermic endpoints, suggesting variation depending on the infectious agent, temperature recording device, and model used. For example, a study evaluating a staphylococcal enterotoxic shock model found that mice reaching a body temperature as low as 23.4 °C had an equal chance of dying or surviving,20 whereas a model of influenza virus infection led to a hypothermic endpoint recommendation of 32 °C.22 The fungal infection model involved intravenous inoculation of Candida and recommended adopting an endpoint of 33.3 °C.21 However, the Candida study21 used a different fungal species and route of inoculation than did our IPA model. Given the variety of endpoints recommended for the various infectious diseases and animal models, we decided that data directly from our model were necessary to independently evaluate the usefulness of a hypothermic endpoint for our particular model of interest. BT measurements were already incorporated into the design of these studies, so we retrospectively analyzed the data in an attempt to identify a specific hypothermic endpoint for this murine model of IPA.
Materials and Methods
All animal procedures were performed in an AAALAC-accredited facility and approved by the City of Hope Beckman Research Institute IACUC. All experiments had taken place before the decision was made to evaluate BT data as a humane endpoint; therefore the current study is a retrospective assessment of mice inoculated with A. fumigatus under several different experimental conditions.
Animals.
Data from 4 independent experiments comprising a total of 122 mice were included in the current study. CF1 female mice were purchased from Charles River Laboratories (Hollister, CA) at 6 to 8 wk of age. Mice were designated by the vendor as SPF for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse norovirus, Theiler murine enchephalomyelitis virus, mouse reovirus type 3, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus 1 and 2, mouse cytomegalovirus, polyoma virus, K virus, mouse thymic virus, Hantaan virus, Prospect Hill virus, cilia-associated respiratory bacillus, E. cuniculi, and Mycoplasma pulmonis. They were also free of Helicobacter spp., Tyzzer disease virus, and endo- and ectoparasites. Mice were kept on a 12:12-h light:dark cycle and allowed to acclimate for at least 1 wk prior to beginning the experiment. They were housed 10 per cage on hardwood bedding (Sani-Chips, PJ Murphy Forest Products, Montville, NJ) in conventional large mouse caging (Allentown, Allentown, NJ) in a BSL2 barrier in our animal facility. They had free access to irradiated diet (Pico-Vac Lab Rodent Diet 5061, LabDiet, St Louis, MO) and water (reverse-osmosis–purified water in bottles). Nesting material (Nestlets, Ancare, Bellmore, NY) and PVC tubes were provided for environmental enrichment.
Study design.
Data were collected retrospectively from 4 independent experiments performed by a single investigator from one research group.
Aspergillus strain and vaccination reagents.
A. fumigatus strain AFCOH1, isolated from a patient with IPA at City of Hope National Medical Center (Duarte, CA), was used for infection and antigen preparations. Conidia were prepared as described by the laboratory in previous publications.3 Vaccinations were performed by using recombinant Asp f3 (residues 15 to 168) with adjuvant (TiterMax, Sigma, St Louis, MO) as previously described.3,4
Vaccinations, immunosuppression, and challenge.
There were 6 different experimental scenarios prior to Aspergillus challenge. Mice subcutaneously received either an aspergillosis vaccine (an initial injection followed by a booster 2 wk later), or PBS plus adjuvant at the same site and time points. Six weeks after injection, mice were either immunosuppressed or not and placed prophylactically on sulfamethoxazole (0.8 mg/mL) and trimethoprim (0.16 mg/mL; Hi-Tech Pharmacal, Amityville, NY) in their drinking water to reduce the risk of opportunistic infections. For immunosuppression, mice either received a subcutaneous injection of cortisone acetate (2.5 mg per mouse; TCI America, Portland, OR) daily for 10 d or a neutrophil-depleting antibody intravenously (antiGR1, Bio X Cell, West Lebanon, NH) 24 h before challenge with A. fumigatus. Thus, the groups were as follows: 1) aspergillosis vaccination + cortisone acetate immunosuppression; 2) aspergillosis vaccination + antiGR1 immunosuppression; 3) aspergillosis vaccination without immunosuppression; 4) PBS + adjuvant + cortisone acetate immunosuppression; 5) PBS + adjuvant + antiGR1 immunosuppression; and 6) PBS + adjuvant without immunosuppression. The day after completion of the immunosuppression regimen, mice were weighed and their BT recorded; mice were then anesthetized with ketamine–xylazine and intranasally inoculated with A. fumigatus (3 to 30 million viable conidia in suspension in 30 μL PBS). The mice were monitored every 2 h during the day for 7 d after inoculation and at least once daily thereafter until euthanasia. Body weight was recorded daily, and BT was recorded as many as 3 times daily. Before they died, mice were euthanized according to subjective and objective criteria including severe dyspnea, 20% weight loss, and unresponsiveness to touch.
