Abstract
OBJECTIVES
The functional aortic valve annulus (FAVA) is a complex unit with proximal (aorto-ventricular junction) and distal (sinotubular junction) components. The aim of our study was to evaluate the impact of the total FAVA remodelling, using a prosthetic ring, on mid-term clinical and echocardiographic results after aortic valve repair.
METHODS
Since February 2003, 250 patients with tricuspid aortic valve insufficiency (AI) underwent aortic valve repair. FAVA dilatation was treated by prosthetic ring in 52 patients, by isolated subcommissural plasty in 62, by subcommissural plasty plus ascending aortic replacement in 57 and by David's reimplantation procedure in 79. Survival rate and freedom from recurrent AI greater than or equal to moderate were evaluated by Kaplan–Meier.
RESULTS
Overall late survival was 90.4%. Late cardiac-related deaths occurred in 15 patients. At follow-up, 36 (16%) patients had recurrent AI greater than or equal to moderate because of cusp reprolapse and/or FAVA redilatation. Freedom from recurrent AI was significantly higher for patients who underwent David's procedure or FAVA remodelling by prosthetic ring than those who underwent isolated subcommissural plasty (P < 0.01) or subcommissural plasty plus ascending aortic replacement (P = 0.02). There was no statistical difference between David's procedure and prosthetic ring annuloplasty (P = 0.26).
CONCLUSION
FAVA remodelling using a prosthetic ring is a safe procedure in aortic valve repair surgery thanks to long-term annulus stabilization and it is a pliable alternative to David's procedure in selected patients. This technique may be used in all patients with slight root dilatation to avoid aggressive root reimplantation. We also recommended total FAVA annuloplasty in all patients who underwent aortic valve repair to improve long-term repair results.
Keywords: Aortic valve, Repair, Statistics, Risk analysis, Echocardiography
INTRODUCTION
The correct function and competency of the aortic valve depend on the integrity of all structural aortic root components: the aortic leaflets, the annulus, the commissures, the sinuses of Valsalva, the sinotubular junction (STJ) and the ascending aorta [1, 2].
The functional aortic valve annulus (FAVA) is a complex unit with proximal (aorto-ventricular junction) and distal (STJ) components. These two anatomical structures, apparently separate, are strictly in contact by the commissures. So, any conditions that affects one of the aortic root component may lead to aortic valve dysfunction and insufficiency.
In patients with aortic root disease and aortic valve insufficiency (AI), surgical management is challenging: patients, in fact, have often similar clinical status, but the different aetiology of AI requires adapted operative techniques. For this reason, aortic valve-sparing and aortic valve repair (AVR) operations were developed to preserve the native aortic valve in patients with aortic root and/or ascending aorta aneurysms with or without AI [3–5].
Understanding the mechanisms of aortic valve dysfunction and the aetiology of lesions has greatly aided surgeons in developing techniques to repair the aortic valve leaflets and roots and to avoid valve replacement [6].
Many surgical approaches have been described for preserving the aortic valve leaflets such as free edge reinforcement by Gore-Tex suture, triangular resection and cusp reconstruction by direct resuturing or by pericardial patch, cusp plication and ‘the chordae technique’ [7–10].
To improve long-term durability of leaflet repair techniques and valve competency, annulus remodelling is, in our opinion, as mandatory as in mitral valve repair surgery. Furthermore, some annulus remodelling procedures have been described [11–13]. The AVA may be addressed by subcommissural plasty with or without STJ remodelling using a Dacron graft. When the aortic root is dilated, David's procedure is the first surgical choice to replace the aortic root saving the aortic leaflets. Moreover, the use of a prosthetic ring may be helpful in patients who underwent isolated AVR to stabilize the FAVA and to improve long-term repair results. Recently, we have introduced the use of a new hand-made prosthetic ring in AVR surgery [14].
The aim of this study was to compare, in a large cohort of patients, the clinical outcomes and durable restoration of the tricuspid aortic valve function after FAVA remodelling, using our prosthetic ring. In addition, we sought to evaluate mid-term predictors of recurrent AI more than moderate and aortic valve reoperation.
MATERIALS AND METHODS
Patients
Since February 2003, 250 patients with tricuspid aortic valve insufficiency underwent AVR in our institutions to treat AI. Age ranged from 38 to 82 years (mean 57 ± 15 years). Demographics and clinical data are illustrated in Table 1. Intraoperative data and outcomes are illustrated in Table 2. The mechanisms of valve dysfunction according to functional classification [15] were the following: Type I in 79 (31.6%) patients, Type II in 138 (55.2%) and Type I + II in 33 (13.2%). Aortic annulus remodelling was performed by our new approach in 52 patients, by isolated subcommissural plasty in 62, by subcommissural plasty plus ascending aortic replacement in 57 and by David's procedure in 79 (Table 3).
