Abstract
Purpose/Background:
The sleeper stretch is a common intervention prescribed for individuals with decreased glenohumeral internal rotation. Passive glenohumeral internal rotation (GHIR) when quantified in sidelying has been suggested to be a more reliable measure as compared to measurments performed in supine with the humerus abducted to 908. Recently, the sidelying position has also been proposed as an evaluative measure to quantify GHIR. Minimal work however has described mean GHIR values in sidelying. Therefore, the objective of this study was to establish preliminary mean passive GHIR values in sidelying for a healthy, college‐age population. Secondary purposes were to ascertain if mean values were affected by upper extremity dominance or sex.
Methods:
Using a standardized protocol, passive GHIR was measured using a digital inclinometer on the dominant and non‐dominant shoulders of 60 healthy, college‐age participants (32 female [20.66±1.15 years, 170.70±14.38 cm, 63.34±7.51 kg] and 28 male [21.50±1.40 years, 183.81±13.17 cm, 90.04±17.91 kg]).
Results:
The sidelying passive GHIR grand mean for all participants bilaterally was 50.4 ± 11.78. Mean passive GHIR values on the non‐dominant side (52.7 ± 10.28) were greater than those on the dominant side (48.0 ± 12.58) (p = 0.002). There was no difference when GHIR values were compared by sex (p = 0.327) and a significant interaction between UE dominance and sex was not apparent (p = 0.693).
Conclusions:
In a healthy college age population, these preliminary data suggest GHIR values are statistically greater on the non‐dominant side and that sex does not significantly affect GHIR measures in a sidelying position.
Level of Evidence:
Level 3c
Keywords: Digital inclinometer, range of motion, shoulder internal rotation, sleeper stretch
INTRODUCTION
Shoulder injuries are common, costly and potentially debilitating.1 In fact, the shoulder is the third most common orthopedic reason for a patient to see a primary care physician.2 In a high school population, when injuries were categorized by body region, the shoulder had the highest mean cost per injury.3 Further, shoulder pain is a frequently cited reason for missing work.4 Hence, additional research geared at measures that may contribute to assessment of the shoulder complex are warranted.
While there are numerous causes of shoulder injuries, posterior glenohumeral joint tissue tightness is often implicated as a primary contributing factor to shoulder pain.5–9 Posterior glenohumeral joint tissue tightness is associated with several specific shoulder pathologies including, subacromial impingement syndrome,6,10–12 glenohumeral internal rotational deficit,9,10,12,13 and SLAP lesions.5,10,11 Given this, it is not surprising that posterior glenohumeral joint tissue stretching is a regularly prescribed intervention by sports medicine professionals for their patients and athletes. Though there are numerous ways by which the posterior glenohumeral joint tissues can be stretched, sidelying glenohumeral internal rotation (GHIR) is often recommended to address this impairment.14–18 In fact, when using a cadaver model, glenohumeral internal rotation with the arm in a flexed posture was able to stress the posterior glenohumeral capsule to a greater extent than when the shoulder was in an abducted position.18
Recently, sidelying GHIR was proposed as an evaluative measure.19 Lunden et al. suggested the sidelying position to be a more reliable measure of passive GHIR when compared to measurements in the standard supine position at 908 of shoulder abduction.20 Specifically, intra‐rater reliability as determined by the intra‐class correlation coefficient in the sidelying position was excellent and ranged from 0.96 to 0.97 compared to 0.85 to 0.90 in the supine position.20 Similarly, inter‐rater reliability in sidelying was good (0.88) while supine inter‐rater values were only reported as moderate (0.69).20 Other work investigating goniometric reliability of GHIR supports these findings.21 Riddle and colleagues identified that while intra‐tester reliability of GHIR was excellent (0.94), inter‐tester reliability was noted to be poor (0.55).21 The authors hypothesized that the poor inter‐rater reliability values may have been a function of the manner in which the therapists controlled scapular motion. In this regard, the sidelying position may be favorable as the assumed posture enhances passive scapular stability given the physical support provided by the exam table or plinth.
