Abstract
OBJECTIVES
Paraplegia is a rare but devastating complication, which may follow thoracoabdominal aortic surgery. Many adjuncts have been developed to reduce this risk including cerebrospinal fluid (CSF) drainage. Acetazolamide (carbonic anhydrase inhibitor) is a drug used to counteract mountain sickness and one of its effects is to reduce CSF production. Here, we report its first postoperative application in thoracoabdominal surgery with the aim of reducing cerebrospinal cord perfusion pressure and reducing risk of paraplegia.
METHODS
We retrospectively reviewed 6 patients who have been treated with this drug between 2011 and 2012 who were undergoing thoracoabdominal aortic surgery. Our indications were decided to include: (i) patients in whom a spinal drain could not be positioned; (ii) patients with blood-stained CSF; (iii) patients in whom the volume of CSF drained was outside guidelines; (iv) patients in whom CSF pressure was elevated; (v) patients with excessive vasopressor usage and (vi) patients with postoperative neurological dysfunction as measured by motor-evoked potentials or clinical examination. All were given 500 mg intravenous acetazolamide, not more than eight hourly, for a duration dependent on response.
RESULTS
In the 6 patients, 2 received a single dose of the drug and responded by an immediate drop in intracranial pressure (ICP) pressure. Of the 4 who received multiple doses of the drug, 1 had an immediate decline in ICP after each of the first six doses, while 3 had no discernable response.
CONCLUSIONS
This is the first report of the efficacy of acetazolamide in reducing CSF production and lowering ICP during thoracoabdominal aortic surgery. We believe that its use will be beneficial in the 6 patient groups described. Our experience suggests there are ‘responders’ and ‘non-responders’, the characteristics of whom are yet to be defined. Its efficacy in reducing not just CSF volume and ICP but also clinically relevant morbidity such as paraplegia, is the subject of a planned randomized controlled trial. This report serves to raise awareness of the possible efficacy of this drug when normal management strategies are limited or exhausted.
Keywords: Thoracoabdominal surgery, Paraplegia, Acetazolamide
INTRODUCTION
Open repair of thoracoabdominal aortic aneurysms carries significant risk of mortality and morbidity including that of paraplegia. Despite a host of adjuncts to reduce risks of paraplegia including: (i) deep hypothermic circulatory arrest (DHCA), (ii) left heart bypass, (iii) sequential clamping, (iv) intercostal artery reimplantation, (v) motor-evoked potential (MEP) monitoring and (vi) cerebrospinal fluid (CSF) drainage, the risk remains even in high-volume centres [1]. CSF drainage has become a standard of care in most centres and is an integral part of the so-called COPS protocol [C (CSF drainage status), O (Oxygen deliver), P (Patient), S (Status)] developed by Professor Safi in Houston, essentially a bundle of measures to try and reverse postoperative neurological dysfunction [2]. On occasions, CSF drainage per hour exceeds recommended levels or the CSF drain is not usable, presenting the clinician with a difficult management dilemma. An alternative approach has been developed by the team at our institution using a drug called acetazolamide. Acetazolamide is a carbonic anhydrase inhibitor used to treat a number of diseases including: acute mountain sickness [3], epilepsy [4] and idiopathic intracranial hypertension [5]. It has also been used in children to reduce CSF shunt flows in hydrocephalus [6]. Acetazolamide has a number of putative mechanisms of action in these scenarios; however, one of its more interesting mechanisms is that of reducing CSF production [6]. Our perceived indications for this drug were:
A CSF drain cannot be inserted for technical reasons.
The CSF is blood-stained precluding use of the drain.
Drainage per hour is outside the protocol guidelines (>20 ml/h).
Intracranial pressure (ICP) remains high despite drainage (>20 mmHg).
Vasopressor use is excessive when attempts are being made to maintain cerebral perfusion pressure (CPP).
There is evidence of neurological impairment (clinical, biochemical or attenuated MEPs).
