Abstract
A 26-y-old male sooty mangabey (Cercocebus atys) was found at necropsy to have a moderate degree of cerebral amyloid β (Aβ) angiopathy in superficial and parenchymal blood vessels of the brain. Senile (Aβ) plaques were absent, as were neurofibrillary tangles and other signs of neurodegeneration. Affected blood vessels were arterial, capillary, and, less frequently, venous in nature. Histologically, the Aβ40 isoform was more prevalent than was Aβ42. As in humans but unlike in squirrel monkeys, the density of lesions in this mangabey increased along a rostral-to-caudal gradient. Therefore mangabeys appear to conform to the general tendency of nonhuman primates by developing cerebral Aβ angiopathy in the absence of other indices of Alzheimer-type neuropathology.
Abbreviations: Aβ, amyloid β; CAA, cerebral amyloid angiopathy; GFAP, glial fibrillary acidic protein; Iba 1, microglia-expressed calcium-binding protein
One of the most common microvasculopathies in the aging human brain is cerebral amyloid angiopathy (CAA), a disorder in which various aggregation-prone proteins accumulate in the walls of parenchymal and meningeal blood vessels.4,9 Most often, the amyloidogenic protein is amyloid β (Aβ), a cleavage product of the Aβ precursor protein and the essential component of senile plaques in Alzheimer disease.13,43 In the brain vasculature, the basal lamina is a primary site of Aβ deposition.25,35 Severely affected arterioles show a loss of smooth muscle cells in the tunica media, a weakening of the vascular wall and a propensity to rupture.3,34 CAA thus increases the risk of intracerebral bleeding and may be responsible for as much as 20% of nontraumatic hemorrhagic stroke in elderly humans.15,18,35 CAA is present to various degrees in virtually all cases of Alzheimer disease,15,16,21 but it also occurs independently.24 As is the case for other proteopathies, advancing age is a significant risk factor for CAA.8,19
In humans, CAA most often affects the arteries and arterioles of the brain, particularly those in the leptomeninges and cortex.2,25 CAA is less frequent in veins and capillaries,25 but capillary CAA can be prominent in some cases.26,33 The occipital lobe is affected most often1,32,37 but all cortical regions are vulnerable. CAA is variable in occurrence in the cerebellum and uncommon in deep telencephalic gray structures, white matter, and the brainstem,36 except in severely affected cases.32
Although its specific role in the pathogenesis of Alzheimer disease remains uncertain, there is now strong evidence that dementia is exacerbated by CAA.14 Furthermore, CAA is independently linked to cognitive decline both in rare familial cases20 and in older humans with idiopathic CAA.2,20 Despite the prevalence of cerebrovascular amyloidosis in elderly humans, surprisingly little is known about its effect on the brain, in part because of a paucity of natural animal models that closely mimic the human disorder.17,38
Nonhuman primates offer a unique opportunity to view CAA from a comparative perspective, given that they normally generate human-sequence Aβ and develop severe cerebral Aβ amyloidosis in old age, generally in the absence of other changes that characterize Alzheimer disease.12 Nonhuman primates have the additional advantage that, compared with humans, their relatively small brains enable exhaustive regional analysis of microscopic lesions, something that, for practical reasons, is seldom undertaken in the human brain. Here we present the first investigation of age-associated brain changes in sooty mangabeys, focusing in particular on Aβ deposition and related abnormalities. One of the 2 aged mangabeys analyzed had Aβ deposition in the brain which was almost exclusively in the form of CAA. Remarkably, the vessel types affected and the regional distribution of CAA more closely resembled the pattern seen in humans than that in other nonhuman primates, particularly squirrel monkeys.6 Differences and similarities in CAA among primate species could provide fresh insights into the development of cerebral amyloidosis and related disorders in older humans.
Materials and Methods
Subjects.
