Abstract
OBJECTIVES
Ivor Lewis minimally invasive oesophagectomy (ILMIE) is a complex surgery aiming to remove an oesophageal tumour and to create a new gastric tube in the abdomen. The objective was to assess the technical and early outcomes of ILMIE for gastric tube construction in the thoracic cavity.
METHODS
A retrospective analysis was conducted in 25 middle or lower oesophageal cancer patients treated with ILMIE between August and December 2012. A gastric tube was constructed in the thoracic cavity in all patients. The gastric tube and the oesophagus were anastomosed using a circular stapler. Clinical data (age, gender, pathological pattern and TNM stage), surgical data (operation time, intraoperative blood loss and intraoperative complications) and follow-up data (postoperative complications, length of stay, thoracic tube drainage time and time before eating) were assessed.
RESULTS
The mean age was 61 ± 8 years. Sixteen patients were male and 9 were female. Oesophageal cancer was located in the middle oesophagus in 5 cases and in the lower oesophagus in 20. No conversion to open surgery was performed. The mean operative time and intraoperative blood loss were 320 ± 63 min and 137 ± 95 ml, respectively. A mean of 2.4 ± 0.5 linear stapler cartridges was used per patient. A mean of 14.6 ± 5.4 lymph nodes was dissected per patient. Postoperative hospital stay was 13.2 ± 2.4 days. Intraoperative and postoperative complications occurred in 12% (3 of 25) and 20% (5 of 25) of patients, respectively, including 1 case of anastomotic fistula. The patients were followed up for a mean of 3.5 ± 1.2 months, and there was no relapse or death.
CONCLUSIONS
The construction of a gastric tube through the thoracic cavity using ILMIE is feasible and safe in patients with middle or lower oesophageal cancer. However, longer follow-up and larger sample sizes are needed to evaluate the oncological efficacy.
Keywords: Oesophageal cancer, Ivor Lewis minimally invasive oesophagectomy, Gastric tube construction, Thoracic cavity, Complications
INTRODUCTION
The original Ivor Lewis oesophagectomy, first reported in 1946, combines an initial laparotomy and construction of a gastric tube, followed by a right thoracotomy to excise the tumour and a gastro-oesophageal anastomosis [1]. It has become one of the main surgical procedures for the treatment of cancers of the middle and lower oesophagus and of the gastro-oesophageal junction. In 1999, Watson et al. [2] reported the use of Ivor Lewis minimally invasive oesophagectomy (ILMIE) with intrathoracic manual anastomosis. In 2001, Nguyen et al. [3] reported the first case of laparoscopic and thoracoscopic ILMIE with thoracic stapling anastomosis.
Classical ILMIE includes the isolation of the stomach from the abdominal cavity and the construction of the gastric tube, followed by an OrVil-assisted anastomosis [4] or a transthoracic Endo-Stitch placement of a stapling anvil [5]. In China, most MIE are now performed using McKeown's approach and cervical anastomosis [6]. However, the final choice depends on the tumour location and the surgeon's preferences.
Ivor Lewis minimally invasive oesophagectomy allows the direct visualization of the oesophagus and a full thoracic lymph nodes excision [3]. Ivor Lewis minimally invasive oesophagectomy is also less traumatic, allows a faster recovery and has long-term outcomes that are comparable with the classical open approach [7–11], as well as having a better cost-effectiveness [12]. However, totally laparoscopic ILMIE is a complex procedure with a steep learning curve [13]. In addition, the disadvantages of ILMIE are the difficulty to achieve oncological negative margins in cases of middle oesophagus cancers [3]. A severe bile reflux occurs in 3–20% of cases; there is also a risk of intrathoracic anastomotic leakage or gastric tube necrosis leading to morbidity and mortality [13–15].
