Abstract
Context:
Athletic trainers recommend and use a multitude of rehydration (REHY) methods with their patients. The REHY modality that most effectively facilitates recovery is unknown.
Objective:
To compare 5 common REHY methods for thermoregulatory and stress hormone recovery after exercise dehydration (EXDE) in trained participants.
Design:
Randomized, cross-over, controlled study.
Patients or Other Participants:
Twelve physically active, non–heat-acclimatized men (age = 23 ± 4 years, height = 180 ± 6 cm, mass = 81.3 ± 3.7 kg, V̇o2max = 56.9 ± 4.4 mL·min−1·kg−1, body fat = 7.9% ± 3%) participated.
Intervention(s):
Participants completed 20-hour fluid restriction and 2-hour EXDE; they then received no fluid (NF) or REHY (half-normal saline) via ad libitum (AL), oral (OR), intravenous (IV), or combination IV and OR (IV + OR) routes for 30 minutes; and then were observed for another 30 minutes.
Main Outcome Measure(s):
Body mass, rectal temperature, 4-site mean weighted skin temperature, plasma stress hormone concentrations, and environmental symptoms questionnaire (ESQ) score.
Results:
Participants were hypohydrated (body mass −4.23% ± 0.22%) post-EXDE. Rectal temperature for the NF group was significantly greater than for the IV group (P = .023) at 30 minutes after beginning REHY (REHY30) and greater than OR, IV, and IV + OR (P ≤ .009) but not AL (P = .068) at REHY60. Mean weighted skin temperature during AL was less than during IV + OR at REHY5 (P = .019). The AL participants demonstrated increased plasma cortisol concentrations compared with IV + OR, independent of time (P = .015). No differences existed between catecholamine concentrations across treatments (P > .05). The ESQ score was increased at REHY60 for NF, AL, OR, and IV (P < .05) but not for IV + OR (P = .217). The NF ESQ score was greater than that of IV + OR at REHY60 (P = .012).
Conclusions:
Combination IV + OR REHY reduced body temperature to a greater degree than OR and AL REHY when compared with NF. Future studies addressing clinical implications are needed.
Key Words: heat stress, heat illness, hypohydration
Key Points
A combination of intravenous and oral fluid replacement after exercise dehydration reduced heat-related symptoms more than a single mode of rehydration.
If intravenous fluid replacement is warranted, the addition of simultaneous oral rehydration seems to add benefit to an efficient recovery.
The importance of rehydration (REHY) after exercise dehydration (EXDE) is emphasized by the many physiologic systems affected by hypohydration. Many of the adverse effects of hypohydration can impair recovery or hamper future exercise performance.1,2 The popular mantra surrounding REHY is that intravenous (IV) fluid is more rapid and advantageous to the athlete. Whether IV fluid administration is necessary in many instances can be questioned.3 Furthermore, it may not be ethical or legal in athletes without a significant medical reason.4
Previous researchers5–14 have identified that cardiovascular and thermoregulatory strain corresponds with greater body fluid losses. When blood volume is limited, evaporative sweat losses are impeded and overall heat dissipation decreases.5,9,14–17 After EXDE, if dehydration is lasting, body temperature decreases at a slower rate, and this can delay recovery.4 Cellular metabolism remains elevated in conjunction with high temperatures, further complicating recovery.15 A delay in exercise recovery could have deleterious implications, hindering the benefits of exercise sessions or increasing the amount and duration of cellular stress.15 Finish-line medical tent staffs treat many patients after endurance races (triathlons, marathons, ultramarathons) for EXDE.3 The recovery time for these patients is the limiting factor in the number of patients that a tent staff can treat at once and should be expedited when possible.3
Decreased water content and heat stress negatively affect cell function and integrity. Circulating catecholamine concentrations are typically used to quantify sympathetic stress.18–22 Previous researchers who compared modes of REHY offer a glimpse of cellular recovery. These studies18,19,21 demonstrate no appreciable differences between modes of REHY on cellular stress recovery. However, no previous authors have included trials during which participants consumed fluids as thirst dictated or combined oral (OR) and IV fluids.
Comparisons of modes of REHY demonstrate the importance of fluid replacement after EXDE.18,19,21,23,24 A lack of REHY delays recovery and profoundly decreases subsequent exercise performance.4 When equal volumes of fluid are provided, IV and OR fluid administration are essentially the same.19,21,23 No previous researchers have tested post-EXDE recovery facilitated by ad libitum (AL) consumption or a combination of IV and OR fluids. Therefore, the purpose of our study was to compare modes of REHY after heat stress and EXDE in recovery of body temperature and sympathetic stress hormone concentrations. We hypothesized that a combination trial, including simultaneous IV and OR fluids, would maximize the rate at which body temperature and hormone levels return to baseline.