Collectively, sidelying GHIR appears not only to place a greater amount of strain on the posterior glenohumeral capsule,18 but may be more reliable when compared to the traditional measure taken in the supine position.20,21 Given the novelty of this measure however, the authors of the current study were only able to identify one study that provided descriptive statistics.20 Hence, further examination of GHIR values in a sidelying position is warranted. These data will provide a benchmark to which injured populations can be compared. The primary purpose of this study, therefore, was to establish mean values for GHIR in a sidelying position in a healthy, college‐age population. Secondary purposes were to determine if values differed between dominant and non‐dominant sides as well as between sexes. The authors hypothesized that in a healthy college‐age sample, non‐dominant sidelying GHIR values would be greater than dominant side values and female sidelying GHIR values would be greater than male values.
METHODS
Identical procedures were performed in a controlled university laboratory setting during a single 30‐minute session for each participant. A non‐experimental, descriptive design was utilized in order to calculate the mean values for GHIR as measured using the sidelying technique. Independent variables were the participant's sex and upper extremity dominance. Upper extremity dominance was defined as the preferred extremity with which the participant chose to throw a ball. The dependent variable was GHIR range of motion measured in the sidelying position. Prior to the initiation of data collection, this study was approved by the University's Institutional Review Board for the protection of human subjects.
Participants
A purposive sample of 60 healthy college‐aged students (Table 1) was recruited from the university community to participate in this study. Inclusion criteria required participants to be between the ages of 18‐25. Participants were excluded from participation in the study if within the last 12 months they had: 1) seen a physician for a shoulder injury, 2) worn a sling for a shoulder injury, 3) engaged in a rehabilitation program for their shoulder, or 4) participated in an organized sport that involved overhead throwing.
Table 1.
Descriptive characteristics of Subjects
| Male (n = 28) | Female (n = 32) | |
|---|---|---|
| Age (years) | 21.50 ± 1.40 | 20.66 ± 1.15 |
| Height (cm) | 183.81 ± 13.17 | 170.70 ± 14.38 |
| Mass (kg) | 90.04 ± 17.91 | 63.34 ± 7.51 |
| UE Dominance | L = 4; R = 24 | L = 2; R = 30 |
UE = Upper extremity; L = Left; R = Right
Procedures
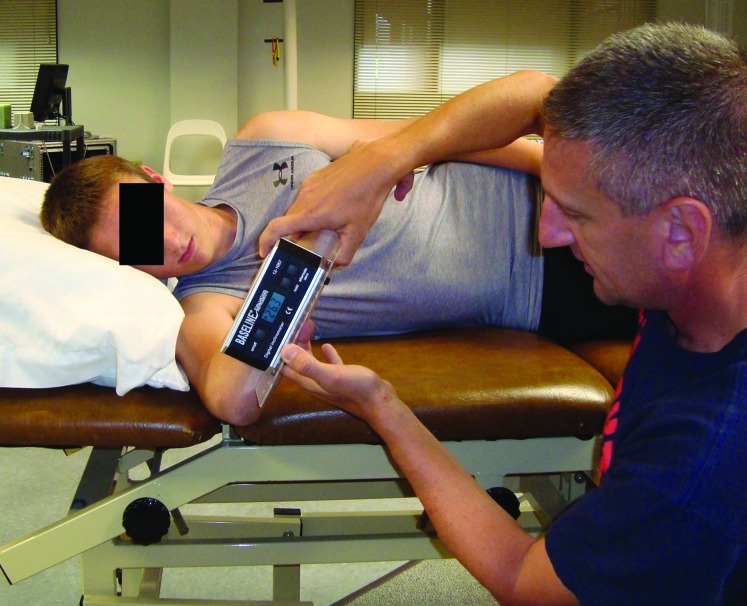
Upon arrival to the laboratory, inclusion and exclusion criteria were reviewed to ensure participants were eligible for the study. Once verified, all experimental details were explained, questions answered, and participants provided written informed consent. Then participants completed the Penn Shoulder Score. The Penn Shoulder Score is a reliable and valid instrument to quantify shoulder pain, satisfaction and function.22 The purpose of gathering the Penn Shoulder Score was to verify the status of the participant's shoulders. The participants then had their height and mass determined using a scientific grade medical beam balance scale (Jarden Corporation; Rye, NY). The