The actual ILMIE approach involves the construction of an abdominal gastric tube [5]. The main disadvantage of the abdominal construction is that a slender gastric tube has a risk of torsion when lifted to the thoracic cavity [3], as well as a risk of gastric volvulus [16]. We are able to perform an Ivor Lewis open oesophagectomy (ILOE) involving the construction of a gastric tube in the thoracic cavity, using transthoracic hand-made purse suture to fix the stapling anvil and performing the anastomosis using a circular stapler. Therefore, we propose a modified totally laparoscopic and thoracoscopic ILMIE approach using a similar procedure for transthoracic gastric tube construction. We hypothesized that this ILMIE approach is equivalent to the ILOE approach, without increasing the rate of surgical complications or decreasing treatment efficacy. The objective was to assess the perioperative and short-term outcomes, as well as the complications of this modified ILMIE.
MATERIALS AND METHODS
Study design and participants
The present study retrospectively analysed 25 patients with middle or lower oesophageal cancer treated with the modified ILMIE between August and December 2012 at the First affiliated hospital of the Nanjing Medical University (Nanjing, China). The inclusion criteria were: (i) definitive diagnosis of middle or lower oesophagus cancer by preoperative ultrasound endoscopy, barium meal and thoracic and abdominal computed tomography (CT); (ii) T1–2 according to the AJCC staging manual, 7th edition; and (iii) adequate pulmonary function allowing the use of a single-lung ventilation. The exclusion criteria were: (i) history of gastric resection; (ii) history of chest surgery; (iii) neoadjuvant chemotherapy; (iv) distant metastasis; (v) impaired cardiac, kidney or liver function or (vi) impaired coagulation.
Surgical approach
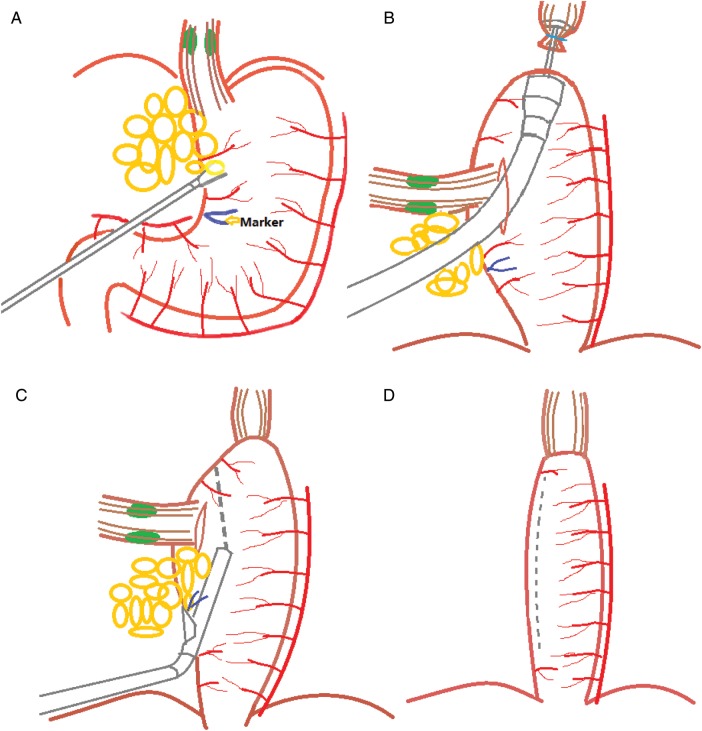
The surgeries were conducted by a group of surgeons who had ILOE experience in 50 cases and McKeown MIE in 90 cases. Double-lumen endotracheal intubation was conducted when the patient was under general anaesthesia. Figure 1 illustrates our modified ILMIE.
Figure 1:
Illustration of the modified ILMIA approach. (A) The adipose tissue and lymph nodes of the lesser curvature are swept up to the cardia from the beginning of the right gastric artery. (B) An incision is made in the anterior aspect of the stomach below the oesophagogastric junction, parallel to the greater curvature. The gastro-oesophageal anastomosis (Ethicon ECS25) is created through the cardiac incision. (C) The articulating endoscopic linear stapler (Ethicon Flex60, nail height 3.8 mm) is fired along the lesser curve below the incision of the cardia. The staple line should be parallel to the greater curvature of the stomach. (D) The conduit measures about 4–5 cm in width. The distance between the top of the lesser curvature cutting line and the anastomosis is kept at least 2 cm.