Three sooty mangabeys (Cercocebus atys) were studied: a 32-y-old female, a 26-y-old male, and a 19-y-old female. All animals were from the colony of the Yerkes National Primate Research Center, and their birthdates were known. The maximal lifespan of sooty mangabeys is unknown, but we are aware of no reports of animals of known age that have lived longer than 32 y. According to the presence of age-associated changes and based on our knowledge of the life history of other Old World simians, particularly macaques,42 we conclude that the 26-y-old and 32-y-old mangabeys can be considered aged, and the 19-y old animal is middle-aged. Given the paucity of information on lifespan in this species, the maximal potential lifespan of mangabeys may be somewhat greater than 32 y.
All 3 animals were diabetic at the time of euthanasia. Due to the difficulty treating diabetes in a colony setting, the decision was made to euthanize these mangabeys. Necropsy revealed marked islet amyloid polypeptide (amylin) amyloidosis in the pancreas of all subjects. In addition to diabetes, the following clinical and pathologic findings were noted. The 32-y-old female was diagnosed with severe age-related kyphosis and acute onset of hindlimb lameness; necropsy revealed the presence of advanced endometrial carcinoma. The 26-y-old vasectomized male was found to have age-related glomerulosclerosis, chronic fibrosing cardiomyopathy, and chronic lymphoplasmacytic colitis. The 19-y old female was diagnosed with endometriosis, lethargy, persistent inappetence, firm abdomen and decreased hematocrit; at necropsy, severe hemoperitoneum and endometriosis were evident.
The mangabeys were euthanized via sodium pentobarbital overdose (2.22 mg/kg IV), and tissues were collected at necropsy, in accordance with federal and institutional guidelines for the humane care and use of experimental animals. The Yerkes Center is fully accredited by AAALAC.
Histochemistry.
The brains were immersion-fixed in 4% paraformaldehyde for a minimum of 2 wk. Subsequently, they were coronally slabbed, dehydrated, embedded in paraffin wax, and sectioned at 10 μm thickness. Tissues were deparaffinized and rehydrated by heating at 60 °C for 45 min followed by immersion in xylene and a graded (descending) series of ethanol to water. Antigen retrieval for Aβ involved treating in 100% formic acid for 10 min, and that for Iba1 and glial fibrillary acidic protein (GFAP) involved heating sections in sodium citrate buffer at 100°C for 30 min.
Coronally sliced tissue sections were matched for the 3 mangabeys and included the following regions: anterior frontal lobe; midfrontal and anterior temporal lobes; posterior frontal, midtemporal, and anterior parietal lobes; occipital lobe; and cerebellum and brainstem. Sections were immunostained with the following antibodies: clone 6E10 (1:10,000) mouse monoclonal antibody (Covance, Princeton, NJ) raised against residues 1 to 16 of the Aβ peptide; rabbit polyclonal antibodies R163 and R165 (both at 1:1000) to the C-terminal amino acids of Aβ40 and Aβ42, respectively; mouse monoclonal antibody CP13 (1:5000) to phosphoserine 202 of Tau (courtesy of Dr Peter Davies, Albert Einstein College of Medicine, Bronx, NY); mouse monoclonal antibody to Iba1 (1:1000; Wako Chemicals USA, Richmond, VA), a calcium-binding protein that is upregulated in activated microglia; and a mouse monoclonal antibody to GFAP (1:1000; Dako, Carpinteria, CA; see reference 27 for details regarding antibodies). GFAP- and Iba1-stained sections were counterstained with thioflavin S to assess the relationship between activated glial cells and thioflavin-positive amyloid deposits.
Briefly, immunohistochemical staining was performed as follows: sections were first incubated in 3% H2O2 and methanol for 10 min and then blocked with 2% normal serum and 0.2% Tween in 1× PBS for 90 min. Subsequently the tissue was incubated in primary antibodies in a blocking solution (2% normal serum and 0.2% Tween in 1× PBS) overnight at 4 °C. Finally, antigen–antibody complexes were enhanced with an avidin–biotin–peroxidase system (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) and visualized by using the chromogen diaminobenzidine. Control sections were run simultaneously, including positive controls (Alzheimer patient brain samples, previously confirmed) and negative controls (omission of the primary antibody).