BT measurement.
BT was measured by using an infrared noncontact thermometer (MiniTemp MT4 IR Thermometer, Raytek, Santa Cruz, CA). The mice were manually restrained, exposing the ventral aspect of the body. BT was measured by aiming the thermometer at 3 different points on the animal's abdomen (Figure 1). The average of all 3 measurements was calculated and recorded.
Figure 1.
The infrared thermometer (MiniTemp MT4, Raytek, Santa Cruz, CA) and demonstration of the method used to record BT.
Statistics.
Data were analyzed by using Prism 6 (GraphPad Software, San Diego, CA) and R (www.r-project.com) software. Independent t tests were used to compare BT over time between survivors (those that survived the Aspergillus challenge and lived until the end of the study) and nonsurvivors (those that were either euthanized or found dead before the study endpoint). A receiver operator characteristics (ROC) curve (true positive rate [sensitivity] against false positive rate [1 – specificity]) was constructed to help identify a diagnostic temperature cut-off. The ROC curve was generated by using data from all A. fumigatus-infected mice, both vaccinated and unvaccinated.
Results
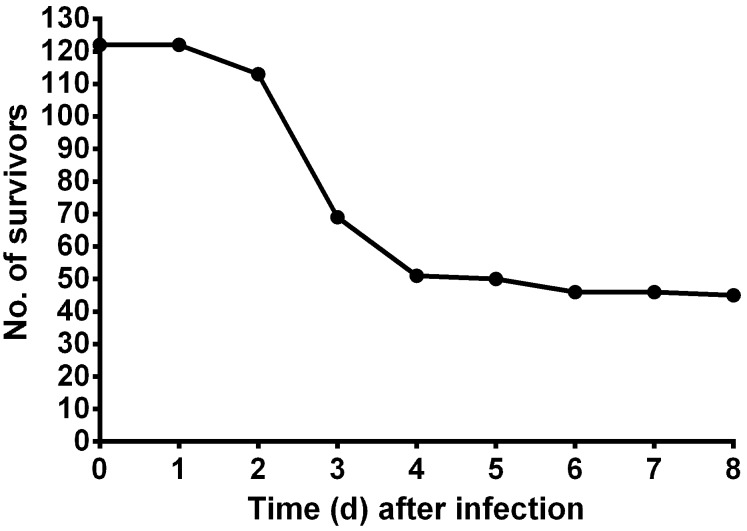
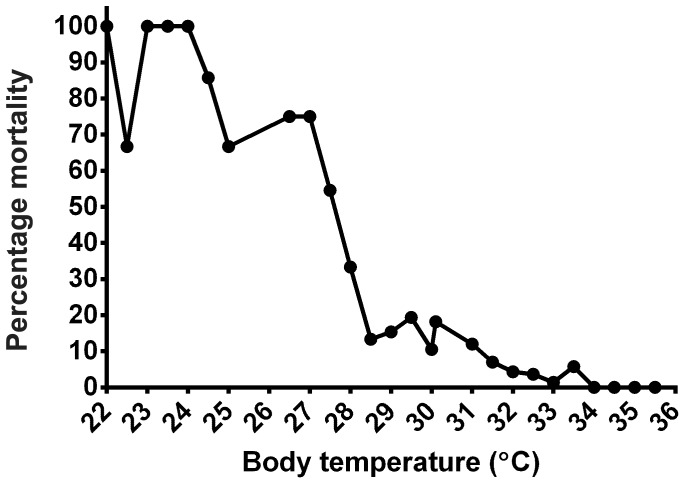
The baseline BT (mean ± SE) of the mice (n= 122) prior to Aspergillus challenge was 34.4 ± 0.067 °C, and the overall BT range recorded after inoculation was 22.0 to 35.5 °C. Mortality occurred soon after inoculation and reached 56% by day 3 after infection (Figure 2). The percentage mortality at any given temperature reading is reported in Figure 3.