Abdominal phase
Patients were maintained in the supine position and the upper body was elevated by 30°. Five laparoscopic ports were placed in the abdominal cavity. A CO2 pneumoperitoneum of 15 mmHg was established. The mobilization of the stomach and lower oesophagus and the dissection of celiac lymph nodes were performed similar to a previously described method [5], without a pyloroplasty. Both crus were divided 0.5–1.0 cm in length to enlarge the diaphragmatic hiatus for an easier delivery of the stomach to the thoracic cavity. Cloth strips 10-cm long and 1-cm wide were used to surround the oesophagus. The cloth was locked by clipping both ends. The cloth was placed in the mediastinum to facilitate the isolation of the oesophagus from the thoracic cavity. Lymph tissue of the lesser curvature was separated (but not divided) from the beginning of the right gastric artery to cardia (Fig. 1A). A suture was made from the third to fourth branch of the right gastric artery of the stomach lesser curvature, serving to mark the starting point of gastric tube construction. Finally, a puncture jejunostomy was conducted at 30 cm from the ligament of Treitz Jenunum.
Thoracic phase
The patient was placed in the left lateral decubitus position with left lung ventilation. Four working ports were created without artificial pneumothorax. Three 1-cm incisions were made at: (i) the anterior axillary line in the third or the fourth intercostal space, (ii) the mid-axillary line in the sixth or seventh intercostal space for the 30° camera and (iii) the infrascapular line in the seventh intercostal space. One 3-cm incision (main port) was made at the posterior axillary line in the eighth or ninth intercostal space for the introduction of a circular stapler. The oesophagus was mobilized with the para-oesophageal lymph nodes in an en bloc manoeuvre. The anticipated location of the anastomosis on the oesophagus was 5–8 cm higher than the superior edge of the tumour. The azygous vein was divided and clamped using a Hem-o-Lok (Teleflex, Research Triangle Park, NC, USA). Level 7 lymph nodes were completely dissected. Left and right para-recurrent laryngeal lymph nodes were selectively dissected. A full-layer pursestring of the oesophagus was hand-sewn 1 cm distal to the anticipated anastomosis. An incision was made transversally in the anterior oesophageal wall, 2 cm distal to the pursestring, and the anvil head was inserted. The purse line was tightened, a knot was made using the knot pusher and the oesophagus was transected.
The stomach was delivered to the chest in the right direction, without torsion or tension. A 3-cm incision of the anterior aspect of the stomach was made 1 cm distal to the oesophagogastric junction, parallel to the greater curvature. The gastro-oesophageal anastomosis (Fig. 1B) was created after putting in the stapler (Ethicon ECS25, Ethicon Endo-Surgery, Cincinnati, OH, USA) from the cardia incision through the main port. An articulating endoscopic linear stapler (Ethilon Flex60, nail height of 3.8 mm, Ethicon Endo-Surgery, Cincinnati, OH, USA) was fired (Fig. 1C) along the lesser curve from the marking suture. The staple line should be parallel to the greater curvature of the stomach and 1 cm lower than the incision of the cardia. Two to three staple cartridges were needed to create the gastric tube. We removed part of the lesser curvature of the stomach and the cardia, as well as the lymphoid adipose tissue of the lesser curvature. These samples were taken out. At least 2 cm was kept between the top of the lesser curvature cutting line and the anastomosis. The conduit measured about 4–5 cm in width (Fig. 1D). The highest anastomosis location was close to the thoracic inlet. The staple line of the conduit and the anastomotic stoma were reinforced with interrupted 4–0 Vicryl sutures at 1.0-cm intervals. A 14-Fr multiporous tube was placed in the oesophageal bed through the posterior axillary line in the eighth or ninth intercostal space. A 32-Fr chest tube was placed through the mid-axillary line in the sixth or seventh intercostal space. A nasogastric tube was routinely placed.