Additional sections were stained with standard histochemical markers, including the Campbell–Switzer silver stain for Alzheimer-associated lesions, Luxol fast blue for myelin, Perls Prussian blue stain for iron, and hematoxylin and eosin.
Analysis.
Sections were analyzed for the presence of Aβ deposits at all coronal levels in the 3 mangabeys. Only one mangabey, the 26-y-old male, showed Aβ deposits, so the lesions in this animal were mapped in representative sections, one for each region (Figure 1) by using a microscope-based mapping system equipped with ImageScope software (Aperio, Vista, CA). All immunoreactive profiles were counted, with the caveat that separate profiles in a given section may represent segments of a single vessel. Vascular lesions were characterized further in regard to the size and location of the vessel. Specifically, superficial blood vessels were defined as any vessels lying outside of the cortex, and intracortical blood vessels were defined as any blood vessels in cortical layers 1 to 6 (that is, between the cortical surface and the white matter; no positive vessels were found in white matter). Capillaries were defined as blood vessels with diameters of approximately 10 μm or less. We also searched all sections for parenchymal Aβ in senile plaques but found none. To estimate the relative numbers of Aβ40- and Aβ-42-positive vessels, a subset of adjacent sections from the 26-y-old monkey were immunostained with antibodies R163 and R165 to Aβ40 and Aβ42, respectively.
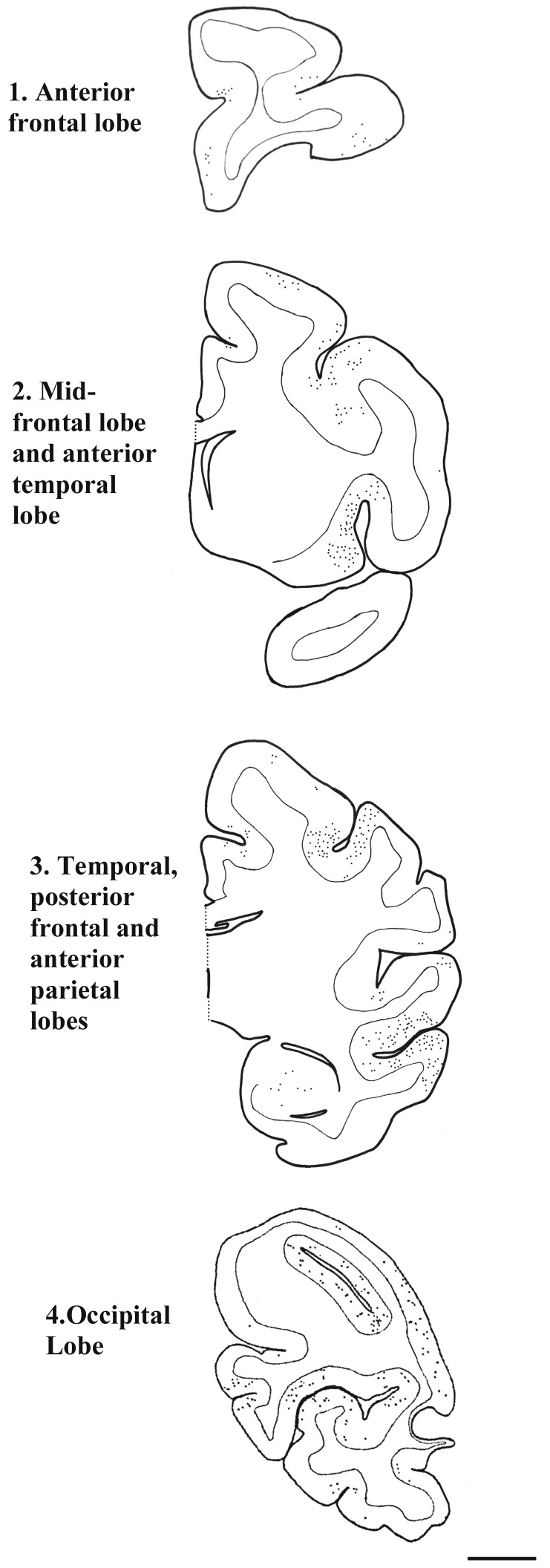
Figure 1.
