Abstract
The purpose of this study was to determine whether chronic exercise alters sensitivity to the conditioned rewarding effects of cocaine. Female rats were obtained at weaning and randomly assigned to either sedentary or exercise conditions. After 6 weeks under these conditions, the effects of cocaine were examined in the conditioned place preference procedure. Cocaine produced a dose-dependent conditioned place preference in both groups of rats. Exercising rats were more sensitive than sedentary rats to cocaine in this procedure, and this effect was most pronounced at the highest dose of cocaine. These data suggest that chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine.
Keywords: cocaine, conditioned place preference, exercise, rat, reward, running wheel
Introduction
Acute bouts of exercise increase central dopamine concentrations [15], and these effects are positively correlated with exercise output [8]. Furthermore, 6-hydroxydopamine (6-OHDA) lesions markedly reduce exercise output whether given intraventricularly [4] or directly in the ventral tegmental area [11]. These effects can be reversed, however, by systemic administration of the dopamine agonist apomorphine [10]. Chronic exercise leads to sustained increases in dopamine concentrations [2] and compensatory alterations in the density of dopamine D2 receptors [14] and the dopamine transporter [7]. Interestingly, very few studies have examined whether these effects have functional consequences for sensitivity to psychomotor stimulants, particularly those with high abuse and dependence liability. As such, the purpose of the present study was to examine the effects of chronic exercise on sensitivity to cocaine in the conditioned place preference procedure, a procedure that provides a measure of the rewarding effects of drugs with high abuse potential [see 19 for review]. Female rats were chosen for the study because they run significantly more than males when given free access to running wheels [5].
Materials and Methods
Animals
Female Long-Evans rats were obtained at weaning (~21 days) and randomly assigned to either sedentary or exercising conditions immediately upon arrival. Sedentary rats (n = 24) were housed individually in standard polycarbonate cages (interior dimensions: 50 × 28 × 20 cm) that permitted no exercise beyond normal cage ambulation. Exercising rats (n = 22) were housed individually in modified cages of equal dimensions, but with a running wheel (35 cm diameter) affixed to the interior of the cage (Harvard Apparatus, Boson, MA, USA). Wheel revolutions were counted continuously by magnetic switches and recorded weekly. Sedentary and exercising rats were housed in their respective conditions for the duration of the study, which lasted approximately 8 weeks. Throughout the study, subjects were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Davidson College. In all procedures, cocaine HCl (generously provided by the National Institute on Drug Abuse) was dissolved in sterile saline and administered intraperitoneally in a volume of 1.0 ml/kg body weight. In order to preserve the ecological validity of the study, no attempt was made to control or manipulate the estrous cycle.
Conditioned Place Preference
Conditioned place preference was assessed with a three-compartment place preference chamber obtained from Med Associates, Inc (St Albans, VT, USA). The chamber consisted of two choice compartments (25 × 20 × 20 cm) separated by a smaller center compartment (13 × 20 × 20 cm). One choice compartment was painted black and had a steel-rod floor covering corncob bedding. The other choice compartment was painted white and had a wire-screen floor covering pine bedding. The center compartment was painted a neutral gray and had a solid PVC floor with no underlying bedding. Each choice compartment was separated from the center compartment by a manually operated guillotine door. Behavior was monitored by a video camera mounted 1.5 m above the chamber.
Six weeks after arrival and one day prior to the first conditioning trial, each rat was given 15 min to habituate to the conditioning chamber. During this habituation session, rats were placed in the center (neutral) compartment and given free access to the entire chamber by opening the guillotine doors separating the two choice compartments from the center compartment. The amount of time spent in each of the three compartments was recorded and summated over the entire 15-min session.
Over the next eight consecutive days, rats received daily conditioning trials in which they were injected with either a selected dose of cocaine or saline and placed into one of the two choice compartments for 30 min. Both guillotine doors were closed during these conditioning trials, and rats were confined to the appropriate compartment for the duration of the trial. Drug and saline administration alternated daily such that each rat received four conditioning trials with both the drug-paired and saline-paired compartments. The drug- and saline-paired compartments were assigned randomly, such that the black compartment served as the drug-paired compartment for half of the subjects, and the white compartment served as the drug-paired compartment for the other half of the subjects. To determine whether a compartmental bias would develop in the absence of drug administration, a subset of rats were conditioned with saline in both compartments. Conditioning trials were conducted with 5.0 mg/kg cocaine, 10 mg/kg cocaine, and saline using separate groups of rats (n = 6 to 8 rats per group).
On the day immediately following the last conditioning trial, place preference was assessed in each rat. During this test session, rats were placed in the center compartment under drug-free conditions and both guillotine doors were opened. Rats had free access to the entire chamber for 15 min, and the amount of time spent in each compartment was recorded.
