Abstract
IRF3 is an innate anti-viral factor whose role in limiting Theiler’s Murine Encephalomyelitis Virus (TMEV) infection and preventing TMEV-induced disease is unclear. Acute disease and innate immune responses of macrophages were examined in IRF3 knockout mice compared with C57Bl/6 mice following in vitro or intracranial infection with either TMEV GDVII or DA. IRF3 deficiency augmented viral infection, as well as morbidity and mortality following intracranial infection with neurovirulent TMEV GDVII. In contrast, IRF3 deficiency prevented hippocampal injury following intracranial infection with persistent TMEV DA. The extent of TMEV infection in macrophages from C57Bl/6 mice was significantly less than that in IRF3 deficient macrophages, which was associated with poor IFN-γ and IL-6 expression in response to TMEV. Reestablishing IRF3 expression in IRF3 deficient macrophages increased control of TMEV replication and increased expression of IFN-γ and IL-6. In addition, IRF3 deficient macrophages failed to exhibit IL-6 antiviral effects, which was associated with inability to sustain IL-6-induced STAT1 activation compared with C57BL/6 macrophages. Altogether, IRF3 contributes to early control of TMEV replication through induction of IL-6 and IFN-γ and support of IL-6 antiviral effects, but contributes to TMEV-induced hippocampal injury.
1. Introduction
Theiler’s murine encephalomyelitis virus (TMEV) is a picornavirus that infects most feral and laboratory strains of mice. Intracranial (i.c.) infection of nearly all mice with as little as 100 PFU of the GDVII strain causes acute severe encephalitis that results in death within 10 days p. i. In contrast, i.c. infection with the DA strain of TMEV causes less severe, acute encephalitis with various outcomes depending upon mouse strain. C57BL/6 (B6) and B10.s mice clear TMEV DA i.c. infections with robust innate and adaptive immune antiviral responses (Monteyne et al., 1999). However, TMEV DA infection in these mice result in hippocampal damage, learning deficits (Buenz et al., 2009; Howe et al., 2012), and recurrent seizures (Libbey et al., 2008). In contrast, SJL/J mice have inadequate innate and adaptive immune responses to TMEV and fail to completely clear TMEV-DA from central nervous system (CNS)-infiltrating macrophages. As a result SJL/J mice develop a chronic TMEV infection in the CNS without acute hippocampal damage (Howe et al., 2012) but develop late demyelinating disease (Lipton et al., 1984). Because TMEV has a short half-life in vivo due to low viral particle production from infected cells and virus-induced apoptosis, viral replication is required to maintain persistence (Lipton et al., 2005). Therefore, resistance to persistent TMEV infection may be related to innate immune control of virus replication in macrophages.
The ability to control TMEV replication in macrophages is related to production of interferon-γ (IFN-γ) (Nguyen et al., 2002) and IL-6 (Moore et al., 2012), and induction of interferon stimulated genes (ISGs). Expression of these antiviral factors depends upon activation of interferon response factor-3 (IRF3), which is constitutively expressed. Activation of IRF3 in TMEV infection of macrophages occurs through TLR3, TLR7(Al-Salleeh and Petro, 2007), and MDA5 signaling pathways(Jin et al., 2011). Once activated, IRF3 functions as a transcription factor to induce IFN-γ, IL-6 and ISGs. The effects of IRF3 on IFN-γ γproduction have been well documented. After its secretion, IFN-γ signals through the type I IFN receptor leading to STAT1 and STAT2 phosphorylation, expression of additional IRFs and interferon stimulated genes (ISGs) (Marijanovic et al., 2007). We have seen that IL-6 signaling through its receptor also leads to STAT1 activation in macrophages (Moore et al., 2012). Although IRF3 is involved in expression of antiviral genes and control of virus replication, it is unclear if it is involved in TMEV-induced IL-6 expression, in the resistance of B6 macrophages to TMEV, and development of TMEV-induced disease.
In this report we demonstrate that replication of the TMEV RNA genome is significantly higher in macrophages from IRF3 knockout (IRF3KO) mice and in the brains of IRF3KO mice following intracranial infection compared with B6 mice. IRF3 deficiency caused greater morbidity and mortality during intracranial TMEV GDVII infection, less TMEV-induced IFN-γ and IL-6 expression, less sustained IL-6 induced STAT1 activation, and less TMEV-DA induced damage to the hippocampus compared to B6 mice. IRF3-expressing plasmids were able to restore IL-6 and IFN-γ expression in response to TMEV and restore control of TMEV replication in IRF3KO macrophages.
2. Results
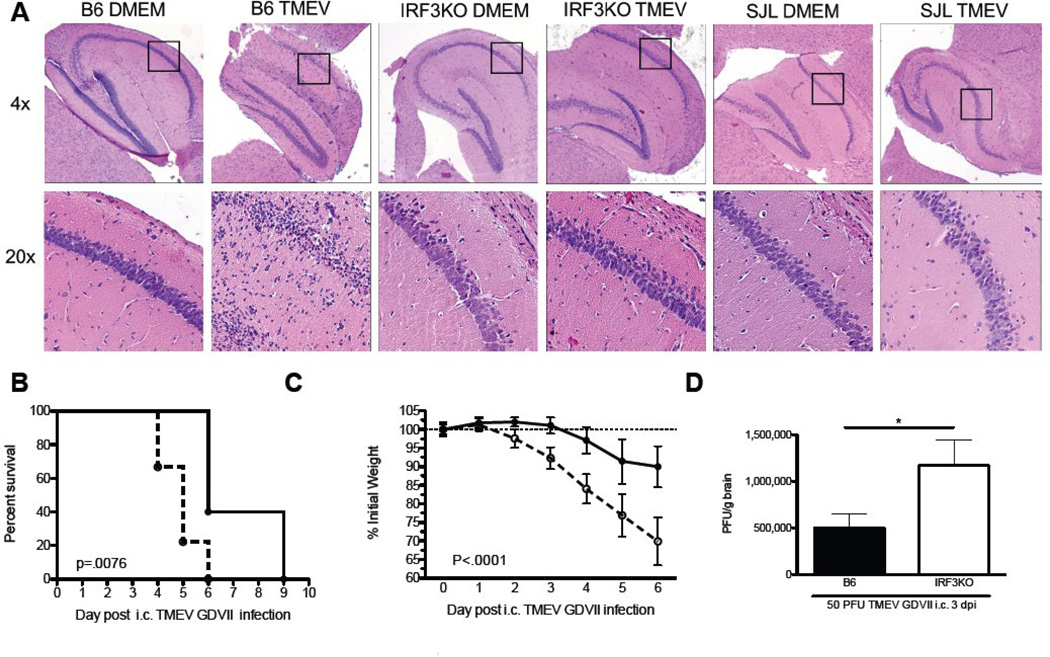
2.1 IRF3 deficiency ameliorates TMEV DA-induced acute hippocampal injury but exacerbates TMEV GDVII-induced acute lethal encephalitis
Intracranial infection of B6 mice with TMEV DA results in immune responses that bring about viral clearance. However, these same immune responses are responsible for hippocampal injury within one week of infection with TMEV DA (Howe et al., 2012). To determine whether IRF3 had a role in TMEV-induced hippocampal injury, B6 and IRF3KO mice were i. c. infected with TMEV DA. SJL/J mice, which have a poor immune response to TMEV DA, were also i.c. infected with TMEV DA and served as a negative control for hippocampal damage. Intracranial infection with TMEV DA resulted in severe hippocampal injury in B6 mice at day 4 p. i. as measured by evaluating CA1 regions of H & E stained hippocampi (Fig. 1A), which is consistent with previous reports (Howe et al., 2012). In contrast, i.c. infection with TMEV DA caused minimal and undetectable hippocampal injury in IRF3KO or SJL/J mice. Therefore, IRF3 is involved in early immune responses to TMEV DA during the encephalitis phase of infection that brings about acute tissue damage.