Follow-up
Patients received standard postoperative care, as well as standard postoperative follow-up after discharge.
Indexes
Clinical data (age, gender, pathological pattern and TNM stage), surgical data (operation time, intraoperative blood loss and intraoperative complication) and follow-up data (postoperative complications, length of stay, thoracic tube drainage time and time before eating) were assessed.
Statistical analysis
Descriptive statistics were used. Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as proportions. Statistical analysis was performed using SPSS (SPSS, Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
Table 1 summarizes the characteristics of the patients. Twenty-five patients underwent modified ILMIE, including 16 males and 9 females, aged 61 ± 8 years (from 49 to 73). Oesophageal cancer was located in the middle oesophagus in 5 cases and in the lower oesophagus in 20. Nineteen patients suffered from squamous carcinoma, 1 from a neuroendocrine carcinoma and 5 from high-grade neoplasia. The anastomosis was higher than the upper edge of the azygos vein in 11 cases, at the same level as the azygos vein in 9 and lower than the azygos vein in 5.
Table 1:
Clinical characteristics of patients
| Clinical characteristics | Minimally invasive (N = 25) |
|
|---|---|---|
| Mean ± SD or n | % | |
| Age (year) | 61 ± 8 (49–73) | |
| Gender (male/female) | 16/9 | |
| Lesion position | ||
| Mid-thoracic | 5 | 20.0 |
| Lower-thoracic | 20 | 80.0 |
| Histological type | ||
| Squamous carcinoma | 19 | 76.0 |
| Neuroendocrine carcinoma | 1 | 4.0 |
| High-degree neoplasia | 5 | 20.0 |
| TNM Stage | ||
| 0 | 5 | 20.0 |
| IA | 3 | 12.0 |
| IB | 4 | 16.0 |
| IIA | 5 | 20.0 |
| IIB | 5 | 20.0 |
| IIIA | 3 | 12.0 |
| Anastomotic height | ||
| Higher than the upper edge of the azygos vein | 11 | 44.0 |
| Azygos vein level | 9 | 36.0 |
| Lower than the lower edge of the azygos vein | 5 | 20.0 |
Peri- and postoperative outcomes
Table 2 presents the peri- and postoperative outcomes of the 25 patients treated with the modified ILMIE. There was no conversion to open surgery. No death occurred within 30 days of operation. The mean overall operation time was 320 ± 63 min (range 210–520 min). The mean intraoperative blood loss was 144 ± 95 (range: 30–800) ml. Two or three linear stapler cartridges were used per case, with a mean of 2.4 ± 0.5. There were 8–21 lymph nodes dissected per case, with a mean of 14.6 ± 5.4. All cases (100%) had negative margins. Patients started eating at 8–12 days after operation, with a mean of 10.0 ± 1.8 days. Postoperative 32-Fr thoracic tube drainage was performed for 2–5 days, with a mean of 3.2 ± 1.3 days. Postoperative 14-Fr thoracic tube drainage was performed for 10–28 days, with a mean of 12.1 ± 1.8 days. Postoperative hospital stay was 11–28 days, with a mean of 13.2 ± 2.4 days. No cases had postoperative pleural effusion that required puncture and aspiration.