Table 1:
Demographic and preoperative patient characteristics
| Variables | All patients (N = 250) |
|---|---|
| Age (years ± SD) | 57 ± 15 |
| Male gender, n (%) | 171 (68.4) |
| Diabetes, n (%) | 26 (10.4) |
| Chronic obstructive pulmonary disease, n (%) | 28 (11.2) |
| Hypertension, n (%) | 160 (64) |
| Creatinine >1.5 mg/dl, n (%) | 18 (7.2) |
| New York Heart Association functional class, n (%) | |
| II | 150 (60) |
| III | 88 (35.2) |
| IV | 12 (4.8) |
| Ascending aortic pathology, n (%) | |
| Atherosclerotic aneurysm | 50 (20) |
| Degenerative | 172 (68.8) |
| Marfan | 28 (11.2) |
| Type of valve dysfunction, n (%) | |
| Type I | 79 (31.6) |
| Type II | 138 (55.2) |
| Type I + II | 33 (13.2) |
| Degree of aortic insufficiency n (%) | |
| I | 16 (2.4) |
| II | 65 (26) |
| III | 71 (28.4) |
| IV | 98 (39.2) |
| Ejection fraction % (mean ± standard deviation) | 0.43 ± 0.11 |
Table 2:
Intraoperative data and early postoperative outcomes
| Variables | All patients (N = 250) |
|---|---|
| Associated surgical procedures | |
| Coronary artery bypass grafting | 25 (10%) |
| Mitral valve repair | 14 (5.6%) |
| Intraoperative data | |
| Bypass time (min) | 102 ± 38 |
| Cross-clamp time (min) | 88 ± 31 |
| Second run for residual aortic insufficiency | 14 (5.6%) |
| Early postoperative outcomes | |
| In-hospital mortality | 6 (2.4%) |
| Stroke | 2 (0.8%) |
| Pneumonia | 4 (1.6%) |
| Sepsis | 1 (0.4%) |
| Reoperation for bleeding | 10 (4%) |
| Permanent pacemaker insertion | 3 (1.2%) |
| Intensive care unit stay (day) | 1.3 ± 0.8 |
| Hospital stay (day) | 8 ± 3 |
Table 3:
Pre- and postoperative aortic insufficiency (AI) grade, cusp repair and root remodelling techniques
| Variables | All patients (N = 250) | Prosthetic ring (N = 52) | Reimplantation (N = 79) | SCP (N = 62) | SCP + AAR (N = 57) |
|---|---|---|---|---|---|
| Preoperative AI grade, n (%) | |||||
| I | 16 (2.4) | 8 (15.4) | 8 (10.1) | 0 (0) | 0 (0) |
| II | 65 (26) | 26 (50) | 32 (40.5) | 2 (3.2) | 5 (8.7) |
| III | 71 (28.4) | 13 (25) | 22 (27.8) | 15 (24.2) | 21 (36.8) |
| IV | 98 (39.2) | 5 (9.6) | 17 (21.5) | 45 (72.5) | 31 (54.4) |
| Preoperative aortic root measures | |||||
| Annulus | 27 ± 3.2 | 27 ± 1.3 | 28 ± 2.1 | 26 ± 1.3 | 27 ± 1.5 |
| Sinuses of Valsalva | 45 ± 2.3 | 41 ± 3.8 | 52 ± 2.6 | 43 ± 2.1 | 44 ± 1.1 |
| Sinotubular junction | 44 ± 1.5 | 40 ± 2.3 | 48 ± 2.5 | 42 ± 1.1 | 47 ± 2.3 |
| Ascending aorta | 47 ± 3.5 | 41 ± 2.1 | 52 ± 5.2 | 41 ± 1.3 | 54 ± 2.5 |
| Postoperative AI grade, n (%) | |||||
| Grade 0 | 225 (90) | 49 (94.2) | 75 (94.9) | 52 (84.8) | 49 (85.9) |
| Trivial to grade I | 25 (10) | 3 (5.8) | 4 (5.1) | 10 (16.2) | 8 (14.1) |
| Postoperative aortic root measures | |||||
| Annulus | 22 ± 2.2 | 22 ± 1.5 | 25 ± 0.5 | 21 ± 1.1 | 21 ± 1.3 |
| Sinuses of Valsalva | 38 ± 3.3 | 40 ± 1.8 | 29.5 ± 1.8 | 41 ± 1.8 | 42 ± 1.2 |
| Sinotubular junction | 33 ± 1.5 | 36 ± 1.6 | 29.5 ± 1.8 | 38 ± 1.1 | 29.8 ± 1.3 |
| Ascending aorta | 35 ± 6.2 | 41 ± 1.3 | 29.5 ± 1.8 | 41 ± 1.5 | 29.8 ± 1.3 |
| Leaflet repair, n (%) | |||||
| Plication | 76 (30.4) | 27 (51.9) | 16 (20.2) | 15 (24.2) | 18 (31.6) |
| Free-edge reinforcement | 60 (24) | 10 (19.2) | 17 (21.5) | 19 (30.6) | 14 (24.6) |
| Plication + free-edge reinforcement | 62 (24.8) | 3 (5.7) | 25 (31.6) | 16 (25.8) | 18 (31.6) |
| Chordae technique | 52 (20.8) | 12 (23.1) | 21 (26.6) | 12 (19.35) | 7 (12.3) |
SCP: subcommissural plasty; AAR: ascending aortic replacement.