participant then performed three active shoulder stretches by clasping their hands together and lifting their arms fully overhead in the sagittal plane. End range position was held for 10 seconds with a 30 second recovery between stretches. This form of ‘warm‐up’ mirrored the methodology previously described by other investigators.17 The participant was then asked to lie on a segmented treatment plinth on their right side. The right arm was placed into the groove between segments of the plinth and the thorax was aligned perpendicular to the plinth and to the right arm. The elbow was then positioned at 908 of flexion and the participant's forearm was palpated to locate the ulnar shaft. The bottom edge of a digital inclinometer (Baseline®, Fabrication Enterprises, White Plains, NY) augmented by a 0.3 cm thick sheet of plexiglass (SABIC Innovative Plastics, Pittsfield, MA) measuring 7 cm by 21 cm was aligned with the ulnar shaft. The digital inclinometer has been: 1) reported by the manufacturer to be accurate to within 0.18 and 2) used in a similar fashion in other studies for quantifying shoulder kinematics23 including GHIR.16 The plexiglass attachment enhanced alignment of the digital inclinometer along the ulnar shaft (Figure 1). The participant's humerus was then passively internally rotated by grasping the participant's distal forearm just proximal to their wrist. The angle between the forearm and the vertical was quantified with the digital inclinometer when glenohumeral motion ceased and just prior to the scapula tilting anteriorly, as monitored visually. Three trials of the aforementioned measure were completed bilaterally. A mean of these three trials was then used for data analysis.
Figure 1.
Passive glenohumeral internal rotation measurement set‐up.
Prior to initiation of the study, reliability of the investigator who performed all measurements was established through a pilot study. Both shoulders of five participants, who did not participate in the larger study, were quantified during two separate sessions using the described methods. The sessions were no less than 48‐hours and no greater than 72‐hours apart. To prevent investigator recall bias, the examiner was blinded to the measure. The intra‐class correlation coefficient (ICC [3,k]) and standard error of the measurement (SEM) were calculated to establish the reliability and precision of the measure. Intra‐rater reliability (ICC) and the SEM for sidelying GHIR by this investigator were 0.92 ± 3.58.
Statistical Analysis
Statistical analysis was performed with commercially available software (SPSS‐19, IBM, Armonk, NY). Descriptive statistics (mean; standard deviation) for sidelying passive glenohumeral internal rotation were compiled collectively for all participants thereby providing an overall mean but were also separately assessed as a function of the independent variables. To augment the descriptive data, a frequency distribution of GHIR values in 108 increments for the dominant and non‐dominant sides was created. A single repeated measures analysis of variance with one within (upper extremity dominance) at two levels (dominant; non‐dominant) and one between factor (sex) at two levels (male; female) was used to assess the dependent measure (sidelying GHIR). Alpha levels for all statistical tests were set a priori at p ≤ .05.