Topographic distribution of vascular Aβ deposits (dots) in a 26-y-old sooty mangabey. The brainstem segments analyzed were devoid of Aβ immunoreactivity and are not pictured. Antibody, antiAβ clone 6E10; bar, 5 mm.
The stereologic point-counting method22 was used to determine the total area of the neocortex in each mapped section, which then served as the 2-dimensional reference space for calculating Aβ lesion density. Briefly, an image of the entire tissue section was captured at low magnification and the cortical areas were outlined using ImageScope software. A symmetrical grid of + symbols was superimposed on an image of the tissue section. Each + represented a micrometer-calibrated square of known area; symbols overlying the area of interest were counted manually to calculate the total area of the cortex in that section.22 The density of Aβ lesions was determined by counting the number of lesions per unit area (mm2) of interest.
Results
Aβ deposition in a male mangabey.
Of the 3 animals analyzed, only the 26-y-old male mangabey displayed β-amyloid deposition, and in the regions sampled the deposits occurred exclusively within the walls of cerebral blood vessels. Senile plaques and tauopathy were not observed in any mangabey, either by immunostaining or Campbell–Switzer silver staining. Because the brains of the 19- and 32-y-old mangabeys showed little frank pathology and no Aβ deposition, our analysis focused on the affected 26-y-old mangabey.
Vessel types affected by β-amyloid deposition.
All Aβ deposits in the 26-y-old mangabey were vascular in nature, affecting both leptomeningeal and intracortical blood vessels. The majority of Aβ-positive superficial vessels were arteries or arterioles, and amyloidotic blood vessels within the cortex were predominantly small arterioles or capillaries (Figures 2 and 3). Given the vessels’ size and the thickness of the vascular walls, it was apparent that a small number of veins were affected also. At more rostral levels, intracortical vessel profiles clearly outnumbered leptomeningeal vessels, but in the occipital lobe the relative amounts of intracortical and leptomeningeal CAA were similar (Table 1). Staining of adjacent sections for Aβ40 and Aβ42 (the 2 main isoforms of Aβ) indicated that most CAA was immunopositive for Aβ40, whereas Aβ42 immunoreactivity was less frequent, as has been reported for other primate species.7
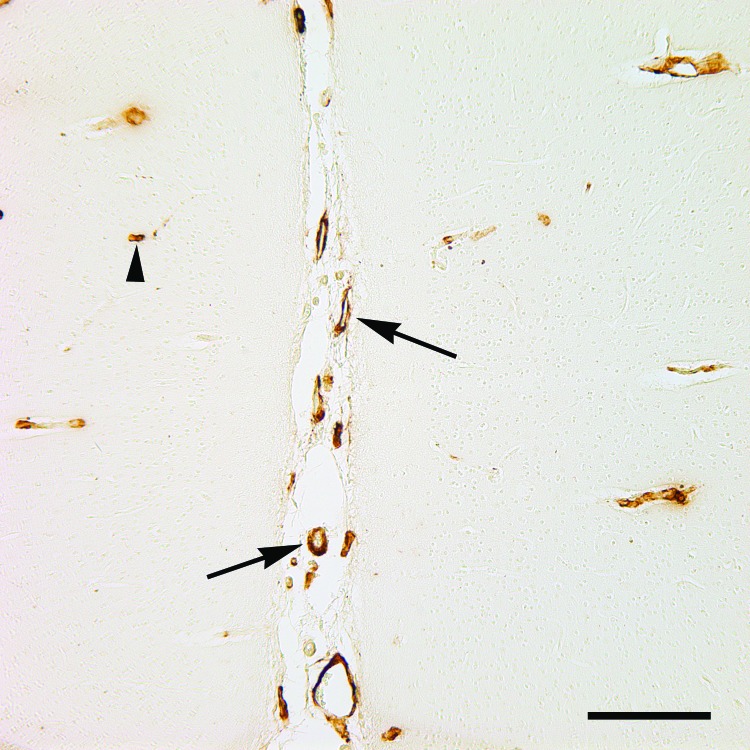
Figure 2.