METHODS
Research Design
We used a randomized, crossover, controlled comparison. All participants completed 5 trials consisting of baseline, EXDE, and REHY components. The only difference between trials was the mode of REHY, which occurred in random order. To establish scientific control, 1 trial included no fluid (NF) but mirrored data collection for all other trials. Other REHY trials consisted of (1) fluid REHY via AL consumption, in which participants were instructed to drink “as little or as much as they desired, according to thirst”; (2) metered OR REHY, which was quantified and separated into equal boluses provided at REHY0 and every 5 minutes throughout REHY (at 0, 5, 10, 15, 20, and 25 minutes); (3) IV REHY, or (4) a combination of IV and OR REHY, in which half of the fluid received was via IV and the other half was OR (IV + OR). Metered fluids were based on a goal of REHY back to −2% body mass.
Dependent recovery variables included rectal temperature (Trec), mean weighted skin temperature (Tmsk), stress hormone levels, and other blood and perceptual measures of stress and thermoregulatory strain. Measurements were recorded according to the experimental flow chart in Figure 1.
Figure 1.

Experimental flow chart. Urine values consist of urine color, specific gravity, and osmolality. Abbreviations: CATS, plasma concentration of norepinephrine, epinephrine, and dopamine; CORT, plasma cortisol concentration; ESQ, Environmental Symptoms Questionnaire; Tmsk, mean weighted skin temperature.
Participants
Twelve well-trained, non–heat-acclimatized men (age = 18–39 years) from the college community were recruited. The men were participating in a moderate fitness program involving a minimum of 8 hours of moderately intense exercise training per week. Screening information was obtained to ensure participants did not have (1) chronic health problems; (2) a previous history of heat illness within the past 3 years; (3) a history of cardiovascular, metabolic, or respiratory disease; (4) a history of consuming any muscle-building supplements within the past year; or (5) a history of smoking within the past 2 years. The participants were instructed to continue regular involvement in their training regimes in order to maintain cardiovascular fitness throughout the study. Physical characteristics of our participants were age = 23 ± 4 years, height = 180 ± 6 cm, mass = 81.3 ± 3.7 kg, V̇o2max = 56.9 ± 4.4 mL·min−1·kg−1, and body fat = 7.9% ± 3%. This study was approved by the University of Connecticut institutional review board, and all participants attended a prestudy briefing during which they had ample time to ask questions. This study was approved by the college institutional review board, and all participants signed a human subjects informed consent form. Participants were financially compensated.
Baseline Testing
Participants initially attended baseline testing and familiarization visits for anthropometric measurements and to ensure an understanding of the experimental protocol. Baseline skin fold calculated body fat percentage (3 site, Jackson and Pollock25) was collected by the same trained clinician. Maximal oxygen uptake (V̇o2max) testing was completed via an incremental run to exhaustion on a treadmill (ParvoMedics, Sandy, UT). The V̇o2max testing occurred in the heat (35°C, 40% relative humidity) to match experimental conditions. Participants also practiced exercising on the treadmill and cycle ergometer to verify exercise intensity for experimental trials as a percentage of V̇o2max and to familiarize themselves with the testing protocol. We chose to use multiple modalities (walking and cycling) for EXDE to prevent boredom and specific muscular fatigue. Euhydrated baseline body mass was collected by having participants report to the laboratory on 3 consecutive days after a 12-hour fast and consuming extra water the night before and morning of baseline measures.26 Euhydration was verified by urine specific gravity (USG) ≤1.020.1,2
Experimental Protocol
The fluid provided in the IV and IV + OR trial via IV was half-normal saline (0.45% NaCl), and the OR fluid for AL, OR, and IV + OR trials was the same with a noncaloric lemon flavoring (Supervalu Inc, Eden Prairie, MN) added. All REHY fluid was stored in the same insulated cooler in the laboratory (ambient temperature ∼22°C) outside the environmental chamber until REHY began to assure no difference in temperature among fluids. The EXDE/REHY trials were separated by at least 6 days to minimize fatigue, allow normal training to continue, and eliminate acclimation (average separation = 10 ± 5 days).