Echocardiographic evaluation of the aortic root lesions
Pre- and postoperative echocardiographic examinations were performed using the iE33 ultrasound imaging system (Philips Medical Systems, Veenpluis, Netherlands). Images from the trans-thoracic parasternal long- and short-axis, and apical five chambers views were acquired. The grade of AI was evaluated semi-quantitatively and was classified into four grades: mild (Grade I), moderate (Grade II), moderate-to-severe (Grade III) and severe (Grade IV). Intraoperative transoesophageal echocardiographic (TOE) examinations were performed in all the patients to identify the mechanisms of valve dysfunction using the fully sampled matrix array TOE transducer (X7-2 T, Philips Medical Systems). Aortic cusps and root lesions were categorized according to the functional classification of AI [15]. The valve dysfunction was described by the cardiologist and confirmed by the surgeon intraoperatively. Three main mechanisms were identified: normal leaflet motion with dilatation of the aortic functional annulus or ascending aorta (Type I), excess leaflet motion including cusp prolapse and free edge fenestrations (Type II) and restrictive leaflet motion with cusp retraction and/or cusp calcifications (Type III). Long- and short-axis views were performed to detect leaflet prolapse. In the long-axis view, the aortic leaflet was defined as prolapsed when parts of the cusp were below the level of the annulus. Cusp prolapse may be partial (prolapsed of the free margin of the cusp or the distal part) or complete (whole cusp prolapsed or eversion). In the short-axis view, the cusp was defined as prolapsed when the free margin was wrinkled in the closure position and a lack in central leaflet coaptation was found. In addition, each examination was completed by the detection of the diameters of the aortic annulus, STJ, Valsalva sinuses and ascending aorta (Table 3). Postoperative echocardiographics controls were performed in all patients to detect valve competency, residual cusp prolapse, size of aortic root components and cups coaptation height.
Surgical technique
In all the patients, longitudinal median sternotomy was made and normothermic cardiopulmonary bypass was started by cannulation of the aorta and the right atrium. After aortic cross-clamping and aortotomy, cardioplegic arrest was induced by infusion of cold blood cardioplegia directly into the coronary ostia. The aortic valve and root components were inspected.
The ascending aorta was replaced when its size was >4.5 cm. David's procedure was applied when the aortic root size was >2.5 cm/m2. Isolated subcommissural plasty was performed when all the components of the aortic root were normal. Recently, we prefer using a prosthetic ring to stabilize the FAVA instead of isolated subcommissural plasty. Moreover, when poor aortic wall quality was present, like in Marfan syndrome, aortic valve-sparing reimplantation or an ascending aorta replacement procedure was preferred.
