Abstract
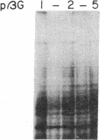
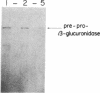
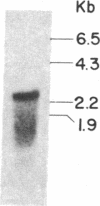
We have selected the rat preputial gland beta-glucuronidase as a model protein to study the sorting of newly synthesized lysosomal hydrolases to the lysosome. The complete coding sequence of beta-glucuronidase messenger RNA was determined from the sequences of a group of overlapping cDNA clones isolated from preputial gland cDNA libraries. The beta-glucuronidase mRNA primary translation product contains 648 amino acids, including an amino-terminal signal sequence of 22 residues. The polypeptide has four potential sites for the addition of asparagine-linked core oligosaccharides. A 376-residue segment of beta-glucuronidase shows extensive homology (23% sequence identity) to a portion of Escherichia coli beta-galactosidase. This homology most likely reflects an evolutionary relationship between the bacterial and eukaryotic enzymes and the conservation of structural features necessary for the glycosidase activity of both proteins. Translation of mRNA synthesized in vitro by transcription of a cDNA containing the entire beta-glucuronidase coding region yielded a polypeptide that was immunoprecipitated with anti-beta-glucuronidase antiserum and had the same electrophoretic mobility as the primary translation product of natural beta-glucuronidase mRNA. In the presence of microsomal membranes, the in vitro-synthesized beta-glucuronidase underwent cotranslational incorporation into the microsomes, as indicated by removal of the signal sequence and the addition of several oligosaccharide chains. The beta-glucuronidase cDNA will provide a useful tool to study the mechanism of mannose phosphorylation and other aspects of the sorting of lysosomal enzymes to lysosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach G., Bargal R., Cantz M. I-cell disease: deficiency of extracellular hydrolase phosphorylation. Biochem Biophys Res Commun. 1979 Dec 14;91(3):976–981. doi: 10.1016/0006-291x(79)91975-2. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., Leary S. L. Detection of early changes in androgen-induced mouse renal beta-glucuronidase messenger ribonucleic acid using cloned complementary deoxyribonucleic acid. Biochemistry. 1983 Dec 20;22(26):6049–6053. doi: 10.1021/bi00295a001. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochemistry. 1983 Oct 25;22(22):5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I., Sinnott M. L., Zabin I. Methionine 500, the site of covalent attachment of an active site-directed reagent of beta-galactosidase. J Biol Chem. 1978 Aug 10;253(15):5283–5285. [PubMed] [Google Scholar]
- Fukushima H., de Wet J. R., O'Brien J. S. Molecular cloning of a cDNA for human alpha-L-fucosidase. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1262–1265. doi: 10.1073/pnas.82.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Kornfeld S. The phosphorylation of beta-glucuronidase oligosaccharides in mouse P388D1 cells. J Biol Chem. 1981 Dec 25;256(24):13060–13067. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guise K. S., Korneluk R. G., Waye J., Lamhonwah A. M., Quan F., Palmer R., Ganschow R. E., Sly W. S., Gravel R. A. Isolation and expression in Escherichia coli of a cDNA clone encoding human beta-glucuronidase. Gene. 1985;34(1):105–110. doi: 10.1016/0378-1119(85)90300-2. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Voss B., Von Figura K. Transport and processing of lysosomal enzymes by smooth muscle cells and endothelial cells. Exp Cell Res. 1981 May;133(1):23–30. doi: 10.1016/0014-4827(81)90352-9. [DOI] [PubMed] [Google Scholar]
- Herrchen M., Legler G. Identification of an essential carboxylate group at the active site of lacZ beta-galactosidase from Escherichia coli. Eur J Biochem. 1984 Feb 1;138(3):527–531. doi: 10.1111/j.1432-1033.1984.tb07947.x. [DOI] [PubMed] [Google Scholar]
- Hieber V. C. Cloning of a cDNA complementary to rat preputial gland beta-glucuronidase mRNA. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1271–1278. doi: 10.1016/0006-291x(82)91387-0. [DOI] [PubMed] [Google Scholar]
- Himeno M., Ohara H., Arakawa Y. Beta-glucuronidase of rat preputial gland. Crystallization, properties, carbohydrate composition, and subunits. J Biochem. 1975 Feb;77(2):427–438. doi: 10.1093/oxfordjournals.jbchem.a130742. [DOI] [PubMed] [Google Scholar]
- Huber R. E., Fowler A. V., Zabin I. Inactivation of beta-Galactosidase by iodination of tyrosine-253. Biochemistry. 1982 Sep 28;21(20):5052–5055. doi: 10.1021/bi00263a031. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., McALLAN A., MARSH C. A. Purification of beta-glucuronidase from the preputial gland of the female rat. Biochem J. 1958 May;69(1):22–27. doi: 10.1042/bj0690022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton P. H., Fisher W. K., Moon K. E., Thompson E. O. Amino acid sequences containing cysteine or half-cystine residues in beta-glucuronidase. Aust J Biol Sci. 1980 Oct;33(5):513–520. doi: 10.1071/bi9800513. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Piekarz R., Neufeld E. F., Shows T. B., Suzuki K. Human beta-hexosaminidase alpha chain: coding sequence and homology with the beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7830–7834. doi: 10.1073/pnas.82.23.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Segundo B., Chan S. J., Steiner D. F. Identification of cDNA clones encoding a precursor of rat liver cathepsin B. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2320–2324. doi: 10.1073/pnas.82.8.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewale J. G., Tang J. Amino acid sequence of porcine spleen cathepsin D. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3703–3707. doi: 10.1073/pnas.81.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Takahashi T., Tang J. Amino acid sequence of porcine spleen cathepsin D light chain. J Biol Chem. 1983 May 25;258(10):6435–6443. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominco S., Paigen K. Egasyn, a protein complexed with microsomal beta-glucuronidase. J Biol Chem. 1975 Feb 10;250(3):1146–1148. [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Winfield S., Barranger J. A., Ginns E. I. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986 Jan 5;261(1):50–53. [PubMed] [Google Scholar]
- Varki A., Reitman M. L., Vannier A., Kornfeld S., Grubb J. H., Sly W. S. Demonstration of the heterozygous state for I-cell disease and pseudo-Hurler polydystrophy by assay of N-acetylglucosaminylphosphotransferase in white blood cells and fibroblasts. Am J Hum Genet. 1982 Sep;34(5):717–729. [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Wiesmann U. N., Lightbody J., Vassella F., Herschkowitz N. N. Multiple lysosomal deficiency due to enzyme leakage? N Engl J Med. 1971 Jan 14;284(2):109–110. doi: 10.1056/NEJM197101142840221. [DOI] [PubMed] [Google Scholar]