Abstract
Several classes of antineoplastic agents are universally referred to as vesicants with ample supporting literature. However, the literature surrounding the taxanes is controversial. While the American Society of Clinical Oncology and Oncology Nursing Society Chemotherapy Administration Safety Standards and the Chemotherapy and Biotherapy Guidelines and Recommendations for Practice identify the risks of extravasation and the parameters surrounding the infusion of known vesicants, recommend administration sites for known agents, and recommend antidotes for particular extravasation cases, they fail to provide specific recommendations for the administration of individual taxanes, or a classification system for antineoplastic agents as vesicants, irritants, or inert compounds. There is also a lack of prescribing information regarding such recommendations. The lack of a formal classification system further complicates the accurate delineation of vesicant antineoplastic agents and subsequent appropriate intravenous administration and extravasation management. There are several factors that make the classification of taxanes as vesicants or irritants challenging. Comprehensive preclinical data describing potential mechanisms of tissue damage or vesicant-like properties are lacking. Furthermore, most case reports of taxane extravasation fail to include the parameters surrounding administration, such as the concentration of medication and duration of infusion, making it difficult to set parameters for vesicant potential. Subsequently, many practitioners default to central venous administration of taxanes without evidence that such administration minimizes the risk of extravasation or improves outcomes thereof. Here, we review briefly the data surrounding taxane extravasation and potential vesicant or irritant properties, classify the taxanes, and propose a spectrum for antineoplastic agent potential to cause tissue injury that warrants clinical intervention if extravasation occurs.
Keywords: cabazitaxel, docetaxel, extravasation, irritant, paclitaxel, taxane, vesicant
Introduction
Extravasation of a vesicant is a potentially disfiguring event associated with many commonly used intravenous antineoplastics. Some chemotherapeutic agents, such as the vinca alkaloids and the anthracyclines, are universally accepted as vesicants with well-described physicochemical properties and supportive literature detailing the consequences of extravasation. However, the delineation of taxanes as vesicants or irritants is poorly defined, posing a clinical controversy, and a challenge in optimal prevention and management of extravasation.
Vesicants are chemicals that cause blistering of the skin or mucous membranes [Polovich et al. 2009]. Irritants cause tissue inflammation or irritation without associated blister formation, and local effects of irritant extravasation resolve with minimal intervention [Polovich et al. 2009]. Two mechanisms of tissue injury following extravasation of vesicants have been proposed. The first involves initial DNA damage with poly (ADP-ribose) polymerase activation, subsequent nicotinamide adenine dinucleotide (NAD+) depletion leading to glycolysis inhibition, and cellular protease cleavage of adherent fibrils connecting the basal epidermal cell layer to the basement membrane [Papirmeister et al. 1985]. The second mechanism involves local glutathione depletion leading to a loss of protection from free radicals, particularly those involved in lipid peroxidation, with direct tissue damage as a result [Gentilhomme et al. 1992]. Neither mechanism has been specifically linked to taxanes.
The incidence and severity of extravasation events has declined over time and has been primarily attributed to increased efforts in staff education, training, early recognition, appropriate response, and an increased use of central venous access devices (CVADs) for the administration of vesicants and irritants [Langstein et al. 2002]. Risk factors and etiology of extravasation from peripheral and CVAD administration and appropriate interventions have been well described [Polovich et al. 2009; Sauerland et al. 2006; Wickham et al. 2006]. As there is no consensus on the classification of taxanes as vesicants or irritants, there are no recommended sites for administration for individual agents.
Are all taxanes vesicants?
There are numerous reports of paclitaxel causing tissue damage including blistering following extravasation [Stanford and Hardwicke, 2003]. Postmarketing data show an incidence of 1.6% (13/812) of all injection-site reactions including those secondary to extravasation [Pfizer, 2011]. The reactions were usually mild and observed more frequently with 24 hour infusions than with 3 hour infusions [Stanford and Hardwicke, 2003; Pfizer, 2011]. The vehicle, polyoxyethylated castor oil, has been suggested as the cause of tissue injury [Kawano et al. 1994], but animal data show greater injury with undiluted paclitaxel than vehicle alone in a dose-dependent manner [Pfizer, 2011; Kawano et al. 1994; Dorr et al. 1996]. The albumin-bound paclitaxel product, Abraxane®, does not contain the polyoxyethylated castor-oil vehicle, but it has been reported to cause tissue injury and necrosis following extravasation (< 1% incidence), supporting the proposition that paclitaxel itself, and not just the vehicle, causes tissue damage [Celgene Corporation, 2012]. The extent of injury and information on the site of administration in cases of extravasation with albumin-bound paclitaxel are unavailable.