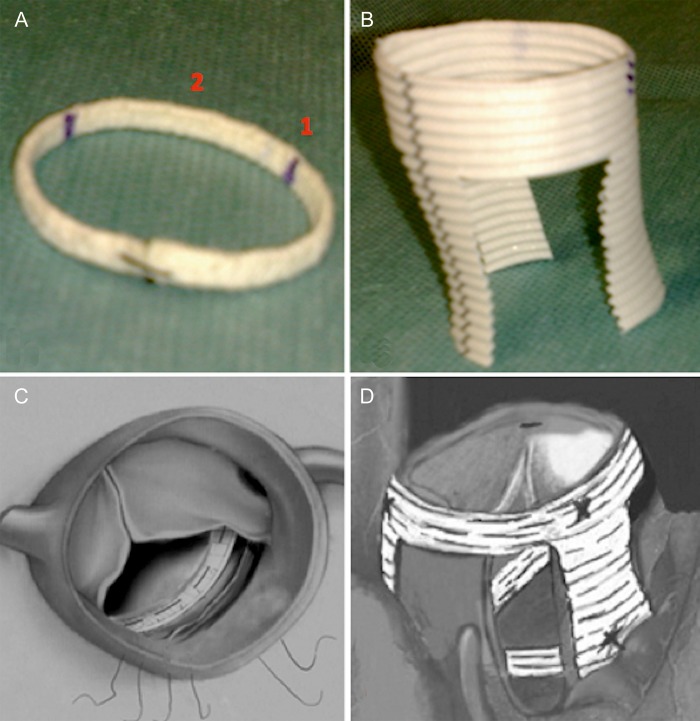
The prosthetic ring components were described previously [14]. The circular ring was fixed into the left ventricular outflow tract just below the aortic valve nadir and the three crown-like shaped ring was sutured to the STJ from the outside of the aortic root. The circular ring was fixed into the left ventricular outflow tract in the subannulus (nadir) position. Ticron 2–0 U stitches (Coviden, Mansfield, MA, USA) were placed first on the prosthetic ring and subsequently under the aortic leaflet nadir. The size (diameter) of the prosthetic circular ring was calculated according to the height of the leaflet measured from the Noduli of Arantius to the base of the cusp [14]. The STJ ring was sutured from outside of the aortic root just at the level of the STJ using a 4–0 Prolene running suture. At the end of the procedure, the three vertical bands of the STJ ring were fixed into the underlying circular ring to stabilize the continuity between the STJ and the nadir of the aortic valve and to avoid future upward displacement of the STJ in cases of ascending aorta or aortic root dilatation (Fig. 1).
Figure 1:
(A) Circular ring for subvalvular aortic annuloplasty: (1) the commissural zone and (2) the intercommissural zone. (B) The three crown-like shaped ring for the sinotubular junction annuloplasty. (C) The circular ring is sutured into the left ventricular outflow tract just under the aortic valve cusps. (D) The sinotubular junction ring is sutured from outside the ascending aorta at the level of the sinotubular junction. The three vertical arms of the sinotubular junction ring were fifixed to the underlying circular ring to stabilize the continuity between the two structures and to reshape the functional annulus.
STJ remodelling was performed with supra-coronary ascending aorta replacement using a Dacron tube graft. The valve reimplantation was performed following the David's reimplantation procedure. Finally, subcommissural annulus plication was performed using a 2–0 Ticron suture reinforced with Teflon pledgets (DuPont, Wilmington, DE, USA) placed at the base of the interleaflet triangle.
Once the aortic root had been reshaped or replaced, the aortic valve was inspected carefully to ascertain additional cusp disease using the systematic approach previously described [8]. In brief, valve analysis was performed using commissural suspension stay sutures; cusp prolapse was identified when the cusp-free margin was lower compared with adjacent normal cusps or when all free margins were at the same level but below the normal AV coaptation level.
Leaflet correction was performed by different techniques (Table 3). The cusp plication technique was performed using a 6–0 Prolene suture (Ethicon, Inc., Hamburg, Germany) placed in the central zone of the elongated-free margin of the cusp and extended perpendicularly from the free margin ∼5 mm through the belly of the leaflet to decrease distension. If excess tissue was found in the plication zone, small resection was performed to avoid postoperative restrictive leaflet motion.
The free edge reinforcement technique was performed using a CV-6 or CV-7 Gore-Tex suture (W.L. Gore and Associates, Flagstaff, AZ, USA) passed in a running fashion over and over, along the entire length of the free margin. Free margin shortening was obtained by applying tension on both suture arms, which were locked at the level of the commissures when appropriate correction was reached. ‘The chordae technique’ was performed by a free margin reinforcement using three Gore-Tex sutures.
Follow-up data
Pre- and postoperative clinical status were determined according to the New York Heart Association (NYHA) functional class for heart failure symptoms. Clinical and echocardiographic follow-up data were obtained for all the patients and were 100% complete. The mean follow-up was 45 ± 30 months for patients with the FAVA procedure and 50 ± 24 months for those with the reimplantation technique (P > 0.05). Follow-up was conducted through our outpatient clinics. Data were obtained from our computerized outcome data collection instrument.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables or as a percentage for categorical variables. The actuarial survival and other time-related events were analysed using the Kaplan–Meier method. The log-rank test was used to compare statistical significance level. P-values of <0.05 were considered statistically significant. The SPSS statistical software (SPSS, Inc., Chicago, IL, USA) was used.