Data Analysis
Difference scores were obtained for each rat by subtracting the amount of time spent in the drug-paired compartment before conditioning (i.e., during the free-access habituation session) from the amount of time spent in the drug-paired compartment after conditioning (i.e., during the free-access place preference test). These scores were then analyzed via two-way ANOVA using the factors of group and dose. Post-hoc tests were conducted using the Bonferroni adjustment for multiple pairwise comparisons.
Results
Exercising rats ran an average of 8565 rev/day (9418 m/day), with a range across rats from 4642 rev/day (5104 m/day) to 12,532 rev/day (13,779 m/day). Running rates increased steadily during the first 4 weeks of exposure to the running wheels before leveling out until behavioral testing commenced. Running rates declined slightly immediately after the initiation of testing, and remained stable thereafter (data not shown).
Cocaine produced a dose-dependent conditioned place preference in both groups of rats (Figure 1). These effects were greater in exercising rats than sedentary rats, and this was most pronounced at the high dose of cocaine (10 mg/kg). Consistent with these observations, a two-way ANOVA revealed significant main effects for dose (F [2, 45] = 3.393; p = 0.044) and group (F [1, 45] = 4.248; p = 0.046). No significant dose x group interaction was observed. Post-hoc tests using the Bonferroni adjustment for multiple pairwise comparisons revealed a significant difference between sedentary and exercising rats in the high dose condition (p = 0.018), but not in the other dose conditions.
Figure 1.
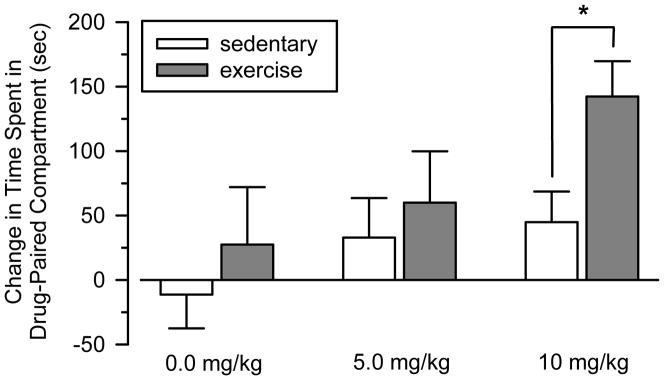
Effects of cocaine and saline in the conditioned place preference procedure. Data reflect time (sec) spent in drug-paired compartment after conditioning minus time (sec) spent in drug-paired compartment before conditioning. Asterisk (*) indicates significant difference between sedentary and exercising rats.
Discussion
The principal finding of this study is that long-term, voluntary exercise increases sensitivity to the rewarding effects of cocaine as measured by the conditioned place preference procedure. This procedure is sensitive to both the positive and negative interoceptive states produced by a drug and provides a measure of reward that reflects the net contribution of these individual states. For instance, cocaine produces both appetitive (e.g., euphorigenic) and aversive (e.g., anxiogenic) effects, and it is the summation of these effects that is responsible for its ability to establish a conditioned place preference. Either an increase in the appetitive effects of cocaine or a decrease in its aversive effects could have contributed to the greater place preference scores seen in the exercising group. Although it was not the aim of this study to determine the mechanism by which exercise alters these effects, several possibilities deserve consideration.
It is possible that the pharmacokinetics of cocaine differ between sedentary and exercising subjects. Indeed, exercising rats have lower body weights, less adipose tissue, and smaller livers than sedentary control rats [17], any of which could lead to differences in the absorption, distribution, and metabolism of cocaine. Consistent with this possibility, Han et al. [9] reported that peak concentrations of an intravenous infusion of cocaine were 69% greater in a group of forced-exercise rats than in a group of rested control rats. In the present study, differences in peak cocaine concentrations during conditioning could account for the differences in sensitivity observed between sedentary and exercising rats in the place preference test.
Alternatively, it is possible that exercise produces pharmacodynamic changes in those neuronal pathways that mediate cocaine’s rewarding effects. It is well established that cocaine increases dopamine transmission in mesolimbic and mesocortical pathways by preventing the reuptake of dopamine by the presynaptic neuron [12]. Similarly, acute bouts of exercise increase central dopamine concentrations [15], and chronic exercise leads to compensatory alterations in the density of dopamine binding proteins [7, 14]. Exercise also increases the expression of the transcription factor ΔFosB in the nucleus accumbens [20]. Similar increases in ΔFosB are also observed after chronic cocaine administration and are believed to contribute to the development of sensitization to cocaine’s behavioral effects [16]. Thus, it is possible that chronic exercise leads to functional changes in mesolimbic and mesocortical neurons that leave an organism more sensitive to the effects of cocaine on measures of conditioned reward.