Fig 1.
IRF3 contributes to hippocampal injury following acute TMEV encephalitis but promotes viral clearance that prevents symptoms of chronic demyelinating disease following TMEV infection. C57BL/6, IRF3KO, and SJL/J mice were infected via the i.c. route with 1 × 106 pfu TMEV DA strain (A) or C57BL/6 and IRF3KO mice were infected via the i.c. route with 50 pfu TMEV GD-VII strain (B,C, D). (A) After 4 days of TMEV DA infection hippocampi were removed from a cohort of infected mice, fixed in formalin, and subjected to H&E stain. 4X images show the Dentate Gyrus, CA3, CA2, and CA1 regions while the 20X inset images show a portion of the CA1 region. Of mice infected i.c. with the TMEV GD-VII strain (B) Kaplan Meyer plots of survival (n=6–9), (C) Percent weight loss in individual mice(n=6–9), (D) TMEV pfu/g brain of individual mice (n=4) at 3 days post infection (dpi) with GD-VII. Data are means +/− SEM. *P<.05.
While i.c infection of B6 mice with TMEV DA results in nonlethal encephalitis that helps to clear the virus but damages the hippocampus, i.c. infection of B6 mice with TMEV GDVII results in severe encephalitis and death within 10 days (Lipton, 1980). To determine the role of IRF3 in lethal encephalitis, B6 and IRF3KO mice were i. c. infected with TMEV GDVII. Intracranial infection with TMEV GDVII resulted in more severe encephalitic outcomes in IRF3KO mice compared with B6 mice as measured by weight loss and the pace at which mortality ensued after infection (Fig. 1B & C). To confirm that the increased mortality rate in IRF3KO mice was due to increased susceptibility to TMEV GDVII infection we extracted brains from B6 and IRF3KO mice that had been i.c. infected 3 days prior, and determined TMEV GDVII pfu in each. The number of pfu/gm of brain tissue was significantly higher in i.c. infected IRF3KO mice compared with B6 mice, correlating disease outcomes with TMEV GDVII infection (Fig. 1D). Therefore activation of IRF3 following TMEV infection lessens the severity of morbidity and mortality during TMEV–GDVII induced encephalitis but contributes to hippocampal damage during TMEV-DA induced encephalitis.
2.2 IRF3 activation controls TMEV replication and promotes early IFN-γ and IL-6 expression in macrophages
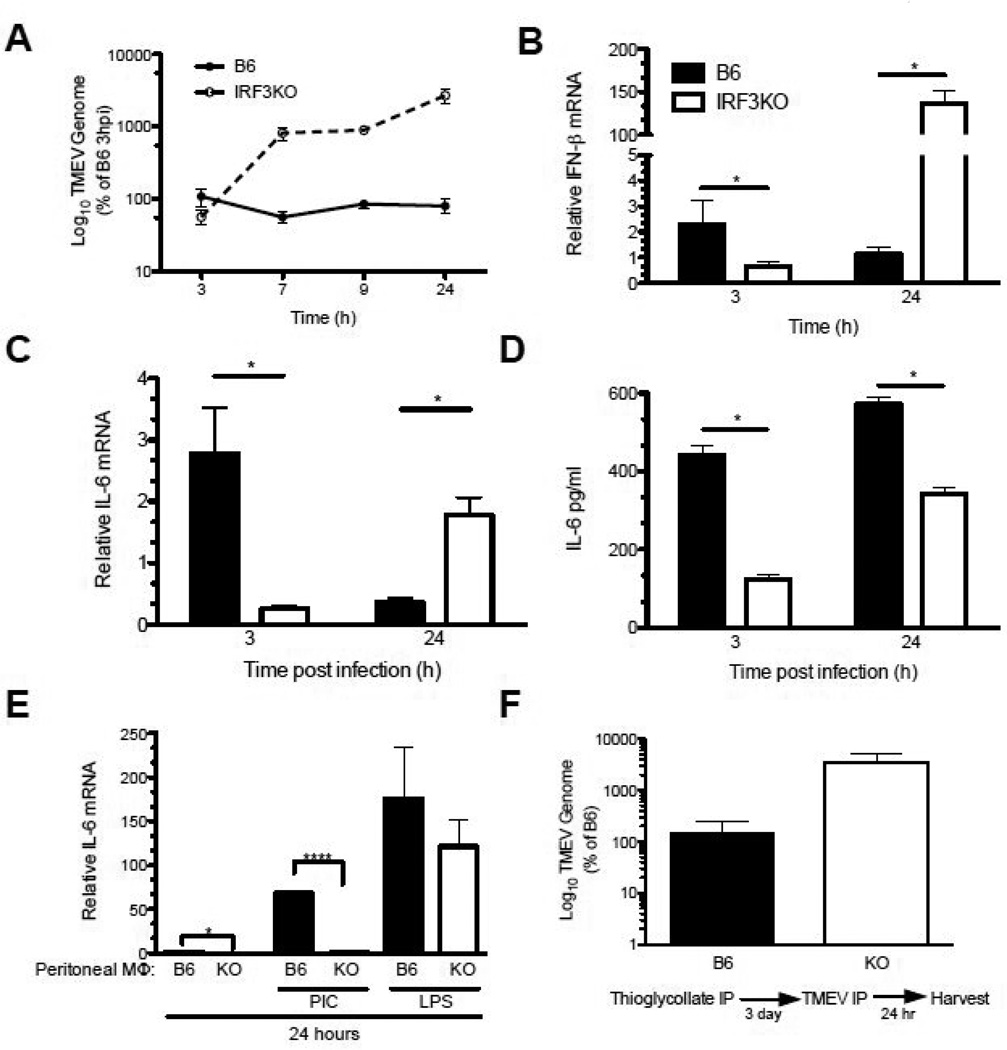
We have previously shown that resistance to TMEV infection in macrophages from B10.S mice is correlated with high levels of IL-6 expression (Moore et al., 2012). We hypothesize that TMEV does not replicate well in macrophages from B6 mice because IRF3 promotes the production of early anti-viral cytokine responses, such as IL-6, which are antiviral but could play a role in hippocampal damage (Sparkman et al., 2006). To investigate the role of IRF3 in controlling TMEV infection and TMEV-induced cytokine expression, sterile thioglycollate-induced inflammatory macrophages were harvested from B6 and IRF3KO mice (Sato et al., 2000) and then infected with TMEV in vitro. Cell lysates were collected at 3, 7, 9 and 24 h p. i. and TMEV genomic RNA was measured by qRT-PCR. TMEV RNA, presented as percent of that found in B6 macrophages at 3 h, was detectable but limited in B6 macrophages at 7, 9 and 24 h p. i. (Fig. 2A). In contrast, TMEV RNA in macrophages from IRF3KO mice was similar to those of B6 macrophages at 3 h but was significantly greater than that found in B6 macrophages as early as 7 h p. i. progressing to even higher levels by 24 h p. i. This indicates that IRF3 is required to resist replication of the TMEV genome. B6 inflammatory macrophages expressed similar basal