RESULTS
Early outcomes
There were 6 (2.4%) in-hospital deaths. Causes of early deaths were bleeding in 3 patients, respiratory failure in 2 and sepsis in 1. Re-exploration for bleeding was needed in 10 (4%) patients. A second pump-run was required in 14 (5.6%) patients to correct residual AI. Mechanisms of residual AI were uncorrected cusp prolapse in 9 patients and residual annulus dilatation in 5. Mean cardiopulmonary bypass and aortic cross-clamp times were 102 ± 38 and 88 ± 31 min, respectively. The mean postoperative hospital stay was 8 ± 3 days. At hospital discharge, post-repair echocardiography revealed no AI in 225 (90%) patients and trace-to-mild AI in 25 (10%). All data are listed in Table 3.
Late outcomes
There were 18 late deaths, including 15 cardiac-related deaths. Causes of late deaths were heart failure in 7 patients, sudden death in 5, malignant arrhythmias in 3, stroke in 2 and unknown in 1. The overall survival rate was 90.4%.
At follow-up, 36 (16%) patients had recurrent moderate or moderate-to-severe AI. Among them, 18 patients had moderate-to-severe or severe AI which led to redo aortic valve operation. In this group of patients, mechanisms of AI were annulus dilatation and/or cusp reprolapse. Eighteen patients with moderate AI were in NYHA functional class I or II and still under clinical follow-up. Three (1.2%) patients had moderate aortic stenosis.
FAVA dilatation occurred only in the group of patients who underwent isolated subcommissural plasty with or without ascending aortic replacement. Freedom from recurrent AI was significantly higher for patients who underwent total FAVA remodelling by prosthetic ring or David's procedure than those who underwent isolated subcommissural plasty (P < 0.01) or subcommissural plasty plus ascending aorta replacement (P = 0.02) (Fig. 2). No statistical difference was found between patients who received either David's procedure or prosthetic ring annuloplasty (P = 0.26).
Figure 2:
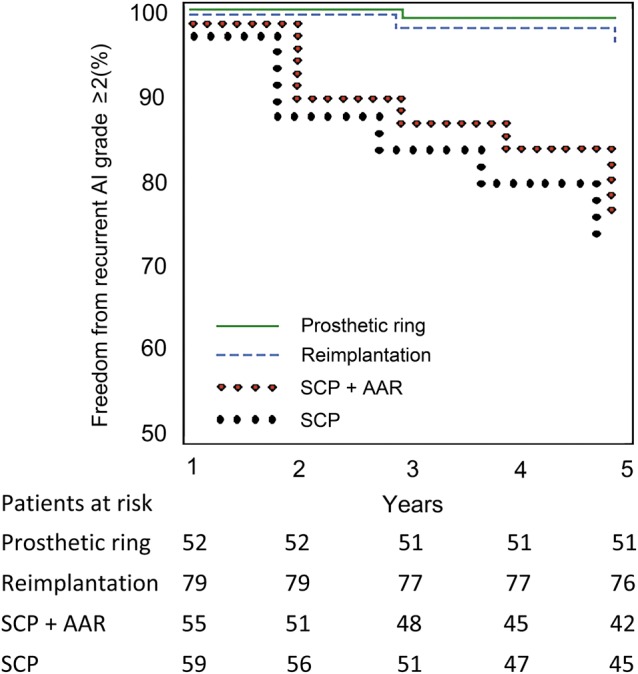
Freedom from recurrent aortic valve insufficiency greater than or equal to moderate for patients who underwent FAVA annuloplasty using our ring (solid line), the reimplantation procedure (dashed line), subcommissural plasty plus ascending aortic replacement (empty circle) and subcommissural plasty (filled circle).
DISCUSSION
Understanding the mechanisms of aortic valve dysfunction and the aetiology of lesions has greatly aided surgeons in the development of repair techniques. Several surgical repair approaches have been described to correct AI, and long-term results for these different operative techniques have been previously reported [7–10, 16].
The use of aortic annuloplasty in addition to cusp repair improves results. As already demonstrated by mitral valve reconstructive operations, concomitant treatment of the leaflet diseases plus annulus reshaping by prosthetic ring emerged as a safe and durable surgical choice and improves long-term repair results. Moreover, annulus remodelling represents a fundamental step in mitral valve repair, because it stabilizes the anatomical shape of the annulus improving the coaptation surface of the leaflets, decreasing closing stress and leading to better outcomes.