Table 2:
Clinical outcomes
| Variable | Minimally invasive (N = 25) |
|
|---|---|---|
| Mean ± SD | Range | |
| Intraoperative bleeding (ml) | 144 ± 95 | 30–800 |
| Overall operation time (min) | 320 ± 63 | 210–520 |
| Laparoscopic gastric isolation and lymph node dissection (min) | 115 ± 43 | 55–230 |
| Laparoscopic jejunostomy (min) | 36 ± 23 | 25–84 |
| Convert position time (min) | 35 ± 8 | 30–40 |
| Thoracoscopy oesophagus isolation, lymph node dissection and anastomosis (min) | 148 ± 36 | 75–260 |
| Linear stapler cartridges | 2.4 ± 0.5 | 2–3 |
| Number of lymph node dissection | 14.6 ± 5.4 | 8–21 |
| Thoracic lymph node | 8.1 ± 3.4 | 4–12 |
| Abdominal lymph node | 6.5 ± 3.2 | 3–11 |
| Time before feeding (days) | 10.0 ± 1.8 | 8–12 |
| 32-Fr thoracic tube drainage time (days) | 3.2 ± 1.3 | 2–5 |
| 14-Fr thoracic tube drainage time (days) | 12.1 ± 1.8 | 10–28 |
| Postoperative hospital stay (days) | 13.2 ± 2.4 | 11–28 |
Complications
Table 3 summarizes the major complications encountered during and after operation. Three (12%) patients suffered from major intraoperative complications. One case had left tension pneumothorax that improved after a thoracic tube was placed, which was due to a pleural rupture during the dissociation of the lower oesophagus, which induced CO2 infusion into the thoracic cavity from the pneumoperitoneum. One case had a haemorrhage of 300 ml from a torn upper spleen. One case suffered from oesophageal bed bleeding of 800 ml. Endoscopic coagulation was performed for all cases with haemorrhage. One of the 3 cases required blood transfusion.
Table 3:
Complications types in patients undergoing modified Ivor Lewis minimally invasive oesophagectomy
| Complication | n | % |
|---|---|---|
| Pyopneumothorax | 1 | 4 |
| Spleen rupture | 1 | 4 |
| Bleeding >500 ml | 1 | 4 |
| Anastomotic leak | 1 | 4 |
| Atrial fibrillation | 2 | 8 |
| Jejunal fistula | 1 | 4 |
| Upper urinary tract infection | 1 | 4 |
Postoperative complications occurred in 20% (5 of 25) of the patients. One case had an anastomotic fistula: the 14-Fr oesophageal bed porous drainage tube produced purulent fluid 4 days after operation and caused fever. The anastomotic fistula was confirmed by oral meglumine diatrizoate angiography and CT and was cured using a 14-Fr drainage tube, enteral nutrition and anti-infection treatment for 28 days. One patient suffered from symptoms of jejunal fistula after removal of the jejunal fistula tube 30 days after operation; his condition improved after gastrointestinal decompression and 3 days of fasting. One case who had a history of chronic pyelonephritis suffered from upper urinary tract infection, and improved after anti-inflammatory treatment. Two cases had atrial fibrillation and were managed using amiodarone therapy. We observed no lung infection, respiratory failure, heart failure or other serious complications. No gastric conduit volvulus, gastric fistula, chylothorax, recurrent laryngeal nerve injury, delayed gastric emptying, diaphragmatic hernia or other complications occurred.
Follow-up
All patients were discharged from hospital and had chest X-ray examination 1 month later. The patients were followed up for 3.5 ± 1.2 months, during which no relapse or death occurred. We could not study the survival rate due to the short follow-up.
DISCUSSION
The objective of the present study was to assess the perioperative and short-term outcomes, as well as the complications, of a modified ILMIE involving the lifting of the stomach to the thoracic cavity for gastric tube construction. Our results showed no conversion to open surgery, only 1 case of anastomotic leak (4%) and no death within 30 days of operation. The total complication rate was 32%. The total surgery time was 320 ± 63 min, and blood loss was small (144 ± 95 ml). Results showed that this modified ILMIE approach was safe and feasible, with a low rate of complications. Short-term outcomes were also favourable.