Experimental trials began with a baseline blood draw. Participants reported to the laboratory after a 12-hour overnight fast (no food or fluid, except water) and 24 hours without exercising, drinking alcohol, or consuming stimulants (including caffeine). To ensure euhydration, participants were asked to drink an extra 36 fluid ounces (1.06 L) of water the night before each baseline visit and 36 fluid ounces of water the morning of each visit before they arrived at the laboratory. Participants provided a small urine sample and voided their bladders, and researchers recorded body mass. Urine was assessed for color, USG, and osmolality via freezing-point depression (model 3250; Advanced Instruments, Norwood, MA). Participants then completed a baseline environmental symptoms questionnaire (ESQ) and thermal sensation scale. Beginning at 1 pm, participants did not consume fluids and were asked to eat low-moisture foods and refrain from ingesting stimulants (including caffeine). After the blood draw, participants left the laboratory and reported back the following day.
Upon arrival, participants were weighed and provided a urine sample to verify hydration state (with a goal of between −1% and −2% hypohydration). They ate a standard meal (bagel, banana, 3 g cream cheese; 490 kcal, 11 g fat, 86 g carbohydrate, 12 g protein, 356 mg Na+) provided by the researchers. We held meal consistent between trials by weighing the volitional amount consumed for trial 1 and weighing subsequent meals to assure the same amount of all food was consumed for the 5 trials. After breakfast, participants had 20-gauge Teflon cannulas inserted into both arms at the antecubital veins that were kept patent with normal saline and heparin (9:1).
Participants then entered the environmental chamber (model 2000; Minus Eleven, Inc, Malden, MA) for the remainder of the testing protocol. Environmental conditions for all trials averaged temperature of 35.5 ± 1.5°C, relative humidy of 33 ± 8%, and barometric pressure of 748 ± 6 mm Hg. After a 15-minute seated equilibration, pre-EXDE blood was drawn. For EXDE, participants alternated between walking on a treadmill at 5.6 to 6.8 km·h−1 with 5% to 10% grade (40%–60% V̇o2max), and cycling on an ergometer at a power output preset to elicit an output of 40% to 60% V̇o2max for 30-minute increments. Body mass was monitored at 30-minute increments. Participants were provided a small snack at EXDE90 (230 or 240 kilocalories with equal amounts of macronutrients, Na+, K+; Powerbar, Glendale, CA). Each participant was provided with the same weight and snack bar flavor in each trial after eating volitionally (up to the provided nutrition information) during the first trial. Participants walked or cycled for 120 minutes with the goal of decreasing total body mass by −4%.
Post-EXDE, participants remained seated and received 1 of 5 REHY methods (except in the NF trial) with a goal of −2% by reducing baseline body mass within 30 minutes. For 30 minutes post-REHY, the participant was observed (Figure 1). Sweat rates were calculated for the EXDE and REHY periods using standard calculations.1
Nutrition Analysis
Dietary records for food intake were completed by the participants for the 3 days preceding the EXDE experimental protocol. Participants were asked to match their diet for these days leading into each trial, and copies of previous records were provided for each trial. Dietary records were entered into nutrition analysis software (Nutritionist Pro, version 4.2.0; Axxya Systems, Stafford, TX) to analyze total caloric, carbohydrate, protein, and fat intake.
Body Temperature Measures
Participants' Trec was measured via flexible thermistors inserted 10 cm beyond the anal sphincter (Yellow Springs Instruments, Yellow Springs, OH). Temperature measures were recorded every 15 minutes during EXDE and every minute during REHY. Skin temperature measurements were recorded (Ototemp 3000; Exergen, Watertown, MA) in succession at the chest, forearm, thigh, and calf. Participants' Tmsk was then calculated using these data according to Ramanathan et al.27
Physiologic Measures
Blood was collected using tubes containing EDTA (Vacutainer; Becton Dickinson and Company, Franklin Lakes, NJ) and then centrifuged at 1500 rpm for 15 minutes for component separation. Plasma was then aliquoted into sealed plasma storage containers and frozen at −80°C until later analysis. Plasma cortisol concentrations ([CORT]) were quantified using a standard, commercially available enzyme-linked immunosorbent assay (ELISA) kit (Assay Designs, Inc, Ann Arbor, MI). Coefficient of variation for [CORT] analysis was 6.4%.
