Abstract
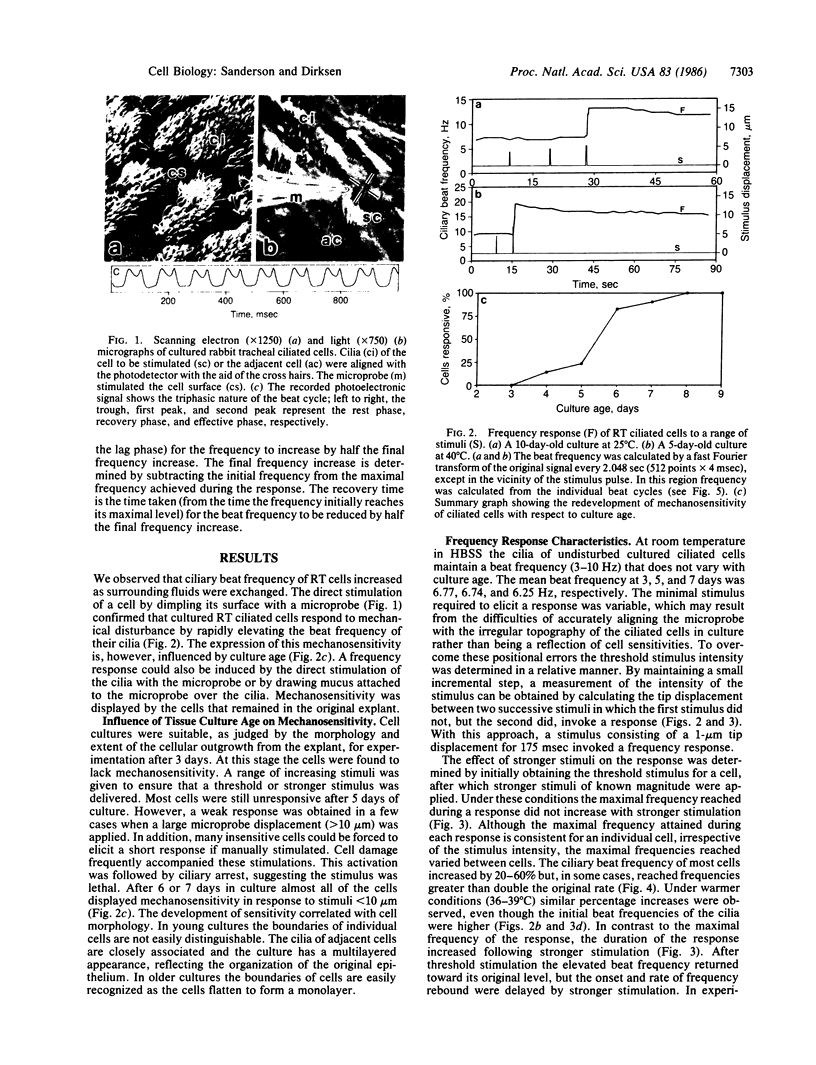
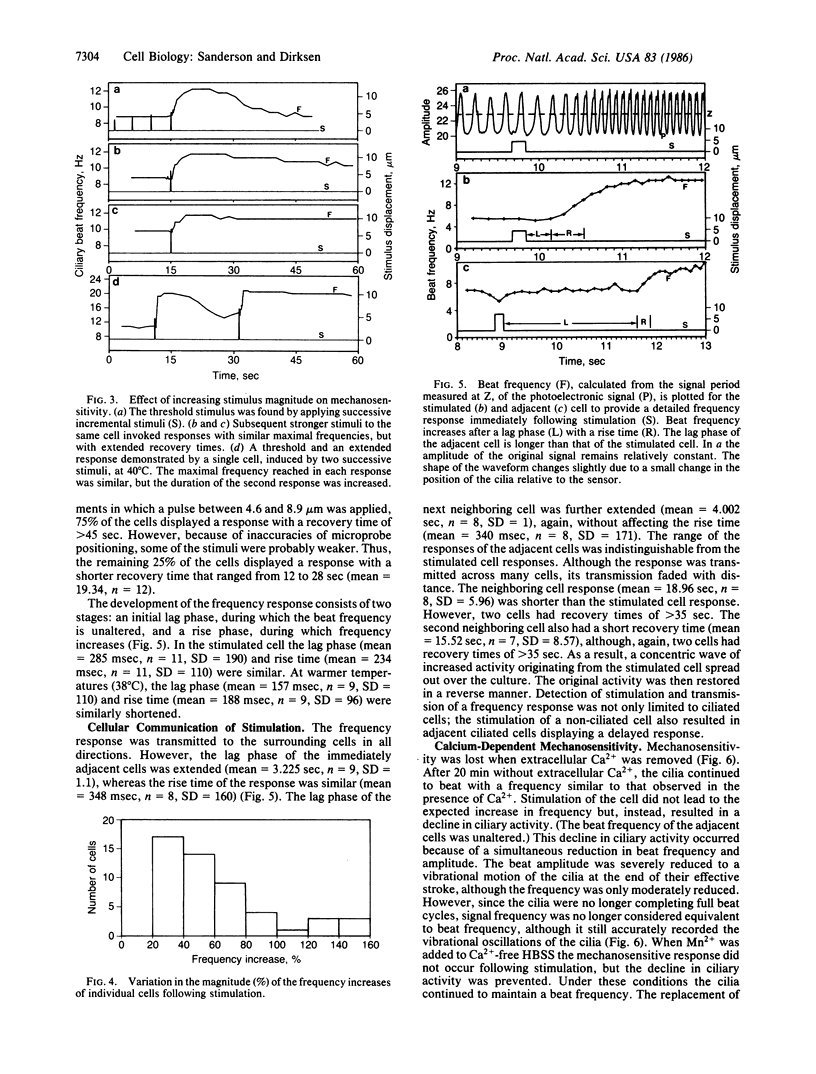
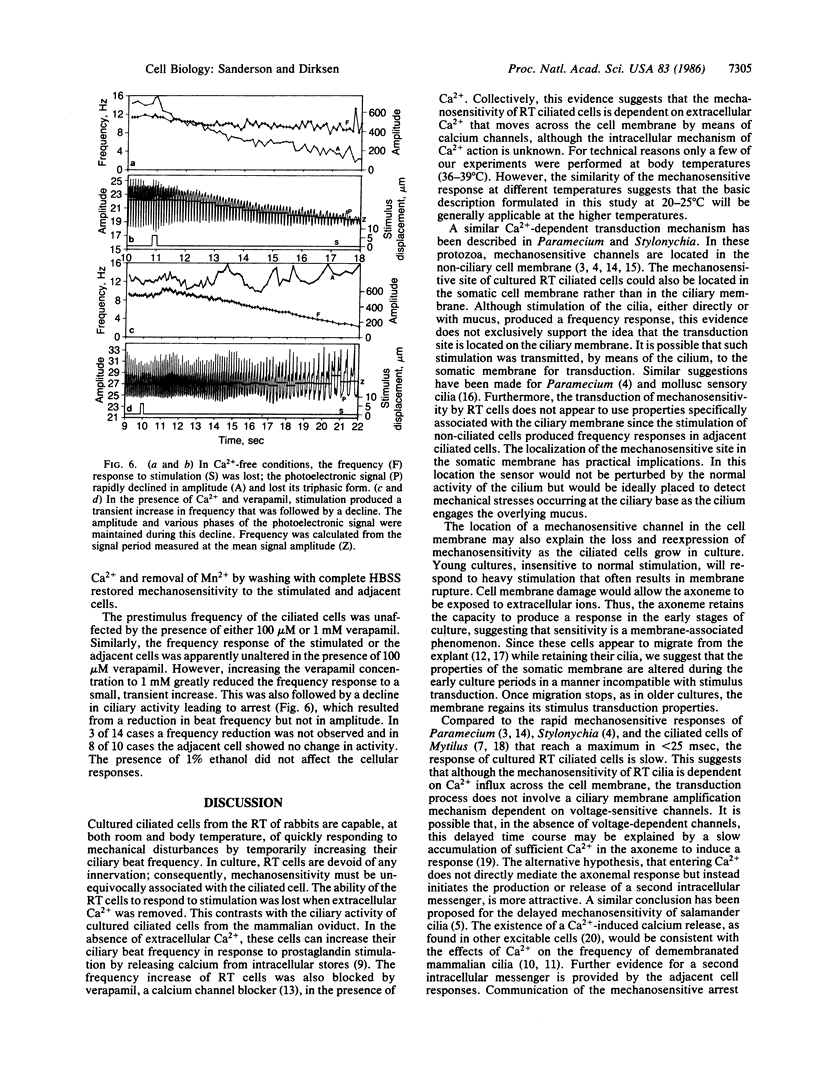
Mechanical stimulation of the cell surface or cilia of cultured ciliated epithelial cells derived from the rabbit tracheal mucosa resulted in a transitory ciliary beat frequency increase of 20% or more. This response was composed of a lag, rise, and recovery phase. The duration of the response, but not the maximal frequency, was increased by stronger stimulation. The ability of these ciliated cells to respond to stimulation depended on culture age; generally, cultures younger than 4 days were insensitive. The frequency response was transmitted to adjacent and more distal cells in all directions. The lag phase but not the rise time of the adjacent cell response was extended. These ciliary responses were lost when extracellular Ca2+ was removed. Replacement of Ca2+ resulted in a restoration of mechanosensitivity. In 1 mM verapamil and Ca2+ the frequency response was also lost. These results suggest that the frequency response is dependent on Ca2+ influx (although its intracellular action is unknown) and imply that mucociliary