Today, subcommissural plasty, initially described by Cabrol et al. [11], represents the first surgical choice in aortic valve annuloplasty, and it is widely used to reduce the circumference of the aortic annulus.
Through this type of annuloplasty, only a component of the aortic annulus (the nadir or aorto-ventricular junction) is corrected, leaving the other components (the STJ) untreated: it is probably the main reason why performing this reparative technique is still controversial. As described by Anderson et al. [2], the AVA does not have a circular biplane shape, but it is a complex tridimensional structure composed of the nadir of the aortic leaflets, the commissures and by the STJ. Conceptually, during an aortic valve annuloplasty, we must be concerned with all the components of the native aortic annulus structure and not only the aorto-ventricular junction. So any type of aortic valve annuloplasty including the use of an aortic prosthetic ring must concern all the components of the functional aortic annulus.
Moreover, using the subcommisural plasty technique, we are not able to decide the exact amount of the annulus diameter reduction. We just include and fix the commissures by the sutures, always considering that their movements are fundamental to preserve valve motion and reduce the stress on the aortic leaflets. Finally, the suture placed in the subcommissural position, between the right coronary cusp and the other cusps, is sited at the level of the interventricular septum, and so it could move down the ventricle muscle. All these situations can be considered reasons why different authors experienced failure of the subcommissural plasty especially, in patients with AI and a dilated left ventricle [10, 17].
Other authors obtained better results performing valve-sparing root replacement (full stabilization of the functional aortic annulus) concomitant with cusp repair rather than isolated cusp repair and subcommissural plasty [3].
Early in the last decade, other surgical techniques were attempted as a possible key to provide stabilization of the aortic annulus over time [11, 12, 14]. Actually, the Lansac ring [12] is the first prosthetic ring available commercially and appears safe and helpful in the surgical valve repair procedure. Anyhow, more clinical results are needed to clarify the long-term efficacy. Recently, Mazzitelli et al. [13] proposed a new prosthetic ring for aortic annuloplasty.
We propose our approach that is supported by the easy technique of suturing the ring in the subvalvular position. The suture line is placed under the aortic leaflets into the left ventricular outflow tract, from inside to outside. This leads to an accurate suturing technique with a correct geometric undersizing of the aortic orifice without any interaction with the cusp motion. The commissures and the interleaflet triangle are above the suture line and are free to move during the cardiac cycle, reducing the stress on the leaflets. The second part is sutured to the STJ and fixed to the nadir ring at the level of the commissures. In this manner, we stabilize the functional aortic annulus from the STJ to the nadir of the aortic valve and we reshape completely the FAVA [13].
Our results showed that recurrent aortic valve regurgitation decreases over time when the entire aortic root is treaded using the reimplantation technique or a prosthetic ring, compared with isolated subcommissural plasty with or without ascending aorta replacement. At follow-up, freedom from aortic valve reoperation and freedom from significant recurrent AI were 92.3 and 84.6%, respectively. All patients without AI have an optimal surface of leaflet coaptation. No restrictive leaflet motion or aortic valve stenosis were observed. In our series, no significant difference was observed between patients who underwent complete FAVA remodelling by prosthetic ring and those who underwent David's operation (P = 0.26); with the current results, we have been able to achieve a valve stability that is comparable with that of David's procedure, whose literature showed a 5-year freedom from reoperation of 90–98% and a freedom from aortic regurgitation of 88–98% [18–22]. Moreover, poor results in terms of freedom from recurrent AI was found for patients who underwent isolated subcommissural plasty with (P = 0.02) or without (P < 0.01) ascending aorta replacement, compared with those who underwent FAVA remodelling by prosthetic ring (Fig. 2).
In conclusion, total FAVA annuloplasty with a prosthetic ring is a pliable alternative to David's procedure for the treatment of AI because it leads to aortic annulus long-term stabilization, and improves repair results. With a careful selection of the appropriate leaflet reconstructive technique and the use of total FAVA stabilization by prosthetic ring, the results of AVR will be improved for long time. The use of an aortic prosthetic ring is a valuable procedure especially in all patients with slight root dilatation to avoid aggressive root reimplantation.
Further study is required to assess the relative importance of the use of an aortic prosthetic ring in patients with aortic annulus dilatation and AI, especially in those with borderline root size.
LIMITATION OF THE STUDY
Considering the small number of patients, it was not possible to perform a propensisty score analysis. Despite the limited number of surgeons involved in these procedures, root-sparing techniques are complex and probably numerous variables play a role in their outcomes.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr E. Lansac (Paris, France): I think this is an important contribution to getting across the message that aside from mitral valve repair, a stable aortic valve repair is achievable when cusp coaptation is restored in combination with aortic annuloplasty which will increase the surface of coaptation and protect the repair.