Standard sample extraction via commercially available methods (ESA, Inc, Chelmsford, MA), followed by high-performance liquid chromatography (HPLC) was used to quantify plasma concentrations of norepinephrine (NE), epinephrine (EPI), and dopamine (DOP). Data analysis after HPLC revealed coefficients of variation of 3.3% for NE, 4.6% for EPI, and 5.0% for DOP.
Perceptual Measures
A previously validated brief environmental symptoms questionnaire (ESQ) was used for perceptual heat stress and hypohydration symptom reporting. The ESQ was administered via laptop computer at the specified time points labeled in Figure 1. The thermal sensation scale was used to quantify perception of the surrounding environment. This scale includes whole and half numbers and verbal cues ranging from 0.0 (unbearably cold) to 9.0 (unbearably hot). The ESQ and thermal sensation scales were used to quantify subjective thermal strain throughout.
Statistical Analysis
We completed an a priori power analysis to establish an estimate of power and to project adequate sample size. Using data from previous studies,18,19,21,22 common dependent variables were considered: Trec and plasma [NE] during EXDE and REHY. A 2-sided test with α = .05 and desired power level of .80 was completed for both variables and the estimated adequate sample size was 9 or 10 participants. Given the findings of these 2 estimates, we recruited 12 participants to adequately power this study.
We used 5 × 5 (trial × time) repeated-measures analysis of variance to compare differences among trials and over time. A t test post hoc comparison with appropriate Bonferroni correction was conducted to detect differences within or among trials when trial × time interactions were present. Select variables were compared using the Pearson r correlation as well. The .05 level of significance was selected for identified differences. Data are presented as mean ± SD, unless otherwise noted.
RESULTS
Environmental conditions were not different among trials (P > .05). Nutritional analysis revealed no differences among trials in kilocalorie, fat, carbohydrate, protein, Na+, or K+ intake (P > .05). Exercise V̇o2 percentages for EXDE treadmill walking and ergometer cycling were not different among trials (P > .05).
Trial body mass measurements are presented in Table 1. Participants lost 4.32% ± 0.22% of their body mass through fluid restriction and EXDE and were rehydrated to −2.13% ± 0.47% body mass in the controlled OR, IV, and IV + OR trials. After the 60-minute REHY period, the NF trial hypohydration level remained constant (4.5% ± 1.2%).The NF group consumed less fluid than in all other trials (P > .05), but there were no statistical differences among other REHY trials (P < .05). Sweat rates were not different among trials for EXDE and averaged 0.96 ± 0.18 L·h−1 (P = .699). Rehydration sweat rates averaged 0.27 ± 0.12 L·h−1 and were not different among trials (P = .675). Urine color (3 ± 1 to 5 ± 1), USG (1.012 ± .006 to 1.024 ± .004), and urine osmolality (405 ± 200 to 889 ± 116 mOsm·L−1) increased from baseline to pre-EXDE (P < .001).
Table 1. .
Body Mass Measurements During Experimental Protocol (Mean ± SD)
| Trial |
Pretrial Body Mass, kg |
Post-EXDE Body Mass, kg |
REHY Fluid Consumed, L |
Post-REHY Body Mass, kg |
Post-REHY Body Mass Loss, % |
| No fluid | 81.2 ± 3.9 | 77.8 ± 3.9 | 0a | 77.6 ± 3.9a | 4.5 ± 1.2b |
| Ad libitum | 81.4 ± 3.8 | 77.9 ± 4.2 | 1.8 ± 0.5 | 79.5 ± 3.9 | 2.3 ± 1.3 |
| Oral | 81.8 ± 3.7 | 78.0 ± 4.0 | 1.8 ± 0.4 | 79.6 ± 3.9 | 2.7 ± 0.7 |
| Intravenous | 81.3 ± 3.9 | 77.9 ± 4.1 | 2.1 ± 0.5 | 79.8 ± 4.1 | 1.9 ± 1.0 |
| Intravenous + oral | 80.9 ± 4.0 | 77.5 ± 4.3 | 2.1 ± 0.4 | 79.5 ± 4.3 | 1.8 ± 0.9 |
Abbreviations: EXDE, exercise dehydration; REHY, rehydration.
No fluid significantly less than all other trials (P < .001).
No fluid significantly greater than all other trials (P < .001).
Body Temperature Measures
Participants' Trec was not different among trials during EXDE (P > .05). Participants' Trec responses during REHY are shown in Figure 2. Data for Tmsk during REHY are shown in Figure 3. Correlative data for Tmsk and Trec throughout REHY are presented in Table 2.