levels of IFN-γ but significantly greater basal expression of IL-6 compared with IRF3KO macrophages. However, B6 macrophages expressed more TMEV-induced IFN-γ (Fig. 2B) and IL-6 (Fig. 2C) at 3 h p. i. compared to IRF3KO macrophages. This difference was apparent up to 9 h p. i. (data not shown). These depressed IL-6 mRNA at 3 h in IRF3KO macrophages was reflected in significantly decreased IL-6 protein accumulation at both 3 and 24 h from IRF3KO macrophages compared with B6 macrophages (Fig. 2D). Interestingly, by 24 h p. i., TMEV-induced IFN-γ and IL-6 mRNA expression in IRF3KO macrophages exceeded that in B6 macrophages, presumably due to higher TMEV RNA levels driving IRF3 independent IL-6 and IFN-γ gene expression (Fig. 2B, 2C). TMEV induced late expression of IRF1 could drive induction of IFN-γ at 24 h p. i. (Dahlberg et al., 2006; Miyamoto et al., 1988). However, increased IL-6 mRNA at 24 h p.i. in IRF3KO macrophages was not reflected in higher IL-6 protein secretion at 24 h (Fig. 2D). These results suggest that IRF3 activation is required for the immediate IL-6 and IFN-γ response of macrophages to TMEV infection. Moreover, the IRF3 role in IL-6 expression may be the basis for its contribution to hippocampal injury during TMEV infection.
Fig 2.
IRF3 activation controls TMEV replication and promotes early IFN-γ and IL-6 in TMEV infected macrophages. Thioglycollate elicited macrophages from B6 or IRF3KO mice were cultured at 1 × 106 cells and then challenged with 1 MOI TMEV DA. B6 (black circle or bar) or IRF3KO (white circle or bar) macrophages infected with 1 MOI TMEV, and (A) TMEV RNA was measured by qRT-PCR at 3, 7, 9, and 24 hours p. i. Data are means of % TMEV genome (log 10) compared to TMEV genome found in B6 macrophages at 3 h. Expression of (B) IFN-γ and (C) IL-6 were measured by qRT-PCR at 3 or 24 hours p. i. Data are means of relative cytokine expression compared to uninfected B6 macrophages at 3 h p. i. (D) IL-6 protein was measure by ELISA at 3 or 24 h p. i.. (F) Macrophages were treated with 50 µg/ml poly I:C or 1 µg/ml LPS for 24 hours and IL-6 mRNA expression was measured by qRT-PCR. (F) B6 or IRF3 KO mice were injected i.p. with 2ml thioglycollate for 3 days and then challenged with 1×106 PFU TMEV for 24 hours and TMEV RNA was measured in peritoneal wash by qRT-PCR. Data are means +/− SEM of 3 to 4 samples each analyzed by a two-tailed t test. *P<.05, ***P<.01, ****P<.001
Because IRF3 is activated through both the TLR3- or TLR4- pathways and the control of certain viral infections requires TLR4 in addition to TLR3 pathways (Ehl et al., 2004), B6 and IRF3KO macrophages were treated with poly I:C, a TLR3 agonist that triggers IRF3 activation, or LPS, a TLR4 agonist that also triggers IRF3 activation. B6 macrophages expressed more IL-6 mRNA than IRF3KO macrophages in response to poly I:C, but not LPS (Fig. 2D), suggesting that IRF3 is involved in the expression of IL-6 in response to TLR3 but not necessarily TLR4 pathways in macrophages.
To determine the impact of IRF3 on TMEV infection of inflammatory macrophages in vivo, B6 or IRF3KO mice were injected i.p. with sterile thioglycollate and three days later challenged with 1×106 PFU of TMEV i.p. After 24 h, peritoneal cells were harvested and TMEV RNA was measured by qRT-PCR. Consistent with in vitro experiments, TMEV RNA was approximately 30 fold higher in thioglycollate-injected IRF3KO mice compared to thioglycollate-injected B6 mice (Fig. 2E). Therefore, IRF3 is required for the control of TMEV infection in macrophages in vitro and in vivo.