Our objective in this manuscript is to report our early experience with the administration of acetazolamide in 6 consecutive patients who underwent thoracoabdominal aortic surgery and had one or more of the six putative indications listed.
METHODS
Operative protocol
Patient preparation
Our patients typically have arterial monitoring, central venous access and urinary catheter. Bladder and nasopharyngeal temperature is recorded. Cerebral saturations are recorded [INVOS Somanetics® Corporation, Troy, USA] as well as bispectral index (Coviden, Mansfield, USA). A double lumen tube is used allowing single-lung ventilation. A CSF drain is inserted at L2–4 and pressure kept below 15 mmHg. Where ICP remains high, CPP is maintained by driving up mean arterial pressure (MAP) with vasopressors (CPP = MAP − ICP). MEP (Medtronic NIM-ECLIPSE system, Minneapolis, USA) monitoring are employed through cases and on intensive care postoperatively.
Conduct
A thoracoabdominal incision is performed entering the thorax through the fifth/sixth space. A retroperitoneal dissection is performed. Cell salvage and rapid infusers (Belmont Instrument Corporation, Billerica, USA Rapid Infuser) are employed. Cases are in general performed either with arteriovenous cardiopulmonary bypass and DHCA (20°C) with full heparinization or with left heart bypass using pulmonary vein and femoral artery or distal aorta as cannulation sites (34°C) with partial heparinization (ACT 250–300). Generally, sequential clamping is employed where possible and intercostal arteries are reimplanted as guided by MEP signals. Where appropriate visceral perfusion is maintained with cold blood supplementation.
Postoperative protocol
Patients are transferred to intensive care postoperatively. MEP and CSF drains continue to be monitored and acted upon for 3 days. We follow an institutional protocol [7] with MAP kept above 90 mmHg and ICP kept below 15 mmHg. Vasopressors are used liberally and where necessary guided by pulmonary artery flotation catheters and MEP readings. Any sign of neurological impairment mandates immediate senior review and implementation of the COPS protocol [2] and imaging as appropriate.
Acetazolamide treatment
Indications
Our indications for use of acetazolamide are:
A CSF drain cannot be inserted for technical reasons.
The CSF is blood-stained precluding use of the drain.
Drainage per hour is outside the protocol guidelines (>20 ml/h).
ICP remains high despite drainage (>20 mmHg).
Excessive vasopressor use while attempting to maintain CPP.
There is evidence of neurological impairment (clinical, biochemical or MEP) despite adhering to the protocol.
As far as our practice is concerned, there are no exclusion criteria.
Dosaging
Patients were administered 250–500 mg acetazolamide intravenously at intervals of not more than eight hourly and for a duration dependent on response.
Definition of a response
Interpretation of a positive response is complicated by the huge number of interacting variables. We chose the definition of a response to include:
A temporally-related drop in ICP after a single dose.
A trending drop in ICP after multiple doses.
A decrease in the volume of CSF required draining to keep within the protocol.
A decrease in the frequency of CSF drainage to keep within the protocol.
A decrease in the requirement for vasopressors.
Institutional approval
The drug was used off licence in this setting and indication. This activity was registered with the appropriate institutional regulatory committee.
RESULTS
Demographics
The patients were treated between November 2011 and May 2012. Their demographics are described in Table 1.
Table 1:
Demographics, risk factors, aneurysm cause, extent of aneurysm, bypass techniques and intra/postoperative management for the 6 patients
| Age range | 42–73 |
| Male sex | 3 |
| Elective | 4 |
| Urgent/emergency | 2 |
| Risk factors | |
| History of hypertension | 3 |
| Coronary artery disease | 0 |
| Current smoking | 1 |
| Diabetes | 0 |
| Aetiology | |
| Chronic dissection | 5 |
| Marfan's syndrome | 1 |
| Extent of aortic replacement | |
| Crawford II | 6 |
| Bypass technique | |
| Deep hypothermic circulatory arrest | 3 |
| Left heart bypass (distal perfusion) | 3 |
| Intra- and postoperative management | |
| Cerebrospinal fluid drainage | 6 |
| Motor-evoked potential monitoring | 5 |
Cases
There were patients who clearly had a dramatic response to acetazolamide (‘Responders’) and those who seemingly were resistant (‘Non-responders’).