Docetaxel, formulated with polysorbate 80, is infused over 1 hour when given at standard doses at a concentration of 0.30–0.74 mg/mL. The incidence of infusion-site reactions, including extravasation, is < 1% and cases are generally mild [Sanofi-aventis US LLC, 2011]. Of the 12 published case reports of docetaxel extravasation [Berghammer et al. 2001; Cifuentes et al. 2012; Kramer et al. 2011; Una et al. 2009; El Saghir and Otrock, 2004; Ho et al. 2003; Raley et al. 2000; Ascherman et al. 2000], 5 were associated with blistering [Una et al. 2009; El Saghir and Otrock, 2004; Raley et al. 2000; Ascherman et al. 2000]. One case of blistering resulted from docetaxel administration at a concentration of 0.72 mg/mL over 1 hour, the other at a concentration of 0.48 mg/mL (infusion duration not reported) [El Saghir and Otrock, 2004; Raley et al. 2000]. The concentration and infusion duration of the other three cases are not reported [Una et al. 2009; Raley et al. 2000]. Although docetaxel does appear to be a vesicant, it should be noted that the reaction following docetaxel extravasation where no blistering occurred has been falsely mislabeled as a vesicant-type reaction [Ho et al. 2003].
Cabazitaxel, also formulated with polysorbate 80, is administered at a concentration up to 0.26 mg/mL over 1 hour [Sanofi-aventis US LLC, 2010]. There have been no reports to date of extravasation injury, but experience is limited compared with other taxanes.
Available evidence suggests that the potential for conventional paclitaxel tissue damage is dependent on concentration and infusion duration, which has not been established with either docetaxel or cabazitaxel. As no formal classification exists for the delineation of antineoplastics as vesicants or irritants, clinical intervention is varied and inconsistent. Criteria based on mechanisms of injury in human and animal models together with a grading system for severity and sequelae of tissue injury would aid appropriate classification of antineoplastic agents as vesicants, irritants, or inert compounds following extravasation. Such a system should also provide clear guidance on treatment and intervention following tissue extravasation of taxanes and other antineoplastic agents.
Is the risk of extravasation dependent on the site of taxane administration?
Although more convenient, CVAD use is associated with bloodstream infections, thrombosis, and increased cost. It is conceivable that vesicant extravasation through CVADs would be more devastating than with peripheral administration due to masking of the extent of damage. Currently there are no data to support taxane administration through CVADs versus peripheral administration to prevent extravasation or improve outcome of extravasation.
Langstein and colleagues reported that 73% of extravasation cases resulted from peripheral administration while 23% resulted from CVAD administration in a retrospective review of 44 patients with chemotherapeutic extravasation at the University of Texas MD Anderson Cancer Center from 1994 to 1999. Of these patients, 26 (61.9%) were referred to plastic surgery, and 10 (23.8%) required surgical intervention. A total o f 15 patients out of the 44 received paclitaxel, but the number of cases per administration site for paclitaxel and outcomes were not described [Langstein et al. 2002]. In a different review of 32 case reports of paclitaxel extravasation with varying degrees of irritation from various institutions, Stanford and Hardwicke reported that 9% of cases (n = 3) received paclitaxel by CVAD administration and 91% (n = 29) by peripheral intravenous administration. Two patients required surgical closure, both of whom received paclitaxel by peripheral intravenous administration [Stanford and Hardwicke, 2003].
Similarly, there have been 12 reported cases of docetaxel extravasation of which only 1 involved CVAD use [El Saghir and Otrock, 2004]. None of the cases required surgical intervention, but one case of peripheral administration required referral to plastic surgery [Raley et al. 2000]. The most common sites of administration in cases of docetaxel extravasation were the hand dorsum (n = 6) [Ho et al. 2003; Raley et al. 2000; Ascherman et al. 2000], followed by the antecubital fossa (n = 4) [Kramer et al. 2011; Una et al. 2009; Ascherman et al. 2000], and cubital fossa (n = 1) [Cifuentes et al. 2012]. All of these sites of administration have been described as risk factors for extravasation [Polovich et al. 2009; Sauerland et al. 2006]. Only one case of extravasation was reported after administration in the medial forearm [Berghammer et al. 2001].
While it is tempting to speculate that CVAD use reduced the incidence of vesicant extravasation, the higher number of cases of extravasation at peripheral venous access sites may simply reflect the higher rate at which these sites are employed for taxane administration rather than inherent safety of the approach [Polovich et al. 2009]. Administration of taxanes by CVADs does not protect from extravasation or subsequent tissue injury. More interesting is the observation that extravasation involving CVADs did not require more invasive surgical intervention for optimal control of tissue injury, as an extravasation in large, central veins could take longer to be recognized and have greater infused volumes over the same time.