Cerebral Aβ angiopathy in the occipital neocortex of a 26-y-old mangabey. The arrows indicate 2 large superficial vessels in a sulcus, and the arrowhead marks a small parenchymal capillary. Antibody, antiAβ clone 6E10; bar, 200 μm.
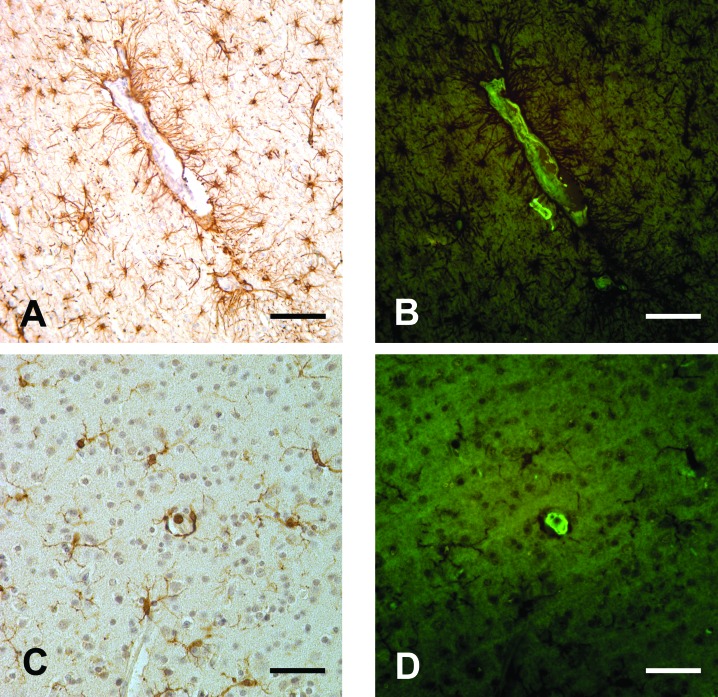
Figure 3.
(A) Astrocytes associated with a thioflavin-positive blood vessel. GFAP immunostain; thioflavin S and hematoxylin counterstain. (B) This image shows the same section as in panel A, but thioflavin S is visualized under fluorescence illumination. (C) Microglial cells near a thioflavin-positive blood vessel. Iba1 immunostain; thioflavin S and hematoxylin counterstain. (D) This image shows the same section as in panel D, but thioflavin S is visualized under fluorescence illumination. Bar, 100 μm (A, B), 50 μm (C, D).
Table 1.
Overall density (no./mm2) of vascular Aβ deposits in the cortex at 4 coronal brain levels of a 26-y-old mangabey
| Cortical region | Superficial | Intracortical | Total |
| Anterior frontal | 0.22 | 0.67 | 0.59 |
| Anterior—mid | 0.56 | 0.94 | 1.5 |
| Posterior—mid | 0.69 | 0.84 | 1.64 |
| Occipital | 1.26 | 1.17 | 2.37 |
See Figure 1 for a description of the cortical regions.
Topographic distribution of CAA.
CAA in the mangabey was distributed in a focal, patchy pattern (Figure 1), with many lesions clustering around various cortical sulci (Figures 1 and 2). The overall density of CAA exhibited an increasing rostral–caudal gradient, with the highest density of CAA in the cortex and leptomeninges of the occipital lobe (Tables 1 and 2). Some CAA was present in the amygdala, but little was seen in the hippocampal formation, and none in the white matter, basal ganglia, diencephalon, cerebellum, or brainstem.
Table 2.