RESULTS
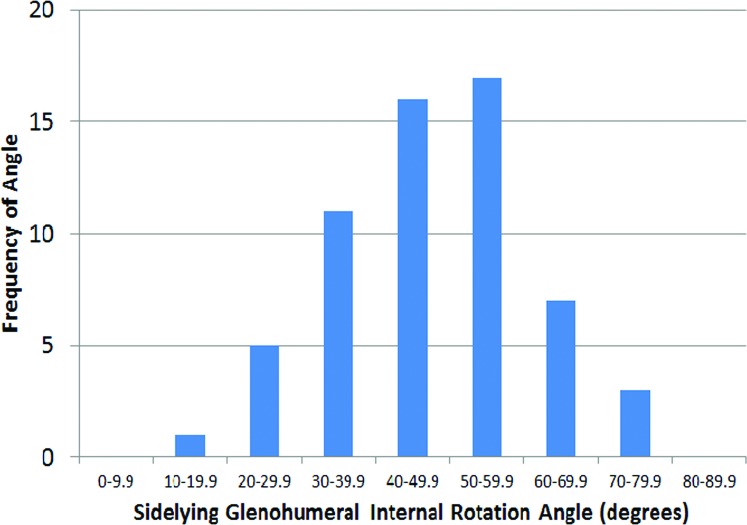
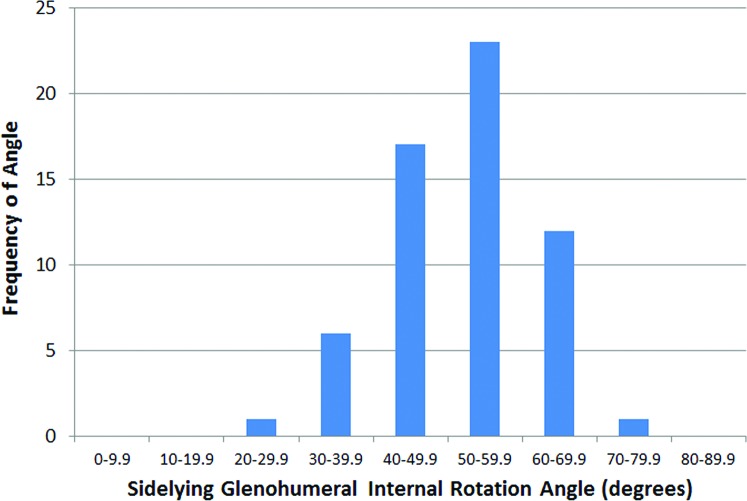
The mean Penn Shoulder Score for participants was 96.9 ± 6.5 out of a maximal score of 100, indicating little to no disability in the participants. The grand mean for sidelying passive GHIR (using bilateral measurements) was 50.4 ± 11.78. The range of passive GHIR measurements on the dominant side was 19.98‐74.08 (Figure 2) while the range on the non‐dominant side was 27.48‐79.08 (Figure 3). Forty (66%) and 52 (86.6%) of the 60 GHIR values were within a 29.98 increment (408‐69.98) on the dominant and non‐dominant side respectively. GHIR values on the non‐dominant side (mean 52.7 ± 10.28) were greater than those on the dominant side (48.0 ± 12.58) (p = 0.002). There was no difference when GHIR mean values (male = 48.9 ± 10.08; female = 51.6 ± 10.18) were compared by sex (p = 0.327; η2 = 0.017). Likewise, the interaction between upper extremity dominance and sex was not significant (p = 0.693).
Figure 2.
Passive glenohumeral internal rotation measurements, dominant side. Mean 48.0 +/‐ 12.5 degrees. Range 19.9 ‐ 74 degrees
Figure 3.
Passive glenohumeral internal rotation measurements, non‐dominant side. Mean 52.7 +/‐ 10.2 degrees; Range 27.4 ‐ 79 degrees”
DISCUSSION
The primary purpose of this study was to identify mean values of passive GHIR in a sidelying position for a healthy, college‐age population. Secondary purposes were to determine if mean values of passive GHIR differed between dominant and non‐dominant sides and also if these values were different between sexes.
The current study established a preliminary mean GHIR baseline of 50.4 ± 11.78 in a healthy collegiate population when measured in the described sidelying position. Utilization of Penn Shoulder Scores validated the participants had minimal to no complaints of shoulder pain, and were satisfied with their shoulder status and function. Given the contemporary use of the sidelying position as a measure for GHIR, the authors were only able to identify one study to which the data from the current study could be directly compared.20 Lunden et al reported sidelying GHIR on the dominant side in a group of 51 healthy participants. Dominant side mean GHIR was 88 greater in the current investigation when compared to the results of Lunden et al.20 While the results are comparable, the small discrepancy in values may be attributed to differences in the population sampled and potentially to the amount of overpressure that was used when recording the measure. Participants in the Lunden et al20 study were on average 8.5 years older than those in the current study. As individuals age, available range of motion at the glenohumeral joint has been shown to decrease.24,25 While an 8.5 year difference in mean subject age between studies is appreciable, it likely does not in of and by itself explain the 88 difference. It is possible the investigator in the current study applied a greater amount of passive overpressure when taking the GHIR measures. This however is only speculation and would have required the use of a hand held dynamometer or similar piece of instrumentation to verify this notion.