Clear responders
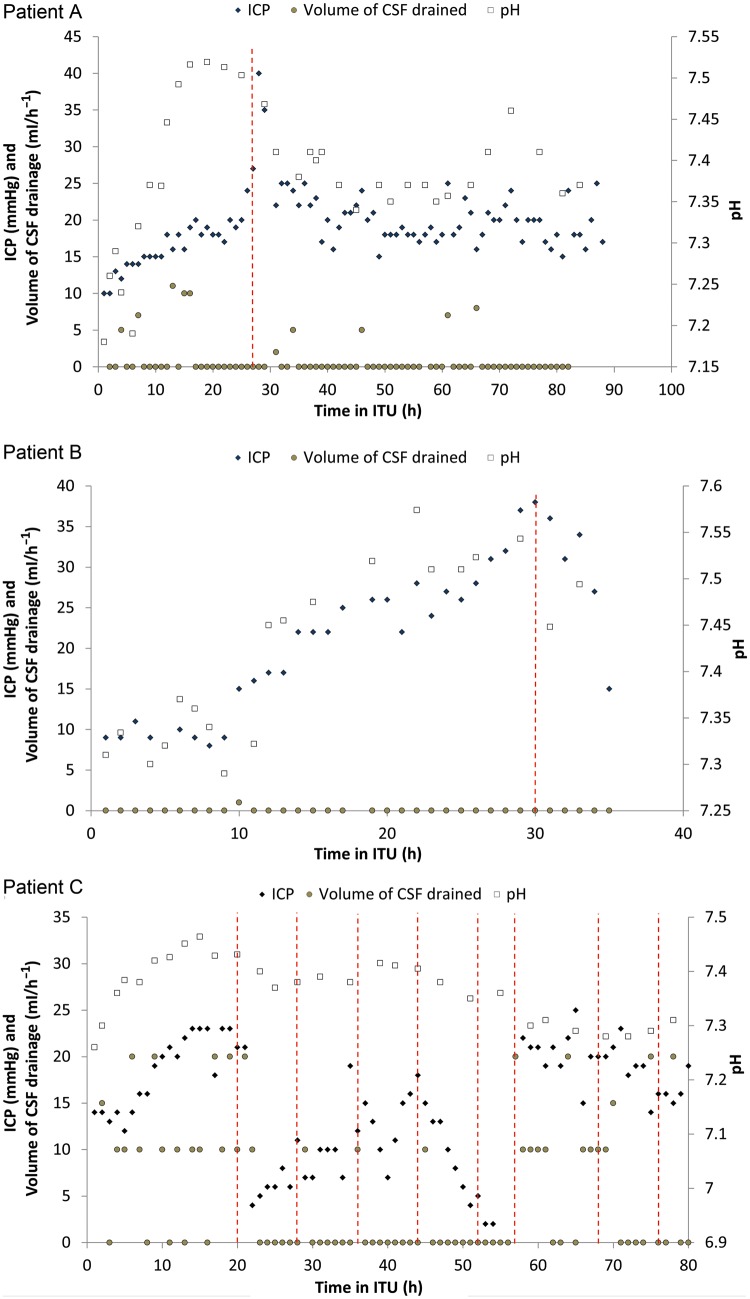
Of the 6 patients, 2 received a single dose of the drug and responded by an immediate drop in ICP. This was accompanied by a similar decrease in pH (Patients A and B, Fig. 1). Patient A had a fairly sudden rise in ICP with red tinging of CSF. Following our protocol further drainage was limited due to concerns about a subarachnoid haemorrhage. CT (Computer Tomography) of the head and spine were normal and acetazolamide was given with dramatic effect. Patient B had a slow rise in ICP but due to a blood-stained CSF fluid was not drained. CT of the head was again unremarkable and acetazolamide was given with good effect. There was no evidence of neurological impairment in either patient at any point.