2.3 IRF3 expression correlates with IL-6 production in TMEV infected macrophages
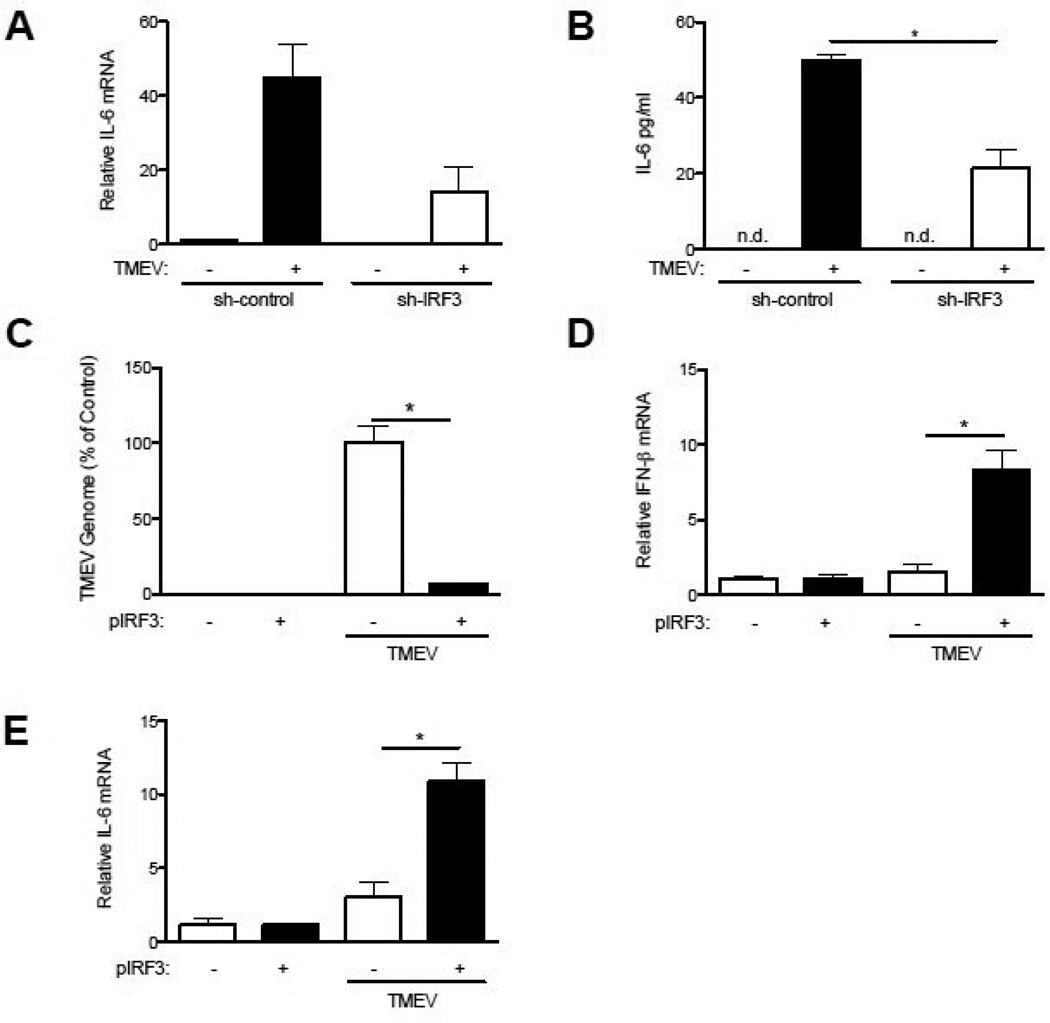
Previously, we have shown that IL-6 is able to directly prevent TMEV replication in macrophages (Moore et al., 2012). To further analyze the role of IRF3 in TMEV induced IL-6 expression we used sh-IRF3 plasmids (Al-Salleeh and Petro, 2008) to knockdown IRF3 expression in the RAW264.7 macrophage cell line, in which TMEV replicates well (Petro, 2005b; Steurbaut et al., 2006). Following transfection of sh-IRF3 plasmids, expression of IRF3 was confirmed by western blot to be approximately 50% of that with transfection of shRNA control plasmid (data not shown) and was consistent with decreased IRF3 expression we previously observed with this sh-IRF3 plasmid (Al-Salleeh and Petro, 2008). Decreased IRF3 expression in TMEV-infected RAW264.7 cells significantly reduced expression of IL-6 mRNA (Fig. 3A) and IL-6 protein (Fig. 3B) compared with RAW264.7 cells transfected with a sh-RNA control plasmid. To confirm that IRF3 contributes to control of TMEV infection and induction of IL-6 expression in response to TMEV infection, IRF3KO macrophages were transfected with an IRF3 expression vector (pIRF3) or pGFP (control expression vector). Transfection rates were ~30–40% as indicated by fluorescent microscopy of GFP+ cells (data not shown). Twenty-four h following TMEV infection, TMEV RNA was significantly decreased in IRF3KO macrophages transfected with pIRF3 compared with IRF3KO macrophages transfected with pGFP (Fig. 3C). In addition, expression of IRF3 in IRF3KO macrophages significantly increased TMEV-induced early expression of IFN-γ (Fig. 3D) and IL-6 (Fig. 3E). These results confirm that IRF3 is a key factor controlling TMEV replication most likely due to immediate-early expression of IFN-γ and IL-6 in response to TMEV.
Fig 3.
IRF3 is required for IFN-γ, IL-6 production and control of TMEV infection in RAW264.7 cells and restores control of TMEV infection in IRF3KO macrophages. RAW264.7 cells were transfected with sh-IRF3 or sh-control plasmids for 48 hours, then infected with TMEV (A,B) for 24 hours and IL-6 mRNA and protein was measured by qRT-PCR and ELISA, respectively. IRF3KO macrophages were transfected with 2 µg pIRF3 or pGFP expression vectors. After 24 hours, transfected IRF3KO macrophages were infected with 1 MOI TMEV for 24 hours and TMEV RNA (C), IFN-γ mRNA (C), or IL-6 mRNA (E) was measured by qRT-PCR. Data are means +/− SEM of 3 to 4 samples each analyzed by a two-tailed t test. *P<.05.
2.4 Exogenous IL-6 cannot reduce TMEV replication in IRF3KO macrophages
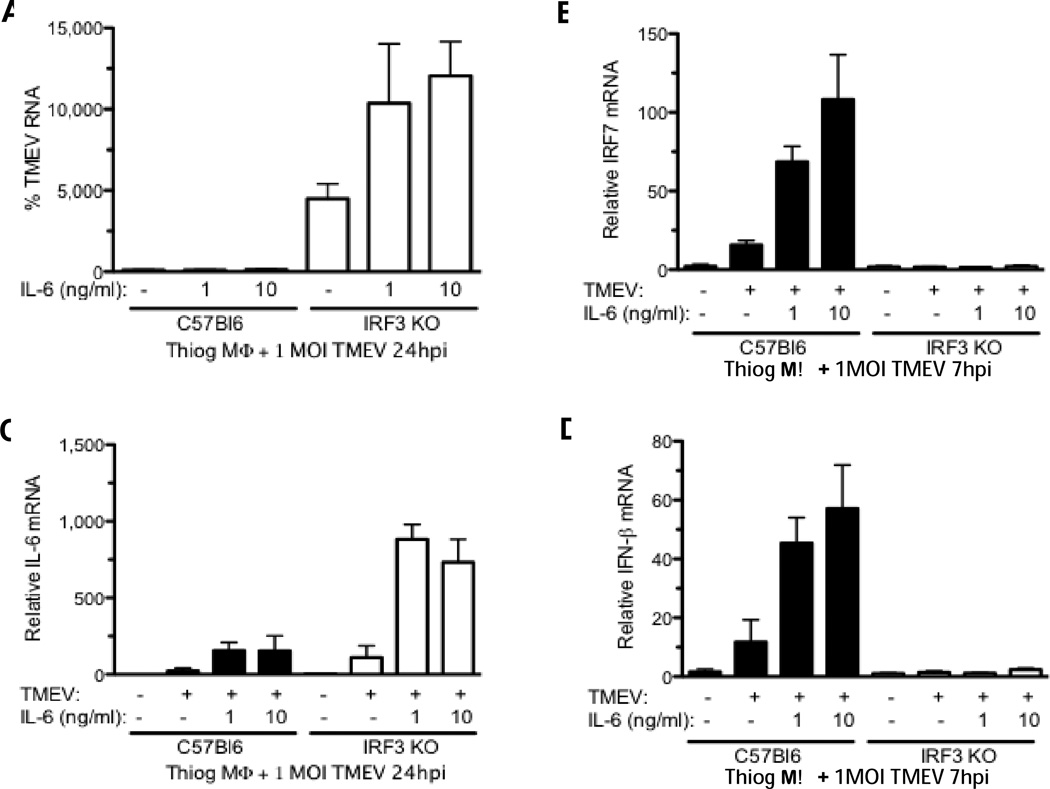
Previously we showed that control of TMEV replication in B10.S macrophages but not SJL/J macrophages is associated with heightened IL-6 expression in B10.S macrophages. Moreover, addition of exogenous IL-6 reduced TMEV replication in SJL/J macrophages (Moore et al., 2012). Therefore we hypothesized that exogenous IL-6 could decrease TMEV replication in IRF3KO macrophages as well. To test this, B6 or IRF3KO macrophages were treated with 1 or 10 ng/ml recombinant IL-6 for 30 min prior to in vitro