The current results support the stated hypothesis that sidelying GHIR values would be greater on the non‐dominant side. Previous investigations which quantified GHIR in supine with the shoulder abducted to 908 have also shown greater GHIR on the non‐dominant side.24,26 Barnes et al reported a mean difference of 11.58 in the general population while Hurd and colleagues reported a 15.08 difference in a collegiate throwing population. While these results parallel the current results, the mean differences reported by these investigators were greater than the 4.78 difference observed in the current study. Undoubtedly the 158 difference noted by Hurd et al. is a reflection of the throwing population studied. The literature clearly supports side‐to‐side differences in rotational range of motion in a throwing population.27–29 It is less clear why Barnes et al recorded a mean difference between dominant and non‐dominant sides just over twice of the value reported in the current study population. Participants who had been engaged in a throwing sport over the last year were consciously excluded from participation in the current study, while no such exclusion criteria were evident in the study by Barnes and colleagues. Similar to Hurd et al., the study by Barnes et al. may have included throwing athletes in their sample. It is also possible the magnitude of the difference is in part due to the dissimilarity between a supine and sidelying measure. Collectively however, these studies suggest that GHIR whether quantified in sidelying or in supine, greater values can be anticipated on the non‐dominant side.
The mean difference between the non‐dominant and the dominant sides noted in the current study, although statistically significant, may have limited clinical value as the intra‐rater SEM for a standard goniometer has been reported to be 48‐78.30 That being said, the difference between non‐dominant and dominant sides in the current study (4.78) was greater than the SEM (3.58) indicating a true difference in GHIR motion was present. Therefore if clinicians can improve the reliability of their goniometric measure, it is more likely true differences will be observed. To minimize measurement error and improve the reliability of standard clinical measures we encourage clinicians not only to consider the sidelying position in lieu of the supine position but also to use a standardized protocol such as the one described herein when goniometrically measuring passive GHIR in their patients or athletes.
The authors hypothesized that female sidelying passive GHIR values would be greater than male values in a healthy college‐age sample. While female internal rotation values were 2.78 greater than males, the difference between sexes was not significant. The authors believe that the lack of a significant difference between groups for this measure was a not a function of a limited sample size or Type II error. Rather, the small effect sizes as portrayed by the calculated eta squared value suggest that additional participants would have done little to change the result of this analysis. The literature is inconclusive with regard to whether sex influences glenohumeral range of motion. Some research has shown a sex difference with respect to glenohumeral range of motion,25,31 while other investigations have not.32 Additional studies are required to determine if a sex difference in shoulder range of motion is evident.
A wide range (19.9–79.08) of passive GHIR values in the sidelying position were observed in this study. The majority (66% of the values on the dominant side; 86% of the values on the non‐dominant side) of the passive GHIR values however were clustered around the mean. Unfortunately, there are very few studies which provide normative data to which our data can be compared. It is unclear what was unique about the participants who scored on either the low or high end of the spectrum. It is possible those with the highest and lowest GHIR scores exhibited characteristics associated with global hyper‐ and hypo‐mobility respectively. However, it is not possible to state this with certainty, as the present study did not assess the participants for these characteristics. Additional descriptive data collection would have been necessary to illuminate more concrete reasons to further explain these scores.