Current guidelines on vesicant designation, route of administration, and extravasation management for taxanes
The American Society of Clinical Oncology and Oncology Nursing Society Chemotherapy Administration Safety Standards recommend extravasation management procedures including the use of antidotes when applicable [Neuss et al. 2013]. However, these professional organizations neither designate chemotherapeutic agents as vesicants or irritants nor recommend specific sites of administration for particular agents. In contrast, the Chemotherapy and Biotherapy Guidelines and Recommendations for Practice recommended against infusing vesicant agents peripherally for more than 30–60 min, but they also fail to categorize specifically chemotherapeutic agents as vesicants or irritants [Polovich et al. 2009]. No current standard or scoring system exists for the classification of a compound as a vesicant, irritant, or inert compound.
Conclusion and recommendations
A formal classification system and reference of chemotherapeutic agents as vesicants or irritants, or a change in the way clinicians view the potential to cause tissue injury, are clearly warranted,. Increased transparency in publishing the results of preclinical studies detailing vesicant properties of antineoplastics will aid appropriate classification and management. The available published literature supports the safety of intravenous administration of taxanes using peripheral venous access at the recommended concentrations and durations [Pfizer, 2011; Celgene Corporation, 2012; Sanofi-aventis US LLC, 2010, 2011]. Professional bodies, moreover, should update practice guidelines to include clinically relevant data (i.e. concentration, infusion duration, optimal site of administration) for taxanes and other chemotherapeutic agents to promote optimal clinical practice and patient safety.
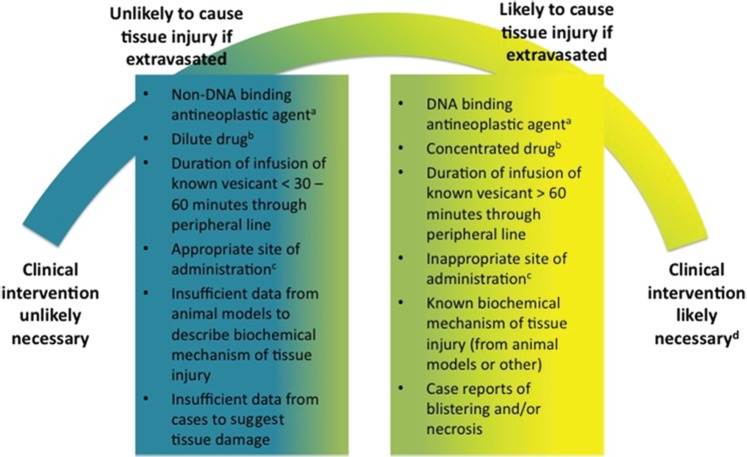
In Table 1 we propose the classification of taxanes based on known physicochemical properties, currently accepted definitions of vesicant and irritant, and data from animal and human exposure described herein. It could be more clinically useful, given current controversies and lack of sufficient data, to view the classification of antineoplastic agents as vesicants or irritants as a spectrum of likelihood to cause tissue damage needing more than minimal intervention rather than strict categorization. We have proposed such a spectrum in Figure 1.
Table 1.
Classification of taxanes.
| Taxane | Classification* | Comment | Level of evidence |
|---|---|---|---|
| Paclitaxel | Vesicant | Blistering reported | Minimal (animal models and patient cases) |
| Albumin-bound paclitaxel | Indeterminate | Tissue injury and necrosis reported, but not further defined or characterized | Low (manufacturer-reported cases of extravasation without mention of blistering) |
| Docetaxel | Vesicant | Blistering reported | Minimal (patient cases) |
| Cabazitaxel | Indeterminate | No cases of extravasation reported | None |
Classification per standard definitions [Polovich et al. 2009].
Figure 1.
Spectrum of antineoplastic agent potential to necessitate clinical intervention if extravasated. aExamples of DNA-binding agents include the anthracyclines. Examples of non-DNA-binding agents include the taxanes. bFor definitions of dilute and concentrated drug, refer to the manufacturer’s prescribing information for individual antineoplastic agents. cFor recommended administration sites, refer to current clinical practice guidelines [Neuss et al. 2013; Polovich et al. 2009]. dClinical intervention may include administration of antidote and/or surgery in addition to the standard application of ice for DNA-binding agents and a warm compress from non-DNA-binding agents.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: RDH receives research funding from Millennium, Celgene, Novartis, Onyx, Synta, and Acetylon. The other authors declare they have no conflict of interest.