Finally, exercise may facilitate the acquisition of Pavlovian associations that are critically involved in place conditioning procedures. Indeed, exercise enhances performance in a variety of learning and memory tasks, particularly those that involve contextual conditioning [1]. Recently, Eisenstein and Holmes [6] reported that chronic, voluntary exercise increased the expression of brain-derived neurotrophic factor in the hippocampus and enhanced the effects of morphine in the conditioned place preference procedure. Similarly, we reported that exercise facilitated the acquisition of a conditioned place aversion to the kappa opioid spiradoline [18]. When considered together with the present findings, these studies suggest that exercise may facilitate the acquisition of Pavlovian associations between interoceptive drug cues and an environmental context in place conditioning procedures, leading to a greater magnitude of conditioned reward in exercising subjects.
Although these data reveal that exercise increases sensitivity to the conditioned rewarding effects of cocaine, it is important to note that these findings do not necessarily imply that chronic exercise leads to a greater susceptibility to drug abuse and dependence. There are numerous biological, pharmacological, and environmental determinants that influence an individual’s propensity to engage in drug-seeking behavior. Although sensitivity to the conditioned rewarding effects of additive substances is one such factor, many other factors are also involved, and it is likely that exercise interacts with a number of these factors to influence the likelihood an individual will use an addictive substance. For instance, drug self-administration studies suggest that exercise may have protective effects on drug-seeking behavior, as concurrent access to a running wheel decreases intravenous cocaine self-administration [3] and reduces the oral consumption of amphetamine [13]. At the present time, it is not known whether long-term, voluntary exercise has protective effects on drug self-administration when a running wheel is not concurrently available. Our laboratory is currently examining this possibility in groups of sedentary and exercising subjects trained to self-administer cocaine under positive reinforcement contingencies.
Acknowledgments
This study was supported by US Public Service Grant DA14255 (to M.A.S). Additional support was provided by the National Science Foundation, the Howard Hughes Medical Institute, the Duke Endowment, and Davidson College. All drugs used in this study were generously supplied by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA). The authors wish to thank Amy Becton for expert animal care and technical assistance. Portions of these data were presented at the 68th annual meeting of the College on Problems of Drug Dependence in Scottsdale, Arizona, USA.
References
- 1.Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- 2.Bauer BA, Rogers PJ, Miller TD, Bove AA, Tyce GM. Exercise training produces changes in free and conjugated catecholamines. Med Sci Sports Exerc. 1989;21:558–562. [PubMed] [Google Scholar]
- 3.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 4.Derevenco P, Stoica N, Sovrea I, Imreh S. Central and peripheral effects of 6-hydroxydopamine on exercise performance in rats. Psychoneuroendocrinology. 1986;11:141–153. doi: 10.1016/0306-4530(86)90049-1. [DOI] [PubMed] [Google Scholar]
- 5.Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 8.Freed CR, Yamamoto BK. Regional brain dopamine metabolism: a marker for the speed, direction, and posture of moving animals. Science. 1985;229:62–65. doi: 10.1126/science.4012312. [DOI] [PubMed] [Google Scholar]
- 9.Han DH, Kelly KP, Fellingham GW, Conlee RK. Cocaine and exercise: temporal changes in plasma levels of catecholamines, lactate, glucose, and cocaine. Am J Physiol. 1996;270:E438–E444. doi: 10.1152/ajpendo.1996.270.3.E438. [DOI] [PubMed] [Google Scholar]
- 10.Heyes MP, Garnett ES, Coates G. Central dopaminergic activity influences rats ability to exercise. Life Sci. 1985;36:671–677. doi: 10.1016/0024-3205(85)90172-9. [DOI] [PubMed] [Google Scholar]
- 11.Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001;899:187–192. doi: 10.1016/s0006-8993(01)02223-5. [DOI] [PubMed] [Google Scholar]
- 12.Julien RM. A primer of drug action. Worth Publishers; New York: 2004. [Google Scholar]
- 13.Kanarek RB, Marks-Kaufman R, D’Anci KE, Przypek J. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav. 1995;51:725–729. doi: 10.1016/0091-3057(95)00022-o. [DOI] [PubMed] [Google Scholar]
- 14.MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92:236–240. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- 15.Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol Scand. 1997;159:335–341. doi: 10.1046/j.1365-201X.1997.00118.x. [DOI] [PubMed] [Google Scholar]
- 16.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitts GC, Bull LS. Exercise, dietary obesity, and growth in the rat. Am J Physiol. 1977;232:R38–R44. doi: 10.1152/ajpregu.1977.232.1.R38. [DOI] [PubMed] [Google Scholar]
- 18.Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav. 2004;77:49–57. doi: 10.1016/j.pbb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 20.Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