Like all studies, this study has some inherent limitations. The mean values reported in the current study are for a healthy, active, college‐age sample. As such, these results should not be generalized to populations of different ages, activity levels or those with shoulder pathologies. The authors did not objectively quantify the amount of overpressure that was used during the sidelying internal rotation measure. Rather, the investigator used what he perceived to be a capsular end feel and visually monitored the scapula ensuring it did not tilt anteriorly. Though an objective measure of overpressure would have provided additional control, the authors felt it was more clinically realistic performing this investigation with the employed methods. Lastly, the authors acknowledge that patients or athletes with a recent shoulder injury or surgical procedure may experience difficulty assuming a sidelying position such as that used in this study.
Although the current study results establish preliminary values in healthy, college‐aged participants, additional research to ascertain mean values for sidelying GHIR for various populations would be of value to clinicians. Specifically, mean values for the physically active individual across the lifespan, both healthy and injured, should be obtained with an emphasis on those engaged in overhead sports. Further, how sidelying GHIR measures are related to the ability of an individual to place their hand behind their back would be of interest. An exploration of the latter would shed light on how much variance in putting the hand behind the back is explained by sidelying GHIR. This information would assist the clinician with how much effort should be expended in restoring sidelying GHIR for those with a limited ability to place their hands behind their back.
CONCLUSION
This study examined passive GHIR in a sidelying position using a standardized protocol in a healthy, collegiate population. The results suggest a wide range of GHIR values may be encountered in the studied population. Passive GHIR was statistically greater on the dominant side although the measure was not influenced by the sex of the participant. The data serves as a benchmark to which other populations (e.g. athletic, injured) may be compared. The sidelying position may be a viable alternative to quantifying passive GHIR compared to the more traditional measure that is performed in a supine position. Further study in patient populations who participate in various types and levels of activity is warranted.
REFERENCES
- 1. Rekola KE, Keinänen‐Kiukaanniemi S, Takala J. Use of primary health services in sparsely populated country districts by patients with musculoskeletal symptoms: consultations with a physician. J Epidemiol Commun H. 1993;47(2):153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57(11):649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knowles SB, Marshall SW, Miller T, et al. Cost of injuries from a prospective cohort study of North Carolina high school athletes. Injury Prev. 2007;13(6):416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. House J, Mooradian A. Evaluation and management of shoulder pain in primary care clinics. Southern Med J. 2010;103(11):1129. [DOI] [PubMed] [Google Scholar]
- 5. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265–277 [DOI] [PubMed] [Google Scholar]
- 6. Harryman DT, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg [Br]. 1990;72‐A(9):1334–1343 [PubMed] [Google Scholar]
- 7. Lin J, Yang JL. Reliability and validity of shoulder tightness measurement in patients with stiff shoulders. Man Ther. 2006;11(2):146–152 [DOI] [PubMed] [Google Scholar]
- 8. Yang J, Chen S, Chang C, Lin J. Quantification of shoulder tightness and associated shoulder kinematics and functional deficits in patients with stiff shoulders. Man Ther. 2009;14(1):81–87 [DOI] [PubMed] [Google Scholar]
- 9. Myers JB. Glenohumeral Range of Motion Deficits and Posterior Shoulder Tightness in Throwers With Pathologic Internal Impingement. Am J Sports Med. 2005;34(3):385–391 [DOI] [PubMed] [Google Scholar]
- 10. Burkhart SS, Morgan CD, Kibler WB, others. The disabled throwing shoulder: spectrum of pathology Part I: pathoanatomy Digital inclinometer, range of motion, shoulder internal rotation, sleeper stretch and biomechanics. Arthroscopy. 2003;19(4):404–420 [DOI] [PubMed] [Google Scholar]