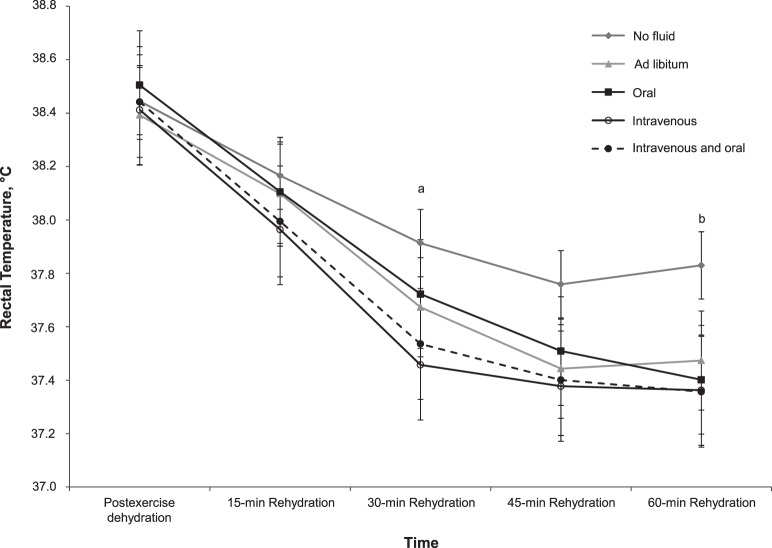
Figure 2.
Rectal temperature responses during rehydration. a No fluid was greater than intravenous (P = .02). b No fluid was greater than oral, intravenous, and intravenous + oral fluids (P ≤ .009) but not ad libitum (P = .07). Overall, independent of trial, rectal temperature was less than after exercise dehydration at all subsequent time points (P < .001).
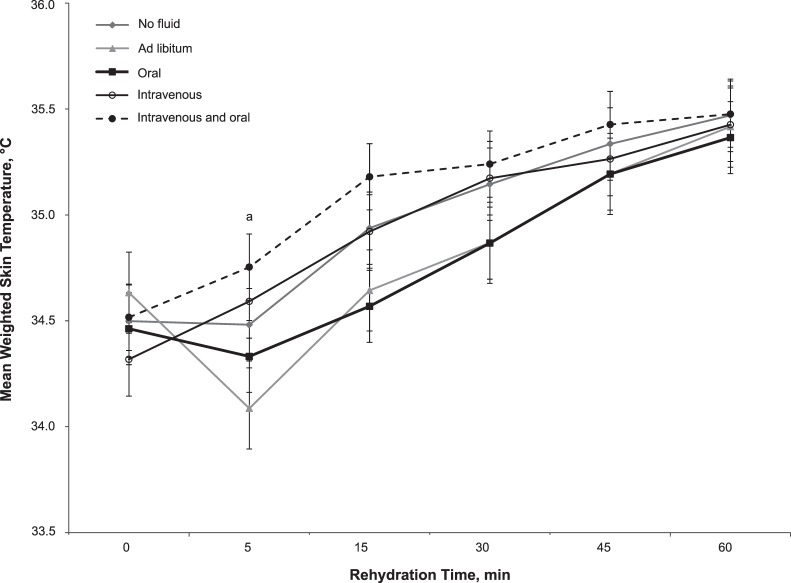
Figure 3.
Mean weighted skin temperature responses during rehydration. a Ad libitum was less than intravenous + oral fluids (P = .02).
Table 2. .
Relationship Between Mean Weighted Skin Temperature and Rectal Temperature According to Rehydration Method
| Trial |
r |
P |
| No fluid | −0.968 | .007 |
| Ad libitum | −0.886 | .046 |
| Oral | −0.955 | .011 |
| Intravenous | −0.974 | .005 |
| Oral + intravenous | −0.945 | .015 |
| Overall | −0.870 | <.001 |
Physiologic Measures
Plasma [CORT] responses during experimental trials are shown in Figure 4. Plasma [NE] demonstrated an increase over time, with post-EXDE greater than at all other time points (P ≤ .037). All REHY time points demonstrated elevated plasma [NE] compared with baseline and pre-EXDE for all trials (P ≤ .018). Plasma [EPI] and [DOP] did not show time or trial differences during our protocol (P ≥ .083).