Figure 2.
Survival over time after intranasal Aspergillus challenge. All mice challenged with A. fumigatus (both vaccinated and unvaccinated) are included (n = 122).
Figure 3.
Percentage mortality at any given temperature reading. Mice were recorded as ‘dead’ when they were either euthanized before the next temperature recording or were found dead between temperature readings or at the next time point.
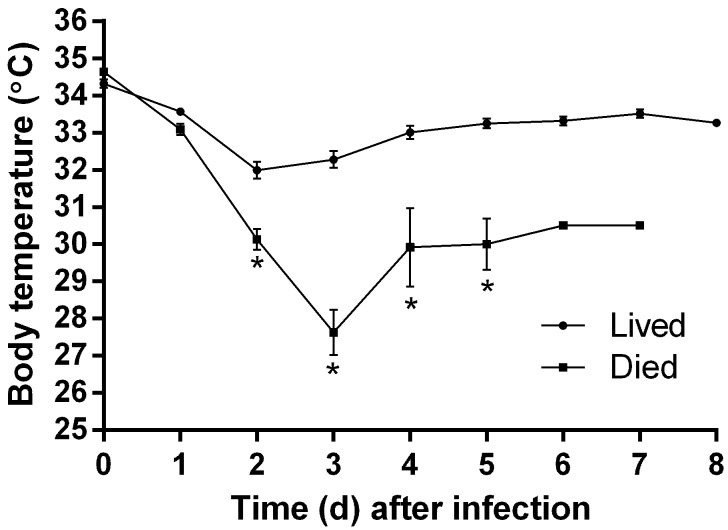
Starting on day 2 and thereafter, the BT of mice that died throughout the experiment was significantly (P ≤ 0.03) different from those that lived (Figure 4). Even though temperature was measured at least twice daily, only the morning temperature is graphed because later temperature measurements were taken at variable times. The vast majority of nonsurviving mice were either euthanized or found dead by day 3 after inoculation, when the greatest dip in BT is evident.
Figure 4.
BT (mean ± SE) of mice that survived (n = 45) compared with those that eventually died over the course of the experiment. The number of animals recorded as dead changed as mice were euthanized or found dead during the study (days 0 and 1, n = 77; day 2, n = 68; day 3, n = 24; day 4, n = 6; day 5, n = 5; days 6 and 7, n = 1); there are no error bars for time points 6 and 7 because only one animal is represented at those times. *, Significant (P ≤ 0.03) difference between groups at that time point.
