Abstract
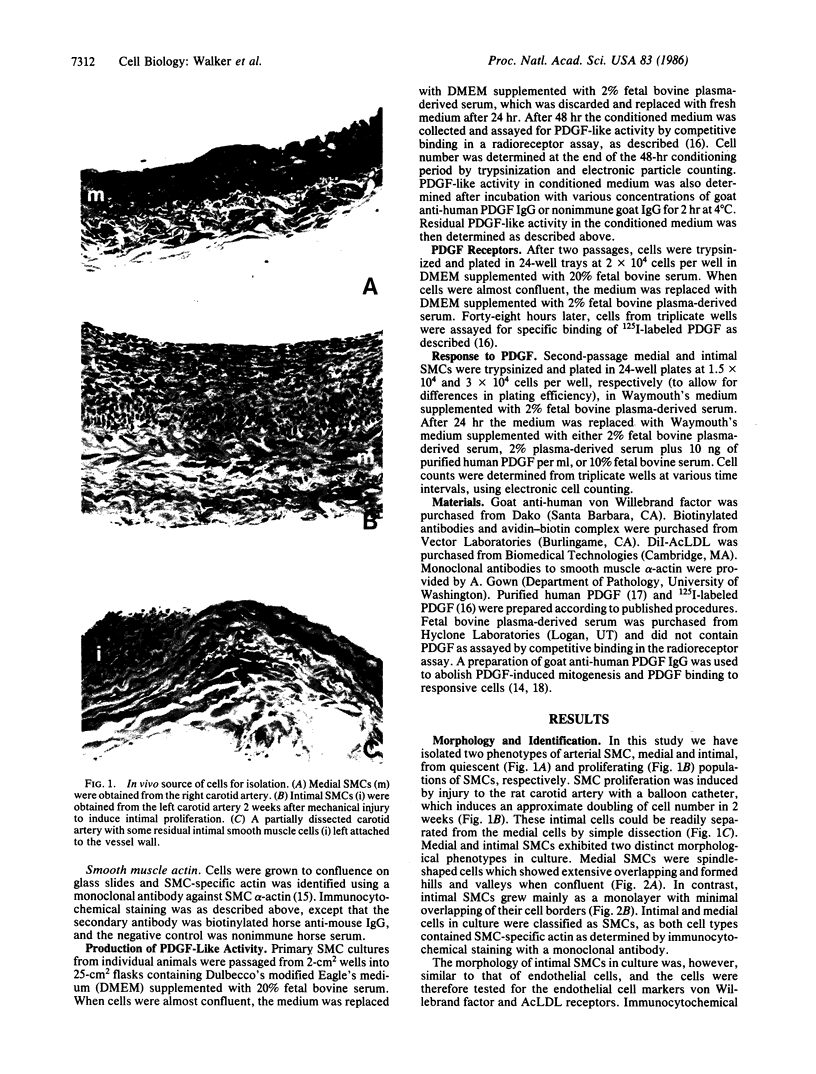
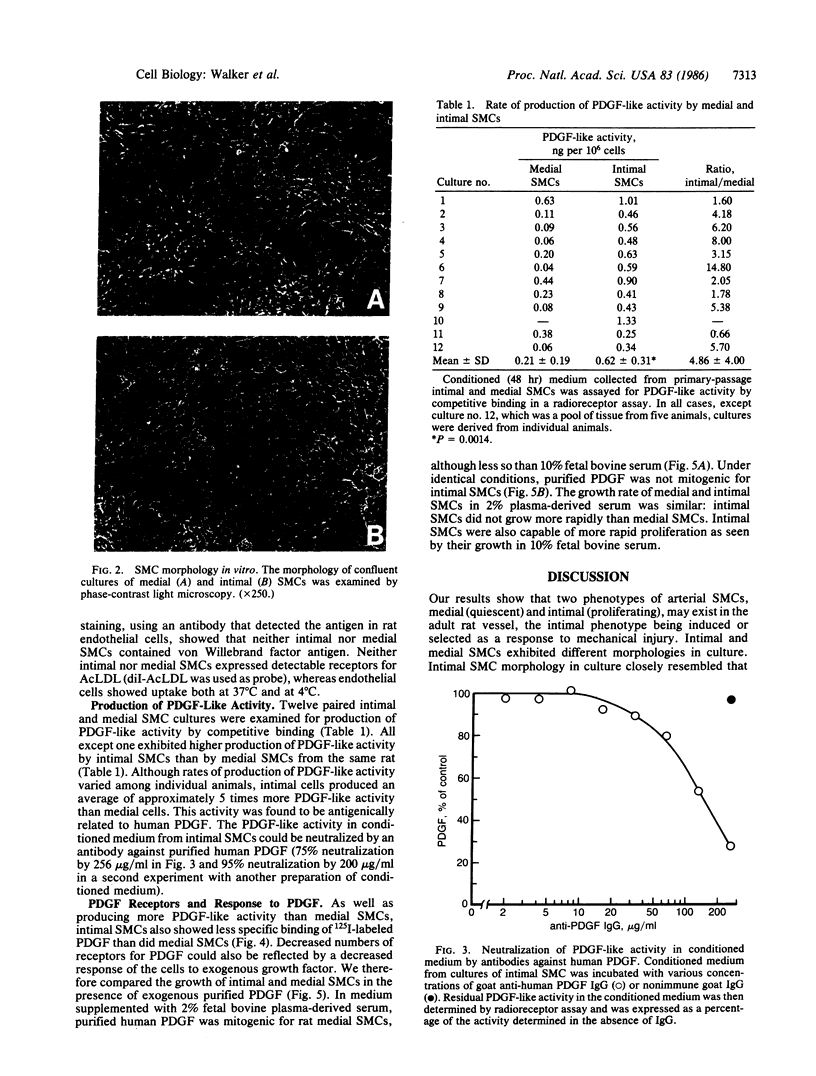
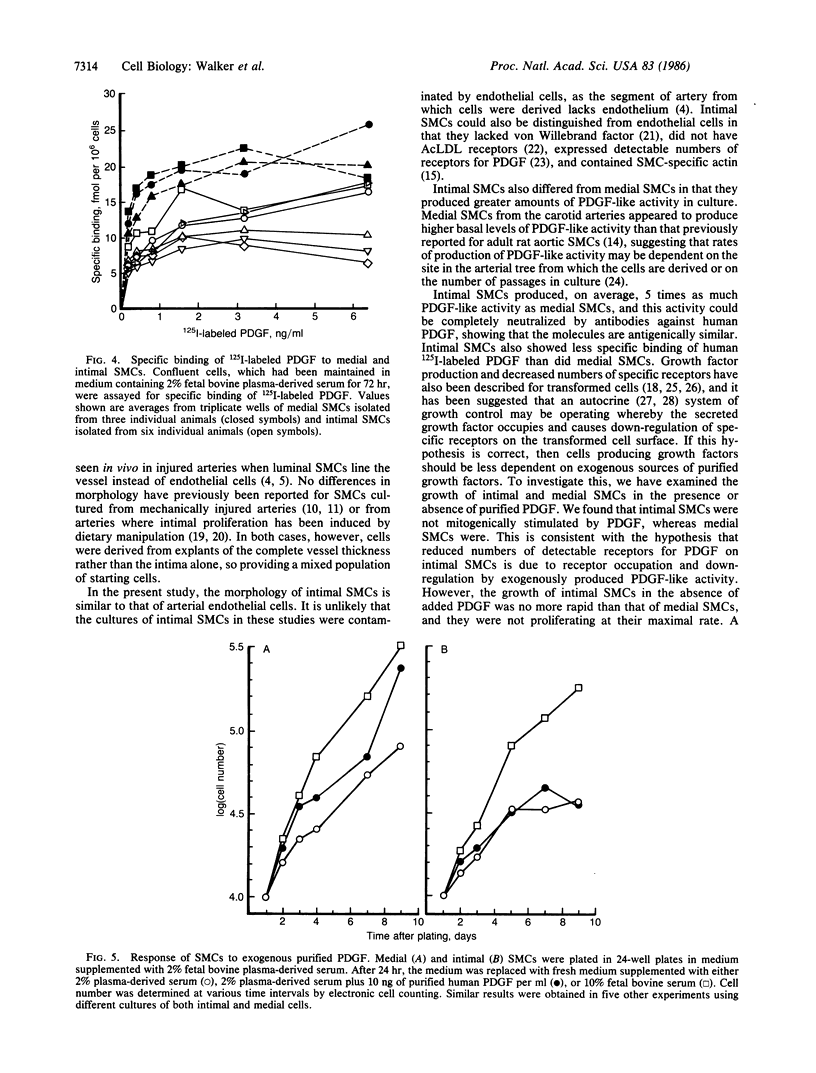
The migration and proliferation of smooth muscle cells (SMCs) within the intima of arteries following mechanical injury is thought to be initiated by vessel wall injury and release of growth factors, in particular the platelet-derived growth factor (PDGF). However, the mechanism by which SMC proliferation is regulated after platelet interaction with the vessel wall has ceased is unknown. Here we show that SMCs derived from the intima of injured rat arteries (intimal SMCs) are phenotypically distinct from SMCs from unmanipulated vessels (medial SMCs). Intimal SMCs secrete 5-fold greater amounts of PDGF-like activity into conditioned medium in culture, have fewer receptors for 125I-labeled PDGF, and are not mitogenically stimulated by exogenous purified PDGF. This study demonstrates that two SMC phenotypes can develop in the adult rat artery and suggests that SMC proliferation in vivo may be controlled, in part, by SMCs that produce PDGF-like molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry C. L., Looker T., Germain J. The growth and development of the rat aorta. I. Morphological aspects. J Anat. 1972 Oct;113(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Methods for studying the platelet-derived growth factor receptor. Methods Enzymol. 1985;109:69–100. doi: 10.1016/0076-6879(85)09078-4. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R. Interaction of circulating cell-derived and plasma growth factors in stimulating cultured smooth muscle cell replication. J Cell Physiol. 1984 Nov;121(2):425–430. doi: 10.1002/jcp.1041210222. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin S. G., Sybers H. D., Lester J. W., Navarro L. T., Gotto A. M., Jr, DeBakey M. E. Human smooth muscle cells cultured from atherosclerotic plaques and uninvolved vessel wall. In Vitro. 1981 Aug;17(8):713–718. doi: 10.1007/BF02628408. [DOI] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Cliff W. J. The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest. 1975 May;32(5):585–600. [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünwald J., Haudenschild C. C. Intimal injury in vivo activates vascular smooth muscle cell migration and explant outgrowth in vitro. Arteriosclerosis. 1984 May-Jun;4(3):183–188. doi: 10.1161/01.atv.4.3.183. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilä K., Nikkari T. Enhanced growth of smooth muscle cells from atherosclerotic rabbit aortas in culture. Atherosclerosis. 1980 Jun;36(2):241–248. doi: 10.1016/0021-9150(80)90232-4. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Reidy M. A., Silver M. Endothelial regeneration. VII. Lack of intimal proliferation after defined injury to rat aorta. Am J Pathol. 1985 Feb;118(2):173–177. [PMC free article] [PubMed] [Google Scholar]
- Rhee C. Y., Herz F., Spaet T. H. Accelerated culture of aortic smooth muscle cells. Thromb Res. 1977 Jul;11(1):90–94. doi: 10.1016/0049-3848(77)90072-x. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster W. S., Bishop S. P., Geer J. C. Experimental aortic intimal thickening. I. Morphology and source of intimal cells. Am J Pathol. 1974 Aug;76(2):245–264. [PMC free article] [PubMed] [Google Scholar]