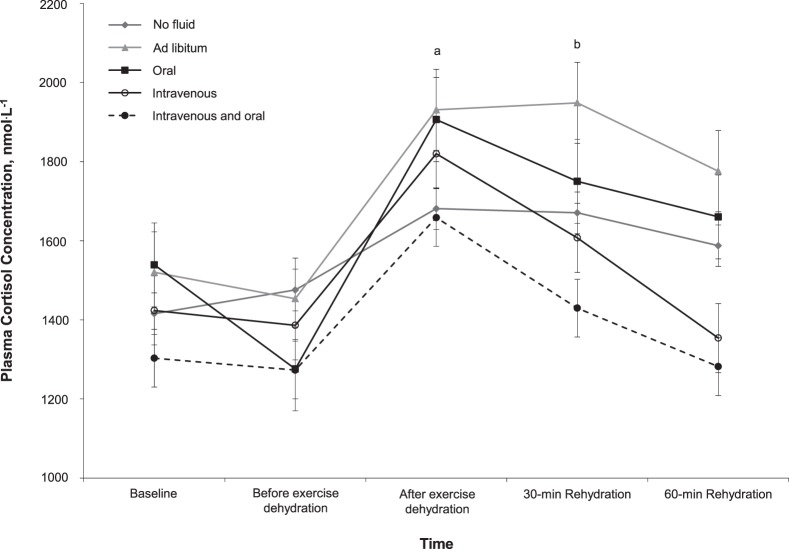
Figure 4.
Plasma cortisol concentration responses during experimental protocol. Ad libitum demonstrated increased plasma cortisol concentration compared with intravenous + oral fluids, independent of time (P = .02). a After-exercise dehydration was greater than baseline (P = .001), before-exercise dehydration (P = .001), and 60-min rehydration (P = .03). b The 30-min rehydration was greater than 60-min rehydration (P = .048).
Perceptual Measures
The ESQ score responses during the experimental protocol are shown in Figure 5. Thermal sensation demonstrated an increase, independent of trial, from pre- to post-EXDE (P = .011). During REHY, thermal sensation decreased over time, independent of trial (P < .05). Thermal sensation at 5 minutes after beginning REHY (REHY5) was greater than at REHY30 through REHY60, and at REHY15 was greater than at REHY60 (P < .05). No significant between-trials differences of thermal sensation during REHY were seen (P ≥ .324).
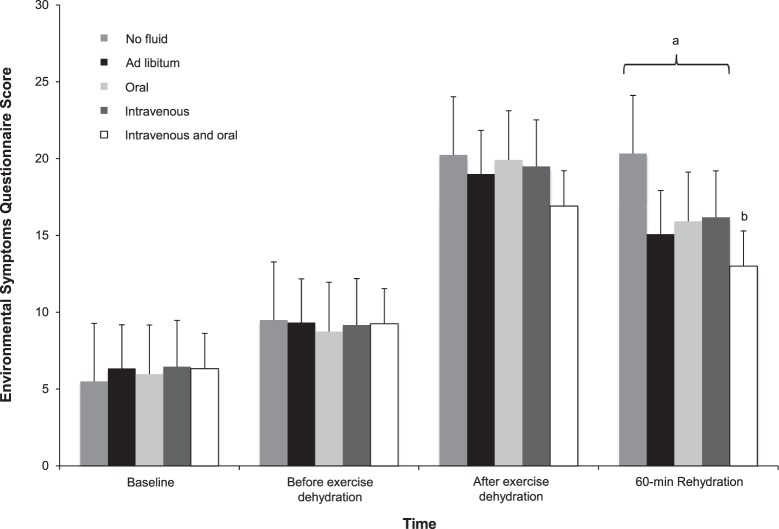
Figure 5.
Environmental symptoms questionnaire responses during experimental protocol. a All trials except intravenous + oral fluids (P = .22) demonstrated greater scores at 60-min rehydration compared with before-exercise dehydration (P < .05). b Intravenous + oral fluids were less than no fluid (P = .01).
DISCUSSION
We are the first to compare common REHY modes (AL, OR, IV, and IV + OR) and the first to focus on how these modes might be applied to acute medical recovery from EXDE and heat stress. Our participants lost more than 4% body mass and were partially rehydrated to −2% body mass loss within 30 minutes; both values represent common scenarios in competitive athletic settings. The most important finding from our study is that supplementing IV with OR REHY during recovery from thermal strain and EXDE gave a slight recovery advantage compared with IV or OR fluid alone.