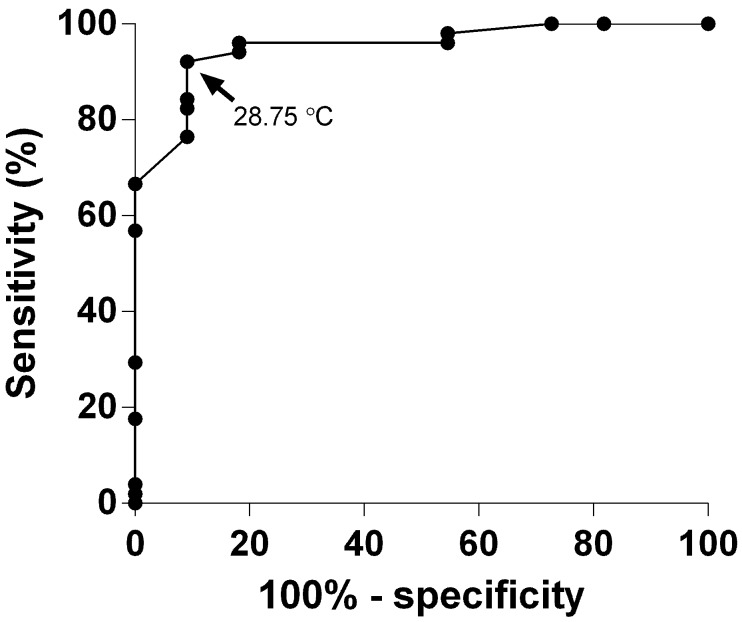
Evaluating the ROC curve (Figure 5) revealed 28.8 °C as the point closest to ideal (that is, where specificity and sensitivity both equal 100%).5 This temperature had a sensitivity of 92.2% and specificity of 90.9% in regard to predicting mortality. Euthanizing animals that had a single reading of 29.0 °C or below would have led to euthanasia of 8.9% (4 of 45) of those that would have survived Aspergillus challenge, whereas increasing the cutoff point to 30.0 °C would have resulted in unnecessary euthanasia of 22.2% (10 of 45) of mice. Using 2 successive BT readings of less than 31.0 °C as the criterion for euthanasia would have achieved early euthanasia of 36.4% (28 of 77) of those that died while only euthanizing 1 mouse that lived to the end of the study. The lowest temperature recorded in a mouse that fully recovered from Aspergillus challenge and survived until the end of the study period was 26.0 °C.
Figure 5.
The sensitivity and specificity associated with using each BT as the diagnostic cut-off were used to construct an ROC curve. These curves are commonly used by the medical community to evaluate diagnostic testing and determine the appropriate values to use to categorize patients as either ‘positive’ (that is, with disease) or ‘negative’ (that is, no disease).5,11,23 In our case, when mice were euthanized or died after a given temperature reading, they were designated as positive. Data from the 72-h time point were used, because they represented the largest number of mice in each designation (that is, positive and negative).
Discussion
This study evaluated the utility of a hypothermic cutoff to enhance the humane endpoint criteria for a mouse model of IPA. Several studies have acknowledged hypothermia as a way to recognize impending death in mice,12,13,15,17-22 however previously published hypothermic endpoints were not appropriate for our needs, due to significant differences in the particular animal model used. This difficulty led us to seek a temperature criterion specific to our animal model of interest.
Our data suggest that low BT can be a useful addition to the endpoint criteria for this IPA mouse model. A BT range of 28.0 to 29.0 °C was consistent with a poor chance of survival, and we found that using this range with signs of morbidity (hunched posture, ruffled fur, respiratory distress) consistently achieves early euthanasia of mice without premature loss of data. Using a combination of subjective and objective criteria can more reliably predict outcome compared with using individual signs only10 and allows research and veterinary staff to optimally refine the experiment to decrease pain and distress. Similar to the previously published literature evaluating hypothermic endpoints, no BT was 100% sensitive and 100% specific for predicting early euthanasia of our mice. Not all mice with clinical signs of Aspergillus infection will have a BT lower than 29.0 °C and, as stated in the Results section, some survivors had BT as low as 26.0 °C. Therefore, we found that the combination of BT and clinical presentation is crucial to ensure an endpoint that is both humane and does not compromise study results. Given that percentage mortality dramatically increased with BT below 28.5 °C (Figure 3), using a single BT endpoint reading of 29.0 °C or less would provide clarity and ease of use while considering both animal welfare and unnecessary euthanasia. Furthermore, in experiments that required tissue or sample collection, such predictions would avoid the loss of data from animals that died before sampling could be completed.