The second message is that following the experience of the El Khoury and Schäfer groups, you clearly demonstrate that partial annuloplasty through subcommissural plication had worse results than a circumferential annuloplasty achieved either through an internal ring or an external one through the proximal suture of the reimplantation, because subcommissural plication was not able to achieve significant annuloplasty or prevent recurrent dilation.
And finally, the last important message is that there was no difference between isolated aortic valve repair when the root was moderately dilated with a circumferential annuloplasty than with the reimplantation of the aortic valve. So in other words, for the non-dilated or moderately dilated aorta, there is no need to take the relative mortality risk of coronary reimplantation.
However, your study would have greatly benefitted from a description of what I would call not the long-term but the mid-term results of each group, and especially by detailing intraoperative failure as well as reoperation for each group.
My first question is more technical. How do you size the internal ring and how do you fix it precisely? And regarding the expansibility of the aortic annulus, why don't you place the ring externally like the proximal suture of the reimplantation that you were using for root replacement? And did you have any reoperation in the ring group? And if ‘yes’, what was the cause of failure and what did the ring look like? Was there any partial dehiscence?
Dr Fattouch: I think that it is still very difficult for the surgeon to choose the internal ring size, because nowadays we haven't any mathematical formula or equation or commercial sizer available. I tried to do this and I published in the Annals of Thoracic Surgery one year ago, one formula for ring sizing. In recent years we learned from Schäfer's group the importance of cusp height in aortic valve repair surgery. So I try to select the radius of my ring equal to or less than about 4–5 mm with respect to the height of the cusp. On the other hand, I think a good measure for the radius ring may be the length of the free margin of the cusp from the nodule of Arantius to the commissure.
In my practice today I use a millimetric sizer such as the Freestyle sizer, and I make this calculation and I tailor my ring and put the sizer in the outflow tract and assess the geometry. For tying, we discussed the modelling with El Khoury. When you use a pliable band or ring, you can shrink the annulus. Therefore I think attention should be paid when tying the stitch to not provoking leaflet prolapse.
Dr E. Mostafa (Cairo, Egypt): Actually, this is the same question that I'm going to ask. What are you going to do if you fix it and then you have a problem with undersizing?
Dr Fattouch: I cut the stitch and I remove the ring.
Dr Mostafa: From the inside, I mean the internal ring.
Dr Fattouch: If I have a problem like undersizing, I cut the band, I remove it, and I place a bigger one.
Dr Mostafa: I have a suggestion for this if you allow me, because actually I'm using it. I do three stitches from outside. And then, my question also is, do you do your sizing after coming off bypass and on a beating heart, or you do it on a still-arrested heart?
Dr Fattouch: No, only on the arrested heart.
Dr Mostafa: Can I advise you to do it while the heart is beating and under echo guidance? Because if you divided the ring into three sutures, and then you tie each one according to the beating heart annulus, which is actually the real annulus, that's why I'm asking whether you fix it on the arrested heart, not on the beating heart. Try it on beating heart with three divided sutures.
Dr Fattouch: I understand your question. Today, I do all my work on the arrested heart because at the beginning of my experience I used the beating heart technique to do annulus remodelling. In two cases I had laceration of the aortic annulus at the level of the right commissure with some complications like bleeding, and one case of a right coronary ostial occlusion, so I avoid working on the beating heart.