infection with TMEV. In contrast to what we previously showed with IRF3+/+ macrophages (Moore et al., 2012), exogenous IL-6 was unable to reduce 24 h TMEV replication in IRF3KO macrophages(Fig. 4A). In fact, a dose-dependent increase in TMEV replication was observed in IRF3KO macrophages treated with exogenous IL-6. In our previous report we showed that the antiviral effect of IL-6 was in part due to its ability to quickly stimulate expression of IFN-γ and IRF7 within 6 h after TMEV infection (Moore et al., 2012). Therefore, we evaluated IRF7 and IFN-γ expression in IL-6-treated B6 and IRF3KO macrophages 7 h post TMEV infection. As we saw previously at 7 h p. i., IL-6 treatment enhanced expression of IRF7 (Fig. 4B) and IFN-γ (Fig. 4D) in TMEV-infected B6 macrophages but IL-6 failed to stimulate any expression of IFN-γ and IRF7 in IRF3KO macrophages that were infected. However, exogenous IL-6 was able to enhance TMEV-induced IL-6 mRNA expression in both B6 and IRF3KO macrophages (Fig. 4C), which is consistent with our data and others showing that IL-6 enhances IL-6 expression (Akira, 1997) . Altogether, these results suggest that IRF3 is a component of the IL-6 receptor signaling pathway that accounts for its antiviral activity.
Fig 4.
Antiviral effect of exogenous IL-6 is absent in IRF3 KO macrophages. B6 or IRF3 KO macrophages were pretreated with 1 or 10 ng/ml IL-6 for 30 minutes then infected with 1 MOI TMEV for 24 hours and TMEV RNA (A), IRF-7(B), IL-6 (C), and IFN-γ (D) were measured by qRT PCR. TMEV genome data are means of % TMEV RNA of untreated TMEV-infected B6 macrophages. Data are means +/− SEM of 3 to 4 samples each analyzed by a two-tailed t test.
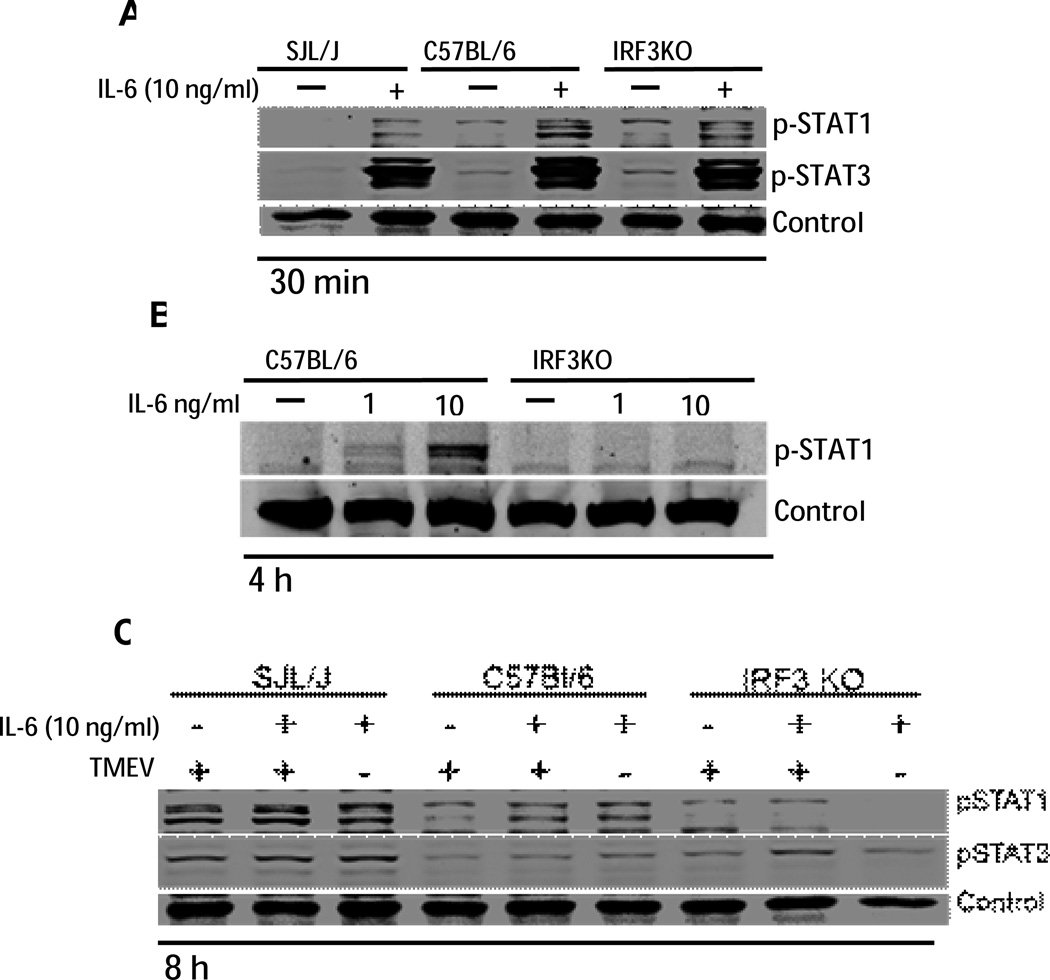
While it is well-established that IL-6 activates STAT3 in macrophages, we have previously shown that IL-6 also stimulates phosphorylation of STAT1, a key transcription factor for several antiviral interferon stimulated genes (ISGs) (Moore et al., 2012). Because IRF3 deficiency in macrophages resulted in loss of the IL-6 anti-viraln effect, it is possible that IRF3 is a component of the IL-6 receptor signaling pathway leading to STAT1 activation. Therefore, B6 or IRF3KO macrophages were treated with or without 1 or 10ng/ml IL-6 for 30 min or 4 h. SJL/J macrophages were used as an additional control. SJL/J, B6, and IRF3 KO macrophages exhibited similar STAT1 and STAT3 phosphorylation at 30 min following addition of exogenous IL-6 (Fig. 5A). STAT1 phosphorylation was sustained for 4 h in B6 macrophages but not IRF3KO macrophages following IL-6 stimulation (Fig. 5B). We also evaluated activation of STAT1 and STAT3 at 8 h post IL-6 treatment with or without TMEV infection. STAT1 and STAT3 activation in SJL/J macrophages was still detectable and was greater than that seen in B6 macrophages at 8 h post IL-6 treatment or post TMEV infection (Fig. 5C). While STAT1 activation in IRF3KO macrophages at 8 h p. i. was detectable, STAT1 activation in IL-6 treated IRF3KO macrophages at 8 h was undetectable. Therefore, IRF3 activation is required to sustain IL-6-induced STAT1 phosphorylation and IL-6 antiviral activity in macrophages.