An important point, and one often overlooked in the humane endpoint literature, is the effect that any given endpoint has on group size. For our particular model, one can expect 58% survival in the vaccinated group and 8% in the unvaccinated group. With these survival rates, a group size of 12 is necessary to obtain a statistically significant difference between the 2 groups. Given that 4 of 45 survivor mice would have been euthanized if 29 °C were used as a stand-alone endpoint (without consideration of clinical signs), then potentially one of the survivor animals in either group would be euthanized. This event would alter the results and obviate a statistically significant difference between groups. Therefore, group size would have to be increased to 13 to again achieve a significant difference. The importance of maximizing animal welfare in contrast to increasing animal numbers can be viewed from various perspectives; however these points should be discussed when determining the optimal endpoint.
Another approach that we felt was practical was the use of 2 subsequent recordings of 31 °C or lower. This practice allows for the investigator to assess the BT trend over time in the context of clinical presentation to judge whether euthanasia is warranted. Using 2 subsequent readings might also be applied as a more ‘concrete’ hypothermic endpoint, given that a mouse experiencing prolonged hypothermia is less likely to recover. Investigators concerned about the accuracy of their data and effects on animal numbers might be more comfortable with this approach. In our case, using 2 successive BT readings of 31 °C or lower to direct euthanasia would have led to premature termination of only 1 of the 45 survivor mice and therefore would be less likely to affect results and require an increase in group size to see statistical significance.
Our recommendations are lower than are the typical hypothermic endpoints recommended in the literature, but most of the previous studies used subcutaneous microchips for BT measurements. The one study we found that evaluated infrared thermometry first calibrated the infrared thermometer by comparing its readings to readings from mice with implanted temperature transponders.21 This calibration in essence eliminated any differences between the microchip and infrared thermometer reading. The infrared thermometer we used recorded an average baseline temperature of 34.4 °C, which is several degrees below the published norm for mice (37.0 to 37.2 °C).9 However, infrared thermometers measure surface temperature, whereas the normal values cited9 represent core body temperature. The consistency and ease of use of infrared thermometry outweigh its lack of accuracy. Reading the temperature takes only seconds, and repeated measurements were very precise with the model of infrared thermometer that we used. In addition, infrared thermometry causes little stress to mice, which is crucial in a model that typically presents with respiratory distress. Given the differences between the animal models and temperature-recording apparatuses that were evaluated previously and our current model and thermometer, one can understand why the temperature endpoint recommendations we report here are a bit lower than those in the literature. Hypothermic endpoints previously published for other animal models might also have been lower had infrared thermometry been used. However, in either case, each animal can act as its own control and be compared with the control group.
The variability of hypothermic endpoints discussed throughout the literature can be frustrating and emphasizes the importance of performing pilot studies with each specific model to ensure that an appropriate temperature is used. If at all possible, minimally invasive monitoring techniques should be used to avoid unnecessary and preventable pain and distress.10 The injection of anesthetized animals with subcutaneous microchips is perhaps ideal but, in our experience, their expense has discouraged their use by many investigators. Rectal measurements require considerable animal handling and are fairly stressful to animals, especially when taking into consideration the serial measurements that are required during studies in which increased morbidity is expected. Infrared thermometers provide a quick, easy, and inexpensive way to include BT recordings. Animal resources centers could purchase several and allow their use by laboratory groups when needed. This practice likely would increase compliance, improve animal welfare, and enhance the overall model by adding consistency to euthanasia guidelines.
A BT endpoint for any specific animal model should be determined by using pilot studies and in discussion with the laboratory group to ensure that the needs of the research are being met while animal pain and distress are alleviated. Numerous factors can influence the BT of mice, including strain, age, time of day, bedding amount and type, and number of cage mates,6,16,19 highlighting the fact that study endpoints must be validated for the specific experimental model of interest. We agree with previous authors19 who affirmed that the publication of similar studies evaluating hypothermia as an endpoint in different animal models would be beneficial for IACUC, veterinary care staff, and investigators to allow a more thorough understanding of its usefulness as an endpoint criterion.
Acknowledgments
The original work that led to this study was supported in part by the Hermann Foundation, the Tim Nesvig Lymphoma Fellowship and Research Fund, and NIH grant AI075239-01.