Density (no./mm2) of Aβ-immunoreactive capillaries and larger vessels in the cortex at 4 coronal brain levels of a 26-y-old mangabey
| Cortical region | Affected capillaries | Affected larger vessels |
| Anterior frontal | 0.86 | 0.03 |
| Anterior—mid | 0.71 | 0.37 |
| Posterior—mid | 1.04 | 0.60 |
| Occipital | 1.38 | 1.04 |
See Figure 1 for a description of the cortical regions.
Other changes in the brain.
Immunostaining for astrocytes and microglia revealed abundant activated glial cells in close proximity to thioflavin-positive vessels, an indicator of a focal inflammatory response (Figure 3). Perls Prussian blue staining revealed a small amount of superficial and intracortical hemosiderin. Luxol fast blue staining showed no obvious abnormalities in myelin.
Discussion
We report the case of an aged male sooty mangabey with cerebral β-amyloid angiopathy that was distributed irregularly throughout many regions of the neocortex. Vascular β amyloid is common in other nonhuman primate species,12,23 but there were 3 unusual aspects of cerebral Aβ deposition in the mangabey. First, we did not detect any senile plaques in the sections that we examined. In squirrel monkeys, which typically develop a preponderance of CAA, senile plaques do occur,6,40,41 and CAA and plaques also coexist in other primate species.12,23,39 It therefore is possible that senile plaques would be present in more severely affected mangabeys. Second, whereas in squirrel monkeys there is a clear rostral predominance of CAA,6 the sooty mangabey had more CAA in the occipital lobe, a pattern resembling stage 1 CAA in humans.32 Finally, compared with squirrel monkeys, the mangabey showed a greater proportion of CAA in larger vessels, often superficial arteries and arterioles. This pattern, in conjunction with the relatively modest overall amount of CAA, is again reminiscent of the earliest stages of human CAA.32
It is not surprising that we failed to detect β-amyloid deposition in the 19-y-old mangabey, given that rhesus monkeys, another Old World species, only begin to show Aβ deposits in their early 20s (although, as in all nonhuman primates, interindividual variation is high).12 However, contrary to expectation, the 32-y-old female mangabey was devoid of Aβ deposition of any kind. The reasons for this discrepancy are unknown at this time. Perhaps Aβ deposition in mangabeys is influenced by their sex, although it is interesting to note that Aβ deposition is more aggressive in female mice that bear a transgene for the human Aβ-precursor protein than in their male counterparts.5 The issue of sex-associated differences in the pathogenesis of Aβ deposition deserves greater attention in all primate species.
Several distinct isoforms of Aβ have been observed in which modifications occur at the C and N termini.44 The most common isoforms vary in length due to differential cleavage at the C terminus of Aβ and accordingly are referred to as Aβ40 (40 amino acids long) and Aβ42. Interestingly, much more Aβ40 than Aβ42 is produced in the brain, but Aβ42 has a stronger tendency to aggregate and appears to be the dominant component of extracellular plaques.11,29 In contrast, Aβ40 generally predominates in CAA in humans.4 Although the Aβ of nonhuman primates is similar in amino acid sequence to that of humans, the Aβ40 isoform is relatively more abundant in the aged simian brain.7,28 Both isoforms of the Aβ peptide, Aβ40 and Aβ42, were present in the affected vasculature of the mangabey we present; as in other primates (including humans), Aβ40 was detected more frequently within the CAA. This finding—that both Aβ40 and CAA are more common in nonhuman primates—suggests that the ratio of Aβ40:Aβ42 may influence the pathobiology of the peptide with regard to the risk for both CAA and Alzheimer disease. As in nearly all other nonhuman primates examined (with the exception of a single chimpanzee),27 none of the mangabeys that we examined showed evidence of human-like neurofibrillary tangles.