Fig 5.
IRF3 KO macrophages do not sustain STAT1 phosphorylation after treatment with exogenous IL-6. SJL/J, B6, or IRF3KO macrophages were pre-treated with 10ng/ml IL-6 with or without TMEV infection for 30 min (A), 4 h (B), or 8 h (C). Total and phospho (p) STAT1 and pSTAT3 were analyzed by western blot. A non-specific band was used as a control. Unstimulated controls for (C) are in (A).
3. Discussion
We have previously shown that TMEV infection of macrophages activates IRF3 through TLR3 and TLR7 (Al-Salleeh and Petro, 2007) and others have shown that IRF3 is also activated by TMEV infection through cytoplasmic MDA5(Jin et al., 2011). The results herein demonstrate that IRF3 deficiency enables high TMEV RNA replication in macrophages of B6 mice, exacerbates acute encephalitis during TMEV GDVII infection, and yet ameliorates TMEV-induced hippocampal injury during acute TMEV-DA infection. These outcomes are similar to vigorous human immune responses that clear virus but cause damage to adjacent tissue (Koyuncu et al., 2013; Virgin et al., 2009). Our data are consistent with a recent report indicating that i.c. TMEV infection in B6 or B10.S mice, but not SJL/J mice, induces hippocampal injury by day 4 p. i. (Howe et al., 2012). In that report, adoptive transfer of B10.S macrophages into SJL/J mice conferred susceptibility to TMEV-induced hippocampal damage. A previous report showed that TMEV-induced hippocampal injury in B6 mice was the result of inflammatory macrophage induction of neuron apoptosis (Buenz et al., 2009; Howe et al., 2012). We show herein for the first time that IRF3 is a significant factor in the hippocampal injury following TMEV DA infection. We also showed that IRF3 deficiency impairs IL-6 expression from infected macrophages. Sustained and heightened IL-6 expression during neuroinflammation has been shown previously to cause damage to the hippocampus (Sparkman et al., 2006). Our data suggest that IRF3 role in TMEV-induced hippocampal damage is through its role in IL-6 expression.
In contrast to i. c. infection with TMEV DA, i. c. infection with the TMEV GDVII causes severe acute encephalitis in nearly all laboratory strains that is exhibited in significant morbidity and mortality within weeks after infection. Here we show that morbidity and mortality to i.c. infection with TMEV-GDVII are significantly earlier in IRF3 deficient mice compared with B6 mice. This enhancement in susceptibility to TMEV GDVII is associated with significantly higher viral titers in the CNS compared with B6 mice. Therefore, during viral infections in the CNS the beneficial aspects of the immune responses to lessen catastrophic outcomes such as morbidity and mortality could contribute to damage due to the immune responses.
We have demonstrated here that IRF3 is involved in the early expression of IL-6 in response to TMEV, which is a cytokine that helps control viral replication but which are also involved in CNS pathology. A recent report showed that besides hippocampal injury IL-6 expression in the CNS in response to TMEV infection may be a contributing factor in seizures that develop in B6 mice following infection (Cusick et al., 2013). It is unclear if the hippocampal damage we observed in B6 mice infected with TMEV-DA is somehow related to the TMEV-induced seizures observed in B6 mice (Libbey et al., 2011).
Nonetheless, even though the IL-6 and IFNγ responses to TMEV at 3 h p. i. are impaired in IRF3KO macrophages, by 24 h p. i. elevated TMEV RNA can induce expression of these cytokines despite the lack of IRF3. Furthermore, elevation of IL-6 and IFN-γ at 24 h has little impact on TMEV because high viral replication is maintained in IRF3KO macrophages. These results suggest that the antiviral effects of both IL-6 and IFN-γ require IRF3 downstream of their receptors and are consistent with recent reports showing that IFN-γ expression exacerbates chronic viral infections when given later during infection (Teijaro et al., 2013; Wilson et al., 2013).
We expected that exogenous IL-6 would be able to control TMEV replication in IRF3KO macrophages because our previous work showed that exogenous IL-6 helped control TMEV replication in SJL/J and RAW264.7 macrophages (Moore et al., 2012). However, the results herein show that exogenous IL-6 was unable to control TMEV replication in IRF3KO macrophages. This suggests that IRF3, in addition to inducing anti-viral factors such as ISG56, IFN-γ , and IL-6, somehow mediates their anti-viral effect as well. We observed that IL-6, which sustained STAT1 phosphorylation in wild-type macrophages, failed to sustain STAT1 phosphorylation in IRF3KO macrophages. It is likely that sustained IL-6-induced STAT1 phosphorylation requires IRF3-dependent expression of IFN-γ , IRFs, or ISGs. However, the precise requirement of IRF3 in IL-6-induced anti-viral activity remains unclear.
We have also demonstrated that overexpression of IRF3 in IRF3KO macrophages restores resistance to TMEV replication. This is important because IRF3KO mice also have a mutation in the BCL2L12 gene, which could be involved in apoptosis (Nakajima et al., 2009). Interestingly, in addition to its role in the nucleus as a transcription factor for induction of anti-viral genes, in the cytoplasm activated IRF3 has also been shown to dimerize with Bax, localize to mitochondria, and induce apoptosis in virus infected cells (Chattopadhyay et al., 2010; Sharif-Askari et al., 2007). Although it is likely that IRF3 controls TMEV replication in macrophages through multiple mechanisms, it is possible that IRF3-dependent virus induced apoptosis also plays a role in controlling TMEV infection.