A recent examination of previous IV versus OR studies concluded that neither mode offered greater benefits compared with the other.4 Oral REHY provides oropharyngeal stimulation, important for perceptual and some physiologic responses.28–30 Bypassing this reflex with IV REHY, however, eliminates the potential disadvantage of delayed gastric emptying or intestinal fluid absorption or discomfort.31–33 Direct comparisons are obviously contingent upon OR fluid tolerance, which can be impaired during significant dehydration.3 These instances require the use of IV fluids, which rapidly increase plasma volume without gastric filling.21,23 Although the suggestion had been presented, REHY approaches including a combination trial of IV + OR fluid had not been directly compared. We are the first to directly research this comparison and provide evidence for any potential, added benefit of IV + OR hydration.
Our study also helped evaluate the efficacy of AL in a comprehensive comparison among different REHY methods. Recently, due to an increase in exertional hyponatremia incidence, some experts34 recommended that athletes consume fluids according to their level of thirst, or AL instead of attempting to replace estimated sweat losses. Ad libitum fluid consumption most often prevents the overdrinking or fluid intoxication that athletes sometimes experience based on abnormal hydration strategies. However, athletes attempting to maximize performance during exercise should not consider dehydration advantageous.1,2,5,10,12 Previous research28,31 comparing metered and AL fluid consumption identified the popular phenomenon of “voluntary dehydration.” However, no investigators have compared physiologic recovery from EXDE with other REHY modes. Our results suggest that AL REHY may not effectively facilitate recovery to homeostasis after EXDE.
Body Temperature Measures
As expected, NF during recovery from EXDE demonstrated a physiologic thermoregulatory disadvantage. This finding provides further evidence to refute arguments that hypohydration does not affect body temperature.35,36 A novel finding was that AL was not statistically better at reducing thermoregulatory strain than NF, although clinical relevance could be questioned here. This is remarkable, however, considering that the amount of fluid consumed during AL was not different from that consumed during OR, which did reduce Trec more than NF. Consistent with previous researchers, we found minimal differences in Trec among OR, IV, and IV + OR throughout REHY. Previous authors18,19,21,23 identified similar marginal and relatively short-term differences among REHY methods. It seems that OR, AL, IV, and IV + OR REHY offer clinically equivalent advantages in facilitating thermoregulatory recovery after EXDE compared with NF.
The most efficient way for humans to dissipate heat is through sweat evaporation. This mechanism is maximized with increased skin blood flow, which transfers internal heat to the periphery for removal. Our results suggest a threshold-initiated oropharyngeal reflex inhibiting skin temperature increase. That is, we identified a graded response in decreased skin temperature with increased OR fluid consumption in acute REHY. At REHY5, IV + OR Tmsk increased in comparison with post-EXDE, whereas OR and AL decreased Tmsk temporarily. More OR fluid consumed initially during REHY was matched with a decreased skin temperature (Figure 3). Participants consumed the most fluid in the first 10 minutes of REHY during AL and exhibited an obvious, but slight, decrease in skin temperature. The OR trial showed a skin temperature decrease but to a lesser extent than AL, and this trial included less acute oropharyngeal stimulation. Our IV + OR trial showed a surprising increase in Tmsk despite OR fluid consumption. This demonstrates a potential threshold for oropharyngeal blunting of the skin temperature increase during recovery. A potential explanation for this response is a limited blood volume after EXDE and an immediate perfusion of gastric blood vessels in order to facilitate fluid absorption.9,13,17 The IV and IV + OR trials demonstrate a mechanism of direct increases in total blood volume, bypassing and potentially overcoming the oropharyngeal reflex.
The Tmsk responses demonstrated an inverse relationship with Trec. This correlation held constant regardless of the acute response of skin temperature, increase or decrease. This finding is consistent with previous results9,13,17 on thermoregulation and heat dissipation in similar environments. Heat dissipation is effectively facilitated via blood flow directed peripherally, increased peripheral temperatures, and sweat evaporation. Increased gastric filling during recovery seems to blunt this response when overall blood volume is limited.
Physiologic Measures
As a means of quantifying the amount of stress our participants underwent with our EXDE protocol, we measured stress hormone responses. We also used stress hormones to quantify the effect of REHY mode on stress recovery. Previous authors18,19 have found that NE and EPI show similar increases in response to sympathetic stress. Varying responses were identified with previous IV versus OR REHY comparisons.4 Catecholamine responses demonstrated similarities among trials, regardless of REHY method. This observation is similar to previous reports and summaries on IV versus OR REHY after EXDE.4,18,19,23 Significant stress was induced by our protocol; however, recovery was equivalent among modes of REHY.