Contributor Information
Meagan S. Barbee, Department of Pharmaceutical Services, Emory Healthcare, and Mercer University College of Pharmacy and Health Sciences, 1364 Clifton Road NE, B712, Atlanta, GA 30322, USA
Taofeek K. Owonikoko, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, USA
R. Donald Harvey, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, USA.
References
- Ascherman J., Knowles S., Attkiss K. (2000) Docetaxel (Taxotere) extravasation: a report of five cases with treatment recommendations. Ann Plast Surg 45: 438–441 [DOI] [PubMed] [Google Scholar]
- Berghammer P., Pohnl R., Baur M., Dittrich C. (2001) Docetaxel extravasation. Support Care Cancer 9: 131–134 [DOI] [PubMed] [Google Scholar]
- Celgene Corporation (2012) Abraxane® Prescribing information. Summit, NJ: Celgene Corporation [Google Scholar]
- Cifuentes L., Ring J., Brockow K. (2012) Extravasation of docetaxel. J Dtsch Dermatol Ges. DOI: 10.111/j.1610-0387.2012.07973.x. [DOI] [PubMed] [Google Scholar]
- Dorr R., Snead K., Liddil J. (1996) Skin ulceration potential of paclitaxel in a mouse skin model in vivo. Cancer 78: 152–156 [DOI] [PubMed] [Google Scholar]
- El Saghir N., Otrock Z. (2004) Docetaxel extravasation into the normal breast during breast cancer treatment. Anticancer Drugs 15: 401–404 [DOI] [PubMed] [Google Scholar]
- Gentilhomme E., Neveux Y., Hua A., Thiriot C., Faure M., Thivolet J. (1992) Action of bis(betachloroethyl) sulphide (BCES)on human epidermis reconstituted in culture: morphological alterations and biochemical depletion of glutathione. Toxicol In Vitro 6: 139–147 [DOI] [PubMed] [Google Scholar]
- Ho C., Yang C., Chu C. (2003) Vesicant-type reaction due to docetaxel extravasation. Acta Derm Venereol. DOI: 10.1080/00015550310013709. [DOI] [PubMed] [Google Scholar]
- Kawano S., Kondoh H., Ishikawa K., Koizumi S., Kadota T., Takahashi N. (1994) Irritability study of paclitaxel in rabbit ear vein. J Toxicol Sci 19: 123– 130 [DOI] [PubMed] [Google Scholar]
- Kramer F., Schippert C., Rinnau F., Hillemanns P., Park-Simon T. (2011) The first description of docetaxel-induced recall inflammatory skin reaction after previous drug extravasation. Ann Pharmacother 45: e11. [DOI] [PubMed] [Google Scholar]
- Langstein H., Duman H., Seelig D., Butler C., Evans G. (2002) Retrospective study of the management of chemotherapeutic extravasation injury. Ann Plast Surg 49: 369–374 [DOI] [PubMed] [Google Scholar]
- Neuss M., Polovich M., McNiff K., Esper P., Gilmore T., LeFebvre K., et al. (2013) 2013 Updated American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards Including Standards for the Safe Administration and Management of Oral Chemotherapy. J Oncol Pract 9: 5s–13s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer (2011) Paclitaxel Prescribing information. New York, NY: Pfizer [Google Scholar]
- Papirmeister B., Gross C., Meier H., Petrali J., Johnson J. (1985) Molecular basis for mustard-induced vesication. Fundam Appl Toxicol 5: S134–S149 [PubMed] [Google Scholar]
- Polovich M., Whitford J., Olsen M. (2009) Chemotherapy and Biotherapy Guidelines and Recommendations for Practice (Third edition). Pittsburg, PA: Oncology Nursing Society [Google Scholar]
- Raley J., Geisler J., Buekers T., Sorosky J. (2000) Docetaxel extravasation causing significant delayed tissue injury. Gynecol Oncol 78: 259-260 [DOI] [PubMed] [Google Scholar]
- Sauerland C., Engelking C., Wickham R., Corbi D. (2006) Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum 33: 1134–1141 [DOI] [PubMed] [Google Scholar]
- Sanofi-aventis US LLC (2010) Jevtana® Prescribing information. Bridgewater, NJ: Sanofi-aventis US LLC [Google Scholar]
- Sanofi-aventis US LLC (2011) Taxotere® Prescribing information. Bridgewater, NJ: Sanofi-aventis US LLC [Google Scholar]
- Stanford B., Hardwicke F. (2003) A review of clinical experience with paclitaxel extravasations. Support Care Cancer 11: 270–277 [DOI] [PubMed] [Google Scholar]
- Una E., Cuadrillero F., Lopez-Lara F. (2009) Drug extravasation: a dreaded complication. BMJ Case Rep. DOI: 10.1136/bcr.09.2008.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham R., Engelking C., Sauerland C., Corbi D. (2006) Vesicant extravasation part II: evidence-based management and continuing controversies. Oncol Nurs Forum 33: 1143–1150 [DOI] [PubMed] [Google Scholar]