- 11. Grossman MG, Tibione JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: A possible etiology of superior labrum anterior‐to‐posterior lesions. J Bone Joint Surg [Br]. 2005;827:824–831 [DOI] [PubMed] [Google Scholar]
- 12. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668–673 [DOI] [PubMed] [Google Scholar]
- 13. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2009;38(1):114–119 [DOI] [PubMed] [Google Scholar]
- 14. Giugale JM, Jones‐Quaidoo SM, Diduch DR, Carson EW. Glenohumeral internal rotation deficit in overhead athletes part 3: stretching techniques. Hart JM, ed. Athletic Training & Sports Health Care. 2010;2(2):50–51 [Google Scholar]
- 15. Izumi T, Aoki M, Muraki T, Hidaka E, Miyamoto S. Stretching positions for the posterior capsule of the glenohumeral joint: strain measurement using cadaver specimens. Am J Sports Med. 2008;36(10):2014–2022 [DOI] [PubMed] [Google Scholar]
- 16. Laudner KG, Sipes RC, Wilson JT. The acute effects of sleeper stretches on shoulder range of motion. J Ath Train. 2008;43(4):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McClure P, Balaicuis J, Heiland D, et al. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Ortho Sport Phys Ther. 2007;37(3):108. [DOI] [PubMed] [Google Scholar]
- 18. Borstad JD, Dashottar A. Quantifying strain on posterior shoulder tissues during 5 simulated clinical tests: A cadaver study. J Ortho Sport Phys The. 2011;41(2):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyler TF, Roy T, Nicholas SJ, Gleim GW. Reliability and validity of a new method of measuring posterior shoulder tightness. J Ortho Sport Phys Ther. 1999;29(5):262–274 [DOI] [PubMed] [Google Scholar]
- 20. Lunden JB, Miffenbier M, Giveans MR, Cieminski CJ. Reliability of shoulder internal rotation passive range of motion measurements in the supine versus sidelying position. J Ortho Sport Phys Ther. 2010;40(9):589–594. 10.2519/jospt.2010.3197. [DOI] [PubMed] [Google Scholar]
- 21. Riddle DL, Rothstein JM, Lamb RL. Goniometric reliability in a clinical setting: shoulder measurements. Phys Ther. 1987;67(5):668–673 [DOI] [PubMed] [Google Scholar]
- 22. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR. The penn shoulder score: reliability and validity. J Ortho Sport Phys Ther. 2006;36(3):138–151 [DOI] [PubMed] [Google Scholar]
- 23. Johnson MP, McClure PW, Karduna AR. New method to assess scapular upward rotation in subjects with shoulder pathology. J Ortho Sport Phys Ther. 2001;31(2):81–89 [DOI] [PubMed] [Google Scholar]
- 24. Barnes CJ, Van Steyn SJ, Fischer RA. The effects of age, gender and shoulder dominance on range of motion at the shoulder. J Shoulder Elbow Surg. 2001;10(3):242–246 [DOI] [PubMed] [Google Scholar]
- 25. Clarke G, Willis L, Fish W, Nichols P. Preliminary studies in measuring range of motion in mormal and painful stiff shoulders. Rheumatol Rehabil. 1975;14(1):39–46 [DOI] [PubMed] [Google Scholar]
- 26. Hurd WJ, Kaplan KM, ElAttrache NS, Jobe FW, Morrey BF, Kaufman KR. A profile of glenohumeral internal and external rotation motion in the uninjured high school baseball pitcher, Part I: Motion. J Ath Train. 2011;46(3):282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609–613 [DOI] [PubMed] [Google Scholar]
- 28. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in major league baseball players. Am J Sports Med. 1988;16(6):577–585 [DOI] [PubMed] [Google Scholar]
- 29. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20–26 [DOI] [PubMed] [Google Scholar]
- 30. Muir SW, Corea CL, Beaupre L. Evaluating change in clinical status: reliability and measures of agreement for the assessment of glenohumeral range of motion. NAJSPT. 2010;5(3):98. [PMC free article] [PubMed] [Google Scholar]
- 31. Allander E, Björnsson OJ, Ólafsson Ó, Sigfússon N, Thorsteinsson J. Normal range of joint movements in shoulder, hip, wrist and thumb with special reference to side: A comparison between two populations. Int J Epidemiol. 1974;3(3):253–261 [DOI] [PubMed] [Google Scholar]
- 32. Kronberg M, Broström LÄ, Söderlund V. Retroversion of the humeral head in the normal shoulder and its relationship to the normal range of motion. Clin Orthop Relat Res. 1990;253:113–117 [PubMed] [Google Scholar]