An intriguing feature of Aβ-amyloidosis in the simian brain is that different species have different patterns of Aβ deposition, despite the fact that all primates studied to date have a human-like Aβ sequence.12,39 Furthermore, Aβ aggregation plays a fundamental role in the development of Alzheimer disease in humans,11,13,30 but no nonhuman species has yet been shown to develop the full clinicopathological phenotype that defines Alzheimer disease.12,17 Even so, CAA can exacerbate cognitive decline in the elderly.2,10,25,32 Comparative analysis of the factors that govern the accumulation of Aβ in the primate brain could yield new insights both into cerebrovascular Aβ amyloidosis and into the uniquely human vulnerability to Alzheimer disease.
References
- 1.Attems J, Quass M, Jellinger KA, Lintner F. 2007. Topographical distribution of cerebral amyloid angiopathy and its effect on cognitive decline are influenced by Alzheimer disease pathology. J Neurol Sci 257:49–55 [DOI] [PubMed] [Google Scholar]
- 2.Auriel E, Greenberg SM. 2012. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep 14:343–350 [DOI] [PubMed] [Google Scholar]
- 3.Bano S, Yadav SN, Garga UC, Chaudhary V. 2011. Sporadic cerebral amyloid angiopathy: an important cause of cerebral hemorrhage in the elderly. J Neurosci Rural Pract 2:87 –91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffi A, Greenberg SM. 2011. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 7:1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. 2001. Augmented senile plaque load in aged female β-amyloid precursor protein-transgenic mice. Am J Pathol 158:1173–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R, Walker LC. 2007. Cerebral β-amyloid angiopathy in aged squirrel monkeys. Histol Histopathol 22:155–167 [DOI] [PubMed] [Google Scholar]
- 7.Gearing M, Tigges J, Mori H, Mirra SS. 1996. Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol Aging 17:903–908 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JJ, Vinters HV. 1983. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke 14:915–923 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SM, Gurol ME, Rosand J, Smith EE. 2004. Amyloid angiopathy-related vascular cognitive impairment. Stroke 35:2616–2619 [DOI] [PubMed] [Google Scholar]
- 10.Grinberg LT, Korczyn AD, Heinsen H. 2012. Cerebral amyloid angiopathy impact on endothelium. Exp Gerontol 47:838–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356 [DOI] [PubMed] [Google Scholar]
- 12.Heuer E, Rosen RF, Cintron A, Walker LC. 2012. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des 18:1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzman DM, Morris JC, Goate AM. 2011. Alzheimer's disease: the challenge of the 2nd century. Sci Transl Med 3:77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C. 2010. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jellinger KA. 2002. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 109:813–836 [DOI] [PubMed] [Google Scholar]
- 16.Joachim CL, Morris JH, Selkoe DJ. 1988. Clinically diagnosed Alzheimer's disease: autopsy results in 150 cases. Ann Neurol 24:50–56 [DOI] [PubMed] [Google Scholar]
- 17.Jucker M. 2010. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 16:1210–1214 [DOI] [PubMed] [Google Scholar]
- 18.Kase CS. 1994. Cerebral amyloid angiopathy. In: Kase CS, Caplan LR, editors. Intracerebral hemorrhage, p 179–200. Boston (MA): Butterworth–Heinemann [Google Scholar]
- 19.Kovari E, Herrmann FR, Hof PR, Bouras C. 2013. The relationship between cerebral amyloid angiopathy and cortical microinfarcts in brain ageing and Alzheimer's disease. Neuropathol Appl Neurobiol 39:498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maat-Schieman M, Roos R, van Duinen S. 2005. Hereditary cerebral hemorrhage with amyloidosis—Dutch type. Neuropathology 25:288 –297 [DOI] [PubMed] [Google Scholar]
- 21.Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, Selman WR. 2012. Cerebral amyloid angiopathy-associated intracerebral hemorrhage: pathology and management. Neurosurg Focus 32:E7. [DOI] [PubMed] [Google Scholar]