Figure 1:
Dose-response curve for Patients A–C following treatment with acetazolamide.
Of the 4 who received multiple doses of the drug, 1 responded to a variable extent repeatedly with each dose up to the sixth (Patient C, Fig. 1). The CSF was drained frequently during the first few hours as the ICP rose. Treatment with acetazolamide was then instituted with dramatic effect. Following doses had a similar although not as dramatic an effect. There was a noticeable drop in frequency of drainage though. Subsequent to the sixth dose the patient became resistant despite dosing and draining. This patient subsequently died of multiorgan failure. This patient had no neurological impairment.
Non-responders
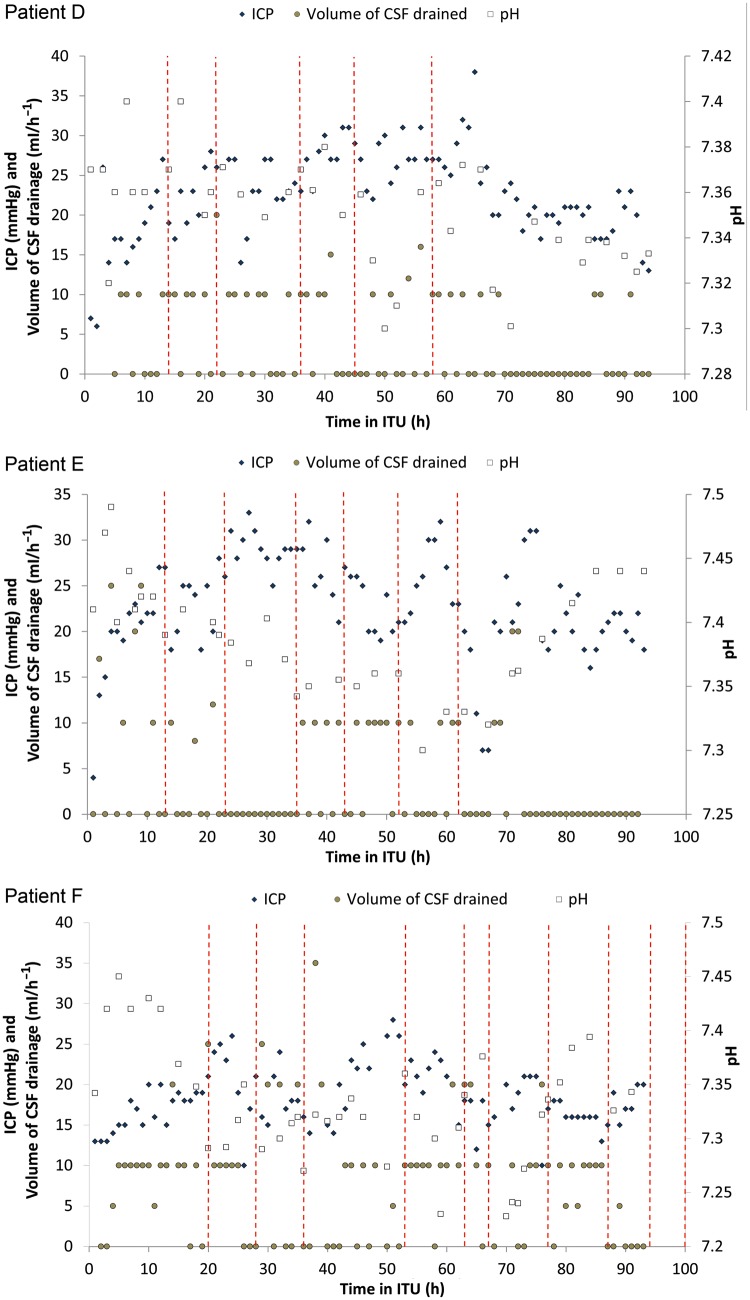
There are non-responders (Patients D, E and F (Fig. 2)). Indication for acetazolamide was high ICP despite drainage in all cases. The effect was variable despite multiple dosing. None of these patients suffered neurological deficit.