Altogether, results herein show that IRF3 is required for early IL-6 and IFN-γ expression, control of virus RNA replication in TMEV-infected macrophages, and severity of TMEV induced morbidity and mortality. In contrast, it appears that IRF3 is also involved in hippocampal damage in mice that clear the virus. These results are important because TMEV GDVII infection of the mouse CNS is a model of lethal virus-induced encephalitis while TMEV DA infection of the mouse CNS is a model of non-lethal viral encephalitis and persistence leading to disease. Furthermore, while IRF3 is involved in early IL-6 expression following TMEV infection of macrophages it appears not to be involved in chronic IL-6 expression. It is postulated that chronic late expression of IL-6 that cannot control TMEV replication contributes to chronic inflammation and disease.
4. Methods
4.1 Mice, virus, cell lines, and reagents
C57BL/6 (B6) mice were obtained from Jackson Laboratories and used at 6–8 weeks age. IRF3 deficient mice (IRF3KO) on the B6 background were offspring of breeder pairs obtained from Dr. Karen Mossman (Sato et al., 2000). SJL/J mice were obtained from Harlan Laboratories and used at 6–8 weeks of age. RAW264.7 cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in DMEM with 10% FBS with 50 µg/ml gentamycin. E. coli LPS O127:B8 was obtained from Sigma Chemical Co.(St. Louis, MO), and poly I:C was obtained from InvivoGen (San Diego, CA). The DA strain of TMEV was obtained from Dr. Kristen Drescher, Department of Medical Microbiology and Immunology, Creighton University, Omaha, Nebraska. The GDVII strain of TMEV was obtained from Dr. Howard Lipton, University of Illinois at Chicago. TMEV was grown in BHK-21 cells. The titer of stock cultures of TMEV was 1 × 107 PFU/ml and macrophage cultures were infected with 1 × 106 PFU of TMEV unless otherwise stated. Mice were infected intraperitoneally (i. p.) or intracranially (i. c.) with 1 × 106 PFU of TMEV DA strain or 50 PFU of the TMEV GDVII strain. Plaque forming units in brains of day 3 GDVII-infected mice were performed by overlaying dissociated brains onto 70% confluent BHK21 cells, incubating at 37°C for 1 h, aspirating media, adding 4% agarose in DMEM with 2% FBS, and incubating at 37°C. After 2 days, plaques were visualized by adding MTT reagent and reincubating for 4 h at 37°C.
4.2 Macrophage preparations
Inflammatory macrophages were elicited by i.p. injection of 2 ml sterile thioglycollate broth into mice. Three days later, the peritoneal cavities were flushed with 2 ml DMEM and cells were incubated at 1 × 106 cells/2 ml of DMEM cell culture medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Invitrogen), and 50 µg/ml gentamycin (Invitrogen). After 24 h, non-adherent cells were removed and 1 ml of culture medium added. Adherent cells were greater than 90% Mac-1+ as determined by FACS analysis (Petro, 2005a). These macrophages were either untreated or pretreated for 30 min with 1 or 10 ng/ml recombinant IL-6 (BD-Pharmingen, San Diego, CA). Untreated or pretreated macrophages were uninfected, infected with 1 × 106 PFU of TMEV, stimulated with 1 µg/ml LPS, stimulated with 50 µg poly I:C or left unstimulated. After 3, 7, 9, or 24 h of infection or stimulation, cell extracts were collected for RNA preparation and qRT-PCR.
4.3 Transfections and RNA interference
Validated inhibitory shRNA targeting mouse IRF3 or control shRNA (Al-Salleeh and Petro, 2008) was transfected into RAW264.7 cells according to manufacturer’s specifications using the nucleofection kit V of Amaxa (Lonza, Cologne,Germany). Transfections were 48 h prior to challenge with TMEV or treatment with poly I:C/IFN-γ . For transfection of primary macrophages, pB10.s-IRF3 (Moore et al., 2011) or pmaxGFP (pGFP), were transfected into thioglycollate-elicited macrophages from IRF3KO mice using the Mouse Macrophage Nucleofector Kit (Amaxa). Cells were rested for 24 hours at 3×105 cells per well, then infected with 1 MOI TMEV, after which cell lysates were collected for qRT-PCR at 24 h p. i.
4.4 RNA preparation and qRT-PCR
RNA was extracted from cells using the PerfectPure kit from 5Prime (Gaithersburg, MD), or the Purelink kit from Ambion/Invitrogen (Carlsbad, CA), according to the manufacturer’s specifications. One-hundred ng to one µg of RNA was reverse transcribed in 0.5 mM each of dATP, dGTP, dTTP, and dCTP, 20 U of RNAse inhibitor with Superscript II reverse transcriptase (Invitrogen) at 42°C for 1.5 h followed by 95°C for 5 min. The cDNA was diluted 1:2 and 1 µL was incubated with 0.4 µM of the following primer pairs (Invitrogen):
IFN-γ sense 5’ ATGAACAACAG GTGGATCCTCC 3’
and anti-sense 5’ AGGAGCTCCTGACATTTCCGAA 3’;
IL-6 sense 5’ ATGAAGTTCCT CTCTGCAAGAGACT 3’
and antisense 5’ CACTAGGTTTGCC GAGTAGATCTC 3’;
TMEV sense 5’ CTTCCCATTC TACTGCAATG 3’;
and antisense 5’ GTGTTCCTGG TTTACAGTAG3’;
or GAPDH sense 5’-TTGTCAGCAA TGCATCCTGCAC-3’;
and antisense 5’-ACAGCTTTCCA GAGGGGCCATC-3’. Quantitative (q) PCR reactions were run on an ABI Prism 7000 thermal cycler at 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95°C for 15 s/60 °C for 30 s. Cycle thresholds (CT) of sample were normalized to the CT of GAPDH for that sample (ΔCT) and then normalized to the average ΔCT of the control samples (ΔΔCT), after which data were expressed as relative levels of mRNA using 2ΔΔCT
4.5 ELISAs
ELISA plates were coated with 1 µg/ml antibodies to mouse IL-6 (MP5–20F3), the plates were blocked with PBS/10% FBS. After washes, cell culture supernatants or serial dilutions of recombinant IL-6 were added to wells. After 2 h, 1 µg/ml biotinylated antibody to mouse IL-6 (MP5–32C11) was added to each well. After 1 h, streptavidin horseradish peroxidase (1:1000) was added for 30 min and then Tetramethylbenzindine substrate/hydrogen peroxide solution was added to each well. All ELISA reagents were purchased from BD-Pharmingen. IL-6 was measured by determining optical densities at OD 450 nm wavelength with reference OD 570 nm using an ELISA spectrophotometric plate reader.
4.6 Clinical evaluation
Four days after i. c. infection with the DA strain of TMEV, brains from individual mice were extracted, inverted, and a region cut from the midbrain to the basal forebrain was placed into 4% formalin, embedded in paraffin, sectioned, rehydrated and stained with hematoxylin and eosin. Evaluation of acute encephalitis following i. c. infection with the TMEV GDVII strain began on day 1 p. i. with determination of percentage weight loss for individual mice and evaluation of percent survivors for each mouse strain(Reddi et al., 2004).