- 22.Mouton PR. 2011. Unbiased stereology: a concise guide. Baltimore (MD): The Johns Hopkins University Press [Google Scholar]
- 23.Ndung'u M, Hartig W, Wegner F, Mwenda JM, Low RW, Akinyemi RO, Kalaria RN. 2012. Cerebral amyloid β42 deposits and microvascular pathology in ageing baboons. Neuropathol Appl Neurobiol 38:487–499 [DOI] [PubMed] [Google Scholar]
- 24.Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C. 2013. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA 110:3089–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, Holton JL. 2003. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol 62:885–898 [DOI] [PubMed] [Google Scholar]
- 26.Richard E, Carrano A, Hoozemans JJ, van Horssen J, van Haastert ES, Eurelings LS, de Vries HE, Thal DR, Eikelenboom P, van Gool WA, Rozemuller AJ. 2010. Characteristics of dyshoric capillary cerebral amyloid angiopathy. J Neuropathol Exp Neurol 69:1158–1167 [DOI] [PubMed] [Google Scholar]
- 27.Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare J, Duong TQ, Hopkins WD, Preuss TM, Walker LC. 2008. Tauopathy with paried helical filaments in an aged chimpanzee. J Comp Neurol 509:259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen RF, Walker LC, Levine H., 3rd 2011. PIB binding in aged primate brain: enrichment of high-affinity sites in humans with Alzheimer's disease. Neurobiol Aging 32:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selkoe DJ. 1999. Biology of β-amyloid precursor protein and the mechanism of Alzheimer disease, p 293–310. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer's disease. Philadelphia (PA): Lippincott Wiliams and Wilkins [Google Scholar]
- 30.Selkoe DJ. 2012. Preventing Alzheimer's disease. Science 337:1488–1492 [DOI] [PubMed] [Google Scholar]
- 31.Smith EE, Greenberg SM. 2009. β-amyloid, blood vessels, and brain function. Stroke 40:2601–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. 2003. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis–lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301 [DOI] [PubMed] [Google Scholar]
- 33.Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. 2002. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–293 [DOI] [PubMed] [Google Scholar]
- 34.Thal DR, Grinberg LT, Attems J. 2012. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47:816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinters HV. 1987. Cerebral amyloid angiopathy. A critical review. Stroke 18:311–324 [DOI] [PubMed] [Google Scholar]
- 36.Vinters HV. 2000. Cerebral microvascular and macrovascular disease in the aging brain; similarities and differences, p 59–80. In: Verbeek MM, de Waal MW, Vinters HV, editors. Cerebral amyloid angiopathy in Alzheimer's disease and related disorders. Dordrecht (The Netherlands): Kluwer Academic Publishers [Google Scholar]
- 37.Viswanathan A, Greenberg SM. 2011. Cerebral amyloid angiopathy in the elderly. Ann Neurol 70:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LC. 1997. Animal models of cerebral β-amyloid angiopathy. Brain Res Brain Res Rev 25:70–84 [DOI] [PubMed] [Google Scholar]
- 39.Walker LC. 1999. The neurobiology of aging in nonhuman primates p 233–243. In: Terry RD, Katzman R, Bick K, Sisodia SS, editors. Alzheimer disease. Philadelphia (PA): Lippincott Williams and Wilkins [Google Scholar]
- 40.Walker LC, Kitt CA, Schwam E, Buckwald B, Garcia F, Sepinwall J, Price DL. 1987. Senile plaques in aged squirrel monkeys. Neurobiol Aging 8:291–296 [DOI] [PubMed] [Google Scholar]
- 41.Walker LC, Masters C, Beyreuther K, Price DL. 1990. Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol 80:381–387 [DOI] [PubMed] [Google Scholar]
- 42.Walker ML, Herndon JG. 2008. Menopause in nonhuman primates? Biol Reprod 79:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe MS, Guenette SY. 2007. APP at a glance. J Cell Sci 120:3157–3161 [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Ma Q, Zhang YW, Xu H. 2012. Proteolytic processing of Alzheimer's β-amyloid precursor protein. J Neurochem 120 Suppl 1:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]