References
- 1.Ben-Ami R, Lewis RE, Kontoyiannis DP. 2010. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol 150:406–417 [DOI] [PubMed] [Google Scholar]
- 2.Clemons KV, Stevens DA. 2005. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy, and virulence. Med Mycol 43:S101–S110 [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Arevalo D, Bagramyan K, Hong TB, Ito JI, Kalkum M. 2011. CD4+ T cells mediate the protective effect of the recombinant Asp f3-based antiaspergillosis vaccine. Infect Immun 79:2257–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Arevalo D, Ito JI, Kalkum M. 2012. Protective effector cells of the recombinant Asp f3 antiaspergillosis vaccine. Front Microbiol 3:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett T. 2006. An introduction to ROC analysis. Pattern Recognit Lett 27:861–874 [Google Scholar]
- 6.Gordon CJ, Becker P, Ali JS. 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262 [DOI] [PubMed] [Google Scholar]
- 7.Gordon CJ, Fogelson L, Highfill JW. 1990. Hypothermia and hypometabolism: sensitive indices of whole-body toxicity following exposure to metallic salts in the mouse. J Toxicol Environ Health 29:185–200 [DOI] [PubMed] [Google Scholar]
- 8.Ito JI, Lyons JM, Hong TB, Tamae D, Liu YK, Wilczynski SP, Kalkum M. 2006. Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun 74:5075–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases in mice, p 35–120. In: Fox JG AL, Loew FM, Quimby FW, editors. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press [Google Scholar]
- 10.Jennings M, Morton DB, Charton E, Cooper J, Hendriksen C, Martin S, Pearce MC, Price S, Redhead K, Reed N, Simmons H, Spencer S, Willingale H. 2010. Application of the Three Rs to challenge assays used in vaccine testing: tenth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Biologicals 38:684–695 [DOI] [PubMed] [Google Scholar]
- 11.Katz D, Foxman B. 1993. How well do prediction equations predict? Using receiver operating characteristic curves and accuracy curves to compare validity and generalizability. Epidemiology 4:319–326 [DOI] [PubMed] [Google Scholar]
- 12.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim 32:260–269 [DOI] [PubMed] [Google Scholar]
- 13.Krarup A, Chattopadhyay P, Bhattacharjee AK, Burge JR, Ruble GR. 1999. Evaluation of surrogate markers of impending death in the galactosamine-sensitized murine model of bacterial endotoxemia. Lab Anim Sci 49:545–550 [PubMed] [Google Scholar]
- 14.Olfert ED, Godson DL. 2000. Humane endpoints for infectious disease animal models. ILAR J 41:99–104 [DOI] [PubMed] [Google Scholar]
- 15.Soothill JS, Morton DB, Ahmad A. 1992. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int J Exp Pathol 73:95–98 [PMC free article] [PubMed] [Google Scholar]
- 16.Toth LA. 2000. Defining the moribund condition as an experimental endpoint for animal research. ILAR J 41:72–79 [DOI] [PubMed] [Google Scholar]
- 17.Toth LA, Hughes LF. 2006. Sleep and temperature responses of inbred mice with Candida albicans-induced pyelonephritis. Comp Med 56:252–261 [PubMed] [Google Scholar]
- 18.Toth LA, Rehg JE, Webster RG. 1995. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol 58:89–99 [DOI] [PubMed] [Google Scholar]
- 19.Trammell RA, Toth LA. 2011. Markers for predicting death as an outcome for mice used in infectious disease research. Comp Med 61:492–498 [PMC free article] [PubMed] [Google Scholar]
- 20.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50:160–166 [PubMed] [Google Scholar]
- 21.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 37:126–131 [DOI] [PubMed] [Google Scholar]
- 22.Wong JP, Saravolac EG, Clement JG, Nagata LP. 1997. Development of a murine hypothermia model for study of respiratory tract influenza virus infection. Lab Anim Sci 47:143–147 [PubMed] [Google Scholar]
- 23.Zou KH. [Internet] 2002. Receiver operating characteristic (ROC) literature research. [Cited June 2013]. Available at: <http://www.spl.harvard.edu/archive/spl-pre2007/pages/ppl/zou/roc.html>