Table 4 summarizes the published series of ILMIE; all these studies were published in 2012–2013. In these studies, the incidence of anastomotic leakage was 0–9.1%, the death rate within 30 days of surgery was 0–6% and the mean operating time was between 360.5 and 499 min, for a blood loss of 40–205 ml [17–21]. These studies also showed an incidence of major complications of 20–36.8%. The results of the present study using a modified ILMIE approach fall within the ranges previously reported, which indicate the safety and feasibility of our modified ILMIE. However, our operating time was considerably shorter. Indeed, the construction of a gastric tube using a transthoracic approach simplifies the surgical procedures. Furthermore, our surgical team already had experience in McKeown MIE and in ILOE, stressing the importance of the learning curve for this procedure.
Table 4:
Published series of Ivor Lewis minimally invasive oesophagectomy
| Author/reference | N | Surgery time (min) | Bleeding amount (ml) | Surgery type | Stomach tube production site | Number of linear stapler cartridge | Stomach tube diameter (cm) | Anastomosis | Anvil fixing method | Number of lymph node dissection | Anastomotic leak | Complications (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sihag et al. [17] | 38 | 360.5 (318–391) | 200 (150–250) | Laparoscopic chest | Abdominal cavity | >3 | 4–6 | Circular stapled | NA | 19 (15–28) | 0 | 36.8 |
| Cerfolio et al. [18] | 22 | 367 (290–453) | 40 | Robot | Abdominal cavity | >3 | NA | Hand-sewn anastomosis | No anvil | 18 | 1 | 36.4 |
| Tapias and Morse [19] | 40 | 364 (285–455) | 205 (100–400) | Laparoscopic chest | Abdominal cavity | >3 | 5–6 | Circular stapled | Pursestring | 21 (11–41) | 0 | 21 |
| Okabe et al. [20] | 26 | 499 (365–645) | 78 (13–210) | Laparoscopic chest | Abdominal cavity | >3 | 5 | Linear stapler | No anvil | 54.8 (37–98) | 1 | 26.9 |
| Merritt [21] | 15 | 468 ± 54 | 182 ± 67 | Laparoscopic chest | Abdominal cavity | >3 | 5–6 | Transorally circular stapled | Linear staple | 11.4 ± 1.1 | 1 | 20 |
| Present study | 25 | 320 ± 63 | 144 ± 95 | Laparoscopic chest | Thoracic cavity | 2–3 | 4–5 | Circular stapled | Hand-sewn pursestring | 14.6 ± 5.4 | 1 | 32 |
NA: not available.
The gastro-oesophageal anastomosis of the ILMIE was located at the azygos vein level, with 44% (11 of 25) of the anastomosis above the upper edge of the azygos vein, with the highest anastomosis done 4 cm higher than the azygos vein and close to the thoracic inlet. This indicates that constructing the gastric tube in the thoracic cavity does not affect the height of the anastomosis. All the cases had negative proximal oesophageal and cardia resection margins. Lymphadenectomy was conducted, and a mean of 14.6 ± 5.4 lymph nodes were sampled per case, which is within the range (11.4–54.8) previously reported for ILMIE in 2012–2013 [17–21]. These results indicate that our modified method satisfies the oncological requirements. The gastric tube resulting from our modified method had a diameter of 4–5 cm and was consistent with the method of abdominal gastric tube construction [17–23].
Constructing a gastric tube in the abdominal cavity has the main disadvantage of the risk of tube reversal when lifted to the thoracic cavity [3]. In 2013, Wang et al. [16] strongly suggested to not separate the gastric tube and the oesophagus after gastric tube construction in the abdominal cavity during McKeown MIE. The reasons he brought up were: (i) to prevent the gastric tube and the oesophagus from separating during lifting; (ii) to avoid gastric tube angulation because the gastric tube has uniformly distributed tension during lifting and (iii) to avoid intra-abdominal suturing and knotting to reduce the difficulty of operation. Our method has all these advantages. Furthermore, our method could reduce the possibility of gastric volvulus.
It is estimated that abdominal gastric tube construction and final removal of excess gastric tube need at least 4–5 linear stapler cartridges, according to previously described methods published in 2012–2013 [17–21]. Our approach used 2.4 ± 0.5 staple cartridges. Compared with gastric tube construction in the abdominal cavity, our method used fewer cartridges and the procedures are simplified.