Figure 2:
Dose-response curve for Patients D–F following treatment with acetazolamide.
DISCUSSION
This is the first report demonstrating the efficacy of intravenous acetazolamide in reducing CSF production in thoracoabdominal aortic aneurysm patients undergoing surgical repair. In all our cases, the drug has been used as a ‘treatment’ when normal management strategies have been limited or exhausted. The effect appears to be variable with ‘responders’ and ‘non-responders’, the reason for which remains to be defined. In addition, there remains a number of questions regarding potential unwanted side-effects, in particular acidosis. In addition, the potential role of the drug given orally as a prophylactic agent has yet to be explored. The potential, however, for a pharmacological intervention to reduce risks of paraplegia during thoracoabdominal aortic aneurysm surgery is tantalizing.
Mechanism of action of acetazolamide and clinical applications
Acetazolamide inhibits the enzyme known as carbonic anhydrase. The mechanism of action of acetazolamide is comprehensively covered in a review by Leaf and Goldfarb [8]. Within the kidney the drug shifts the equilibrium (CO2 + H2O <-> H2CO3 <-> H+ + HCO3−) to the right. HCO3− is excreted in the urine and H+ and Cl− are absorbed causing systemic hyperchloraemic metabolic acidosis with hypokalaemia. This effect, and a number of other putative actions, has a number of consequences resulting in other uses in acute mountain sickness [3], epilepsy [4], idiopathic intracranial hypertension [5] and hydrocephalis [6].
Documented clinical applications for reducing CSF
The documentation of the drug effect in reducing CSF production in hydrocephalus and mountain sickness is of most interest to this work. Carrion et al. [6] published an article in which two children with hydrocephalus were treated with acetazolamide. Both children had suffered shunt failures and had externalized ventriculostomy drains as a temporary measure prior to formal ventriculopleural shunts. Following treatment with acetazolamide clinicians noted dramatic reductions in shunt CSF output of 48% with a dose of 75 mg/kg/day.
A more common application of acetazolamide with the therapeutic aim of reducing CSF production is in its use in acute mountain sickness. Its effects in this scenario are thought to be multiple [8] including (i) a metabolic acidosis which stimulates respiration and improves oxygenation, (ii) diuresis and (iii) reducing CSF production. The drug is thought to act by altering blood flow to the choroid plexus in this later context.
Current practice for maintaining CPP during aortic surgery
Currently, a CSF drain is inserted in every patient undergoing aortic intervention on the descending portion unless there is a good indication not to. ICP is monitored as well as MAP and from this the CPP is calculated. In order to maximize the cord perfusion pressure clinicians aim to keep ICP below 15 mmHg through drainage and MAP >90 mmHg through the use of vasopressors as required. Should there be signs of neurological impairment, either through MEP measurement or through clinical observation, the so-called COPS protocol is instituted [2, 7]. This represents a set of measures to attempt to optimize cord perfusion by further improving blood supply and oxygen delivery. On occasion, despite frequent CSF drainage, it has been impossible to reduce ICP to a lower value, i.e. <10 mmHg. This has led to excessive vasopressor use to maintain CPP. In addition, scenarios have been encountered where the drain could no longer be used such as evidence of blood staining or blockage. Up until the use of acetazolamide, there has been little else that could be done in this scenario. Acetazolamide has given clinicians in this hospital a further measure to attempt to reduce ICP and maintain CPP.
Observed clinical responses with acetazolamide in aortic surgery
We have observed a mixed clinical response to acetazolamide in our 6 patients. Full interpretation is confounded by the vast number of variables in our patients who are acutely unwell following major thoracoabdominal aortic surgery. There are some patients (Patients A and B) who are clear responders (Methods) after a single dose, while a third patient (C) had responses following multiple doses. There was a predictable change in the pH following administration with a relative acidosis observed (Patients A–C, Fig. 1). The drug allowed us to stay within protocol limits in these three patients, but we can draw no conclusions on other endpoints as none of these patients had overt neurological issues.