4.7 Statistical analyses
Statistical analyses were performed using GraphPad Prism Software. Student’s two-tailed unpaired t test was used to determine the significance of differences between means; p < 0.05 was considered significant. For clinical evaluation Anova was used to determine the significance of main effects; p < 0.05 was considered significant.
Highlights.
IRF3 is essential to control acute TMEV infection
IL-6 expression in response to TMEV infection depends on IRF3
IRF3 contributes to TMEV induced hippocampal damage
STAT1 activation in response to IL-6 requires IRF3
Acknowledgements
The authors wish to thank Marian Schmid for her excellent animal care and animal technical services. This work was supported by funding from the University of Nebraska Medical Center College of Dentistry and University of Nebraska Lincoln, School of Biological Sciences, and supported by Award Number P30GM10350903 and P20GM103489 from the National Institute of General Medicine, a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- IFN
interferon
- IRF
interferon response factor
- TMEV
Theiler’s murine encephalomyelitis virus
- i. c.
intracranial
- i. p.
intraperitoneal
- KO
knockout
- ISGs
interferon stimulated genes
- B6
C57BL/6
- p. i.
post infection
- CNS
central nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S. IL-6-regulated transcription factors. Int J Biochem & Cell Biol. 1997;29(12):1401–1418. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Al-Salleeh F, Petro TM. TLR3 and TLR7 are involved in expression of IL-23 subunits while TLR3 but not TLR7 is involved in expression of IFN-beta by Theiler's virus-infected RAW264.7 cells. Microbes Infect. 2007;9(11):1384–1392. doi: 10.1016/j.micinf.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Al-Salleeh F, Petro TM. Promoter Analysis Reveals Critical Roles for SMAD-3 and ATF-2 in Expression of IL-23 p19 in Macrophages. J Immunol. 2008;181(7):4523–4533. doi: 10.4049/jimmunol.181.7.4523. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175(2):668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, Williams BRG, Sen GC. Viral apoptosis is induced by IRF-3-mediated activation of Bax. Embo J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating Macrophages Are Key to the Development of Seizures following Virus Infection. J Virol. 2013;87(3):1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A, Auble MR, Petro TM. Reduced expression of IL-12 p35 by SJL/J macrophages responding to Theiler's virus infection is associated with constitutive activation of IRF-3. Virology. 2006;353(2):422–432. doi: 10.1016/j.virol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Ehl S, Bischoff R, Ostler T, Vallbracht S, Schulte-Monting J, Poltorak A, Freudenberg M. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34(4):1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- Howe CL, Lafrance-Corey RG, Sundsbak RS, Sauer BM, Lafrance SJ, Buenz EJ, Schmalstieg WF. Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci Rep. 2012;2:545. doi: 10.1038/srep00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Kim SJ, So EY, Meng L, Colonna M, Kim BS. Melanoma Differentiation-Associated Gene 5 Is Critical for Protection against Theiler's Virus-Induced Demyelinating Disease. J Virol. 2011;86(3):1531–1543. doi: 10.1128/JVI.06457-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu Orkide O, Hogue Ian B, Enquist Lynn W. Virus Infections in the Nervous System. Cell Host & Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, Produced by Resident Cells of the Central Nervous System and Infiltrating Cells, Contributes to the Development of Seizures following Viral Infection. J Virol. 2011;85(14):6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49(6):1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Kratochvil J, Sethi P, Dal Canto MC. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology. 1984;34(8):1117–1119. doi: 10.1212/wnl.34.8.1117. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Kumar AS, Trottier M. Theiler's virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 2005;111(2):214–223. doi: 10.1016/j.virusres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Marijanovic Z, Ragimbeau J, van der Heyden J, Uze G, Pellegrini S. Comparable potency of IFNalpha2 and IFNbeta on immediate JAK/STAT activation but differential down-regulation of IFNAR2. Biochem J. 2007;407(1):141–151. doi: 10.1042/BJ20070605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Monteyne P, Bihl F, Levillayer F, Brahic M, Bureau JF. The Th1/Th2 balance does not account for the difference of susceptibility of mouse strains to Theiler's virus persistent infection. J Immunol. 1999;162(12):7330–7334. [PubMed] [Google Scholar]
- Moore TC, Al-Salleeh FM, Brown DM, Petro TM. IRF3 polymorphisms induce different innate anti-Theiler's virus immune responses in RAW264.7 macrophages. Virology. 2011;418(1):40–48. doi: 10.1016/j.virol.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TC, Bush KL, Cody L, Brown DM, Petro TM. Interleukin-6 control of early Theiler's Murine Encephalomyelitis Virus replication in macrophages occurs in conjunction with STAT1 activation and nitric oxide production. J Virol. 2012;86:10841–10851. doi: 10.1128/JVI.01402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Nishimura K, Nakaima Y, Oh T, Noguchi S, Taniguchi T, Tamura T. Cell type-dependent proapoptotic role of Bcl2L12 revealed by a mutation concomitant with the disruption of the juxtaposed Irf3 gene. Proc Natl Acad Sci U S A. 2009;106(30):12448–12452. doi: 10.1073/pnas.0905702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169(8):4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Petro TM. Disparate expression of IL-12 by SJL/J and B10.S macrophages during Theiler's virus infection is associated with activity of TLR7 and mitogen-activated protein kinases. Microbes Infect. 2005a;7(2):224–232. doi: 10.1016/j.micinf.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Petro TM. ERK-MAP-kinases differentially regulate expression of IL-23 p19 compared with p40 and IFN-beta in Theiler's virus-infected RAW264.7 cells. Immunol Lett. 2005b;97(1):47–53. doi: 10.1016/j.imlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Reddi HV, Kumar ASM, Kung AY, Kallio PD, Schlitt BP, Lipton HL. Heparan Sulfate-Independent Infection Attenuates High-Neurovirulence GDVII Virus-Induced Encephalitis. J Virol. 2004;78(16):8909–8916. doi: 10.1128/JVI.78.16.8909-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13(4):539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Sharif-Askari E, Nakhaei P, Oliere S, Tumilasci V, Hernandez E, Wilkinson P, Lin R, Bell J, Hiscott J. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology. 2007;365(1):20–33. doi: 10.1016/j.virol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, Johnson RW. Interleukin-6 Facilitates Lipopolysaccharide-Induced Disruption in Working Memory and Expression of Other Proinflammatory Cytokines in Hippocampal Neuronal Cell Layers. J. Neurosci. 2006;26(42):10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurbaut S, Rombaut B, Vrijsen R. Persistent infection of RAW264.7 macrophages with the DA strain of Theiler's murine encephalomyelitis virus: An in vitro model to study viral persistence. J Neurovirol. 2006;12(2):108–115. doi: 10.1080/13550280600714120. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining Chronic Viral Infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]