In 2012, Zhang et al. [23] published a similar method, except that they placed the anvil and the stapler in the third or fourth intercostal space at the right posterior axillary line, which was, in the present study, in the eighth or the ninth intercostal spaces. With our approach, the stapler's direction of travel was then more parallel to the longitudinal axis of the oesophagus. In addition, this method facilitates oesophageal anastomosis in situ and the smooth progress of the stapler. This approach is not difficult for skilled thoracoscopic surgeons. In the first 10 cases, it took ∼30 min from intrathoracic anvil placement to anastomosis completion. The same process took ∼15 min in the later 15 cases. Importantly, this did not affect the progress of the surgery.
We performed conventional full-thickness suture of gastric tube cutting lines and anastomosis with 4–0 vicryl. Endoscopic suturing requires high technical skills, and this process usually takes 20–30 min. However, even if costly in time, this approach is worthwhile to reduce the fistula incidence [24]. In addition, in order to reduce the risk of anastomotic leak, we placed a 14-Fr porous drainage tube behind the anastomotic stoma and oesophageal bed. One case suffered from anastomotic leakage in this study, which was cured by the drainage tube. Because of placement of the drainage tube, it did not cause encapsulation of fluids, and the tube was removed after 28 days.
Our modified ILMIE approach ensured the resection of abdominal cavity lymph nodes. Indeed, during the laparoscopic phase, the fat tissue of the lesser gastric curvature was separated to the cardia, and the fat tissue around the cardia was resected. When the gastric tube was constructed in the thoracic cavity, the cardia and part of the lesser gastric curvature were resected, including the lymph nodes and fat tissues. In addition, the use of an articu-lating linear stapler was critical for the construction of the gastric tube in the thoracic cavity, to make the cut lines of the lesser gastric curvature parallel to the greater gastric curvature and thus to control the width of the gastric tube.
This study suffers from some limitations. First, the sample size was small, but comparable with most of previously published series (Table 4). Secondly, the follow-up was short, preventing us from observing the oncological outcomes of the procedure. In future studies, larger sample size and more comprehensive follow-up assessment are needed, and this modified approach will need to be compared with classical ILMIE and open surgery approaches.
Our ILMIE technique for gastric tube construction in the thoracic cavity had efficacy and safety profiles comparable with those of the classical abdominal approach in terms of operative time, blood loss, complications and mortality rates. This indicates that our approach is safe and feasible for the treatment of middle and lower oesophageal cancer. Furthermore, our modified ILMIE has apparently shorter operative time. Gastric tube construction in the thoracic cavity reduced the use of linear stapler and simplified surgical procedures.
Conflict of interest: none declared.