Three other patients had an equivocal or no discernable response (Patients D–F, Fig. 2). At first sight it seems difficult to explain this apart from these patients are to a variable extent in a critically ill state with vast systemic inflammatory responses requiring different levels of organ support, i.e. at dissimilar starting points. However, it is interesting to note the variable responses documented in its use in heart failure. The approach in heart failure has been to ‘look and see’ what effect the first dose elicits with subsequent doses omitted or staggered [9]. Recommendations are that when used as a diuretic it should be given on alternate days to allow a ‘day of rest’. Indeed, failures of therapy may be due to overdosage or over frequency [10]. Thus, complex pharmacokinetics and pharmacodynamics superimposed on a complex and changing clinical picture may account for the variable response in our patient group. We have yet to define these aspects of the treatment in detail.
Potential adverse consequences
There are a number of potential adverse consequences very well documented from its use in other clinical scenarios centring on metabolic acidosis, hypokalaemia and hypocabnia [9, 10]. Our patients are administered this drug in an intensive care setting with at least hourly assessments of haemodynamic and respiratory function, including arterial blood gas analysis. Some patients are ventilated and some are on haemoflitration. Needless to say these consequences are easily corrected when encountered. A theoretical consequence of this drug is that lowering ICP excessively causes tearing of vessels in the subarachnoid space. Our experience is that the drug does not have such a dramatic effect and at best lowers ICP into a physiological range.
Future work
The work to date has focused on using this drug as a treatment when certain conditions are encountered. The drug may also be given orally and there is a potential application of this drug preoperatively given prophylactically. At present, we are engaged in setting up a randomized controlled trial to test for efficacy as a prophylactic agent.
CONCLUSION
This is the first report of the use of acetazolamide to reduce production of CSF in thoracoabdominal surgery. Our initial observations are that the drug is efficacious in certain patients only. We have yet to determine the pharmacokinetics and pharmacodynamics in this setting.
Conflict of interest: none declared.
REFERENCES
- 1.Coselli J, LeMaire S, Conklin L, Koksoy C, Schittling Z. Morbidity and mortality after Extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2002;73:1107–16. doi: 10.1016/s0003-4975(02)03370-2. [DOI] [PubMed] [Google Scholar]
- 2.Estrera AL, Sheinbaum R, Miller C, Azizzadeh A, Walkes J-C, Lee T-Y, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg. 2009;88:9–15. doi: 10.1016/j.athoracsur.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102:1313–22. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- 4.Reiss WG, Oles KS. Acetazolamide in the treatment of seizures. Ann Pharmacother. 1996;30:514–9. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 5.Lee AG, Pless M, Falardeau J, Capozzoli T, Wall M, Kardon RH. The use of acetazolamide in idiopathic intracranial hypertension during pregnancy. Am J Opthalmol. 2005;139:855–9. doi: 10.1016/j.ajo.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 6.Carrion E, Hertzog JH, Medlock MD, Hauser GJ, Dalton HJ. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child. 2001;68:68–71. doi: 10.1136/adc.84.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field ML, Doolan J, Safar M, Kuduavlli M, Oo A, Mills K, et al. The safe use of spinal drains in thoracic aortic surgery. Interact CardioVasc Thorac Surg. 2012;13:557–65. doi: 10.1510/icvts.2011.272211. [DOI] [PubMed] [Google Scholar]
- 8.Leaf D, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl. 2007;102:1313–1322. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- 9.Acetazolamide for injection. http://www.drugs.com/pro/acetazolamide.html. (1 July 2013, date last accessed)
- 10.Acetazolamide information from drugs update. http://www.drugsupdate.com/generic/view/10. (1 July 2013, date last accessed)