clearance is a localized self-regulating process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carafoli E., Penniston J. T. The calcium signal. Sci Am. 1985 Nov;253(5):70–78. doi: 10.1038/scientificamerican1185-70. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Hogg J. C. Intercellular junctions of the tracheal epithelium in guinea pigs. Lab Invest. 1974 Jul;31(1):68–74. [PubMed] [Google Scholar]
- Kakuta Y., Kanno T., Sasaki H., Takishima T. Effect of Ca2+ on the ciliary beat frequency of skinned dog tracheal epithelium. Respir Physiol. 1985 Apr;60(1):9–19. doi: 10.1016/0034-5687(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Kennedy J. R., Ranyard J. R. Morphology and quantitation of ciliated outgrowths from cultured rabbit tracheal explants. Eur J Cell Biol. 1983 Jan;29(2):200–208. [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Murakami A., Eckert R. Cilia: activation coupled to mechanical stimulation by calcium influx. Science. 1972 Mar 24;175(4028):1375–1377. doi: 10.1126/science.175.4028.1375. [DOI] [PubMed] [Google Scholar]
- Reed W., Satir P. Spreading ciliary arrest in a mussel gill epithelium: characterization by quick fixation. J Cell Physiol. 1986 Feb;126(2):191–205. doi: 10.1002/jcp.1041260207. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Dirksen E. R. A versatile and quantitative computer-assisted photoelectronic technique used for the analysis of ciliary beat cycles. Cell Motil. 1985;5(4):267–292. doi: 10.1002/cm.970050402. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Sleigh M. A. Ciliary activity of cultured rabbit tracheal epithelium: beat pattern and metachrony. J Cell Sci. 1981 Feb;47:331–347. doi: 10.1242/jcs.47.1.331. [DOI] [PubMed] [Google Scholar]
- Stommel E. W., Stephens R. E., Alkon D. L. Motile statocyst cilia transmit rather than directly transduce mechanical stimuli. J Cell Biol. 1980 Dec;87(3 Pt 1):652–662. doi: 10.1083/jcb.87.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P. Ca2+-dependent hormonal stimulation of ciliary activity. Nature. 1980 Feb 21;283(5749):764–765. doi: 10.1038/283764a0. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Raess B. V., Villalon M. The role of calmodulin in the regulation of ciliary movement in mammalian epithelial cilia. J Submicrosc Cytol. 1983 Jan;15(1):95–96. [PubMed] [Google Scholar]