REFERENCES
- 1.Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31. doi: 10.1002/bjs.18003413304. [DOI] [PubMed] [Google Scholar]
- 2.Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc. 1999;13:293–7. doi: 10.1007/s004649900969. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Follette DM, Lemoine PH, Roberts PF, Goodnight JE., Jr Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2001;72:593–6. doi: 10.1016/s0003-4975(00)02261-x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TN, Hinojosa MW, Smith BR, Gray J, Reavis KM. Thoracoscopic construction of an intrathoracic esophagogastric anastomosis using a circular stapler: transoral placement of the anvil. Ann Thorac Surg. 2008;86:989–92. doi: 10.1016/j.athoracsur.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2010;89:S2159–62. doi: 10.1016/j.athoracsur.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, Jin K. Minimally invasive esophagectomy for esophageal cancer in the People's Republic of China: an overview. Onco Targets Ther. 2013;6:119–24. doi: 10.2147/OTT.S40667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncological outcomes to open techniques? A systematic review. J Gastrointest Surg. 2012;16:486–94. doi: 10.1007/s11605-011-1792-3. [DOI] [PubMed] [Google Scholar]
- 8.Sgourakis G, Gockel I, Radtke A, Musholt TJ, Timm S, Rink A, et al. Minimally invasive vs open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55:3031–40. doi: 10.1007/s10620-010-1153-1. [DOI] [PubMed] [Google Scholar]
- 9.Zingg U, McQuinn A, DiValentino D, Esterman AJ, Bessell JR, Thompson SK, et al. Minimally invasive vs open esophagectomy for patients with esophageal cancer. Ann Thorac Surg. 2009;87:911–9. doi: 10.1016/j.athoracsur.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Dolan JP, Kaur T, Diggs BS, Luna RA, Schipper PH, Tieu BH, et al. Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surg Endosc. 2013;27:4094–4103. doi: 10.1007/s00464-013-3066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwameis K, Ba-Ssalamah A, Wrba F, Birner P, Prager G, Hejna M, et al. The implementation of minimally-invasive esophagectomy does not impact short-term outcome in a high-volume center. Anticancer Res. 2013;33:2085–91. [PubMed] [Google Scholar]
- 12.Lee L, Sudarshan M, Li C, Latimer E, Fried GM, Mulder DS, et al. Cost-effectiveness of minimally invasive vs open esophagectomy for esophageal cancer. Ann Surg Oncol. doi: 10.1245/s10434-013-3103-6. 2013 Jul 10. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am. 2012;92:1265–85. doi: 10.1016/j.suc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Griffin SM, Shaw IH, Dresner SM. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management. J Am Coll Surg. 2002;194:285–97. doi: 10.1016/s1072-7515(01)01177-2. [DOI] [PubMed] [Google Scholar]
- 15.Ramage L, Deguara J, Davies A, Hamouda A, Tsigritis K, Forshaw M, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl. 2013;95:329–34. doi: 10.1308/003588413X13629960045751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang BY, Tsao LC, Cheng CY, Lin CH, Shih CS, Liu CC. Experiences with a simple laparoscopic gastric tube construction. J Cardiothorac Surg. 2013;8:14. doi: 10.1186/1749-8090-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sihag S, Wright CD, Wain JC, Gaissert HA, Lanuti M, Allan JS, et al. Comparison of perioperative outcomes following open vs minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg. 2012;42:430–7. doi: 10.1093/ejcts/ezs031. [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg. 2013;145:90–6. doi: 10.1016/j.jtcvs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Tapias LF, Morse CR. A preliminary experience with minimally invasive Ivor Lewis esophagectomy. Dis Esophagus. 2012;25:449–55. doi: 10.1111/j.1442-2050.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 20.Okabe H, Tanaka E, Tsunoda S, Obama K, Sakai Y. Intrathoracic esophagogastric anastomosis using a linear stapler following minimally invasive esophagectomy in the prone position. J Gastrointest Surg. 2013;17:397–402. doi: 10.1007/s11605-012-2009-0. [DOI] [PubMed] [Google Scholar]
- 21.Merritt RE. Initial experience of total thoracoscopic and laparoscopic Ivor Lewis esophagectomy. J Laparoendosc Adv Surg Tech A. 2012;22:214–9. doi: 10.1089/lap.2011.0429. [DOI] [PubMed] [Google Scholar]
- 22.Bizekis C, Kent MS, Luketich JD, Buenaventura PO, Landreneau RJ, Schuchert MJ, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2006;82:402–6. doi: 10.1016/j.athoracsur.2006.02.052. discussion 406–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang RQ, Xia WL, Kang NN, Ge W, Chen AG, Zhu KC. Pursestring stapled anastomotic technique for minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2012;94:2133–5. doi: 10.1016/j.athoracsur.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 24.Boone J, Rinkes IH, van Hillegersberg R. Gastric conduit staple line after esophagectomy: to oversew or not? J Thorac Cardiovasc Surg. 2006;132:1491–2. doi: 10.1016/j.jtcvs.2006.08.017. [DOI] [PubMed] [Google Scholar]