REFERENCES
- 1.Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair: from structure to function. Part I. Circulation. 2004;109:942–50. doi: 10.1161/01.CIR.0000115633.19829.5E. Part II. Circulation 2004;109:1064–72. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RH, Devine WA, Ho SY, Smith A, McKay R. The myth of the aortic annulus: the anatomy of the subaortic outflow tract. Ann Thorac Surg. 1991;52:640–6. doi: 10.1016/0003-4975(91)90966-t. [DOI] [PubMed] [Google Scholar]
- 3.Langer F, Aicher D, Kissinger A, Wendler O, Lausberg H, Fries R, et al. Aortic valve repair using a differentiated surgical strategy. Circulation. 2004;110(Suppl II):II-67–73. doi: 10.1161/01.CIR.0000138383.01283.b8. [DOI] [PubMed] [Google Scholar]
- 4.Haydar HS, Guo-Wei HE, Hagop H, Mc Irvin D, Douglas H, Starr A. Valve repair for aortic insufficiency: surgical classification and techniques. Eur J Cardiothorac Surg. 1997;11:258–65. doi: 10.1016/s1010-7940(96)01014-7. [DOI] [PubMed] [Google Scholar]
- 5.David TE, Christopher MF. An aortic valve-sparing operation for patients with aortic incompetence and aneurysms of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617–22. [PubMed] [Google Scholar]
- 6.Carr JA, Savage EB. Aortic valve repair for aortic insufficiency in adults: a contemporary review and comparison with replacement techniques. Eur J Cardiothorac Surg. 2004;25:6–15. doi: 10.1016/j.ejcts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Ahn H, Kim K-H, Kim YJ. Midterm result of leaflet extension technique in aortic regurgitation. Eur J Cardiothorac Surg. 2002;21:465–9. doi: 10.1016/s1010-7940(01)01150-2. [DOI] [PubMed] [Google Scholar]
- 8.Fattouch K, Sampognaro R, Bianco G, Navarra E, Moscarelli M, Speziale G, et al. Implantation of Gore-Tex chordae on aortic valve leaflet to treat prolapse using ‘the chordae technique’: surgical aspects and clinical results. Ann Thorac Surg. 2008;85:2019–24. doi: 10.1016/j.athoracsur.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 9.de Kerchove L, Boodhwani M, Glineur D, Poncelet A, Rubay J, Watremez C, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg. 2009;88:455–61. doi: 10.1016/j.athoracsur.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 10.Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Noirhomme P, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. Thorac Cardiovasc Surg. 2010;140:276–84. doi: 10.1016/j.jtcvs.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Cabrol C, Cabrol A, Guiraudon G, Bertrand M. Treatment of aortic insufficiency by means of aortic annuloplasty. Arch Mal Coeur Vaiss. 1966;59:1305–12. [PubMed] [Google Scholar]
- 12.Lansac E, Di Centa I, Raoux F, Bulman-Fleming N, Ranga A, Abed A, et al. An expansible aortic ring for a physiological approach to conservative aortic valve surgery. J Thorac Cardiovasc Surg. 2009;138:718–24. doi: 10.1016/j.jtcvs.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Mazzitelli D, Nöbauer C, Rankin JS, Badiu CC, Krane M, Crooke PS, et al. Early results after implantation of a new geometric annuloplasty ring for aortic valve repair. Ann Thorac Surg. 2013;95:94–7. doi: 10.1016/j.athoracsur.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Fattouch K, Sampognaro R, Speziale G, Ruvolo G. New technique for aortic valve functional annulus reshaping using a handmade prosthetic ring. Ann Thorac Surg. 2011;91:1154–8. doi: 10.1016/j.athoracsur.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 15.El Khoury G, Glineur D, Rubay J, Verhelst R, d'Acoz Yd, Poncelet A, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol. 2005;20:115–21. doi: 10.1097/01.hco.0000153951.31887.a6. [DOI] [PubMed] [Google Scholar]
- 16.Fattouch K, Murana G, Castrovinci S, Nasso G, Mossuto C, Corrado E, et al. Outcomes of aortic valve repair according to valve morphology and surgical techniques. Interact CardioVasc Thorac Surg. 2012;15:644–50. doi: 10.1093/icvts/ivs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aicher D, Langer F, Adam O, Tscholl D, Lausberg H, Schäfers HJ. Cusp repair in aortic valve reconstruction: does the technique affect stability? J Thorac Cardiovasc Surg. 2007;134:1533–8. doi: 10.1016/j.jtcvs.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Leontyev S, Trommer C, Subramanian S, Lehmann S, Dmitrieva Y, Misfeld M, et al. The outcome after aortic valve-sparing (David) operation in 179 patients: a single-centre experience. Eur J Cardiothorac Surg. 2012;42:261–6. doi: 10.1093/ejcts/ezs011. [DOI] [PubMed] [Google Scholar]
- 19.David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S14–9. doi: 10.1016/j.jtcvs.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Pacini D, Settepani F, De Paulis R, Loforte A, Nardella S, Ornaghi D, et al. Early results of valve-sparing reimplantation procedure using the Valsalva conduit: a multicenter study. Ann Thorac Surg. 2006;82:865–71. doi: 10.1016/j.athoracsur.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 21.De Paulis R, Scaffa R, Nardella S, Maselli D, Weltert L, Bertoldo F, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140:23–7. doi: 10.1016/j.jtcvs.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 22.Shrestha M, Baraki H, Maeding I, Fitzner S, Sarikouch S, Khaladj N, et al. Long-term results after aortic valve-sparing operation (David I) Eur J Cardiothorac Surg. 2012;41:56–62. doi: 10.1016/j.ejcts.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]