Plasma [CORT] was increased post-EXDE, indicating that participants were under stress from the heat and EXDE. During REHY, IV + OR reduced plasma [CORT] by a greater amount than AL. This indicates a potential advantage in overall stress reduction for IV + OR above and beyond that of AL fluid consumption. The differences we identified were slight but may indicate future areas for study. A potential explanation for our results is that participants during AL consumed more fluid than in other trials within the first 10 minutes of REHY. Our data suggest this may actually increase sympathetic stress for a brief period of time before the return to homeostasis. Oral REHY did not demonstrate this increase, but the amount of fluid provided was metered and equal every 5 minutes. Previous researchers18,19,21 comparing IV versus OR REHY after EXDE showed no differences in plasma [CORT].
Perceptual Measures
Our participants demonstrated an increased ESQ score during EXDE. We found a difference, with IV + OR being less than NF at the end of REHY. This demonstrates accentuated perceptual recovery from EXDE with multiple REHY methods. No other trial offered a decreased ESQ score compared with NF. Concomitant REHY with IV + OR limited heat stress and dehydration symptoms better than other means of REHY.
Thermal sensation during EXDE increased and then decreased for all trials during REHY. Participants were no longer creating metabolic heat with muscular contractions during REHY and were rehydrated during 4 of the 5 trials, contributing to decreased thermal sensation. Previous authors24 did identify minor thermal sensation differences, with IV being greater than OR during subsequent exercise. However, others21 identified no differences between REHY modes. Interestingly, our lowest value was seen with IV + OR, followed by IV, although this was not significant. This suggests potential clinical relevance, as participants felt better with rapid IV + OR versus IV or OR alone.
Practical Implications
Body temperature recovery after EXDE is important for restoring homeostasis so that body systems can function normally. Rapid REHY using IV offers slight advantages over other modes, but these are short-term benefits. The benefits of REHY on the thermoregulatory system are seen with AL, OR, IV, and IV + OR REHY after EXDE, allowing the body to restore body temperature within minutes. The amount of stress on the body after EXDE seems to be minimized favorably by IV + OR beyond that of other methods. Interestingly, environmental symptoms are also attenuated with this method of REHY. Clinically, this study demonstrated a potential interplay of mechanisms contributing to recovery from EXDE, facilitated by oropharyngeal reflexes, coupled with rapid plasma volume expansion above and beyond that of a solitary mode of REHY.
CONCLUSIONS
The importance of REHY in acute recovery from EXDE should not be downplayed, as the potential benefits are profound and numerous. The mode of REHY during acute recovery had a limited effect. The few differences among methods of REHY were short lived and inadequate to alter recommended care after EXDE for athletes. However, if IV fluids are used for EXDE, OR REHY should be used to potentially accentuate benefits and more rapidly replace fluid deficits. Future research should be conducted to determine if the recovery benefits of IV and IV + OR offer ergogenic advantages compared with OR REHY.
REFERENCES
- 1.Casa DJ, Armstrong LE, Hillman SK, et al. National Athletic Trainers' Association position statement: fluid replacement for athletes. J Athl Train. 2000;35(2):212–224. [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine. Sawka MN, Burke LM, et al. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 3.Pyne S. Intravenous fluids post marathon: when and why? Sports Med. 2007;37(4–5):434–436. doi: 10.2165/00007256-200737040-00042. [DOI] [PubMed] [Google Scholar]
- 4.Casa DJ, Ganio MS, Lopez RM, McDermott BP, Armstrong LE, Maresh CM. Intravenous versus oral rehydration: physiological, performance and legal considerations. Curr Sports Med Rep. 2008;7(4):S41–S49. [Google Scholar]
- 5.Armstrong LE, Maresh CM, Gabaree CV, et al. Thermal and circulatory responses during exercise: effects of hypohydration, dehydration, and water intake. J Appl Physiol. 1997;82(6):2028–2035. doi: 10.1152/jappl.1997.82.6.2028. [DOI] [PubMed] [Google Scholar]
- 6.Candas V, Libert JP, Brandenberger G, Sagot JC, Amoros C, Kahn JM. Hydration during exercise: effects on thermal and cardiovascular adjustment. Eur J Appl Physiol. 1986;55(2):113–122. doi: 10.1007/BF00714992. [DOI] [PubMed] [Google Scholar]
- 7.Claremont AD, Costill DL, Fink W, Van Handel P. Heat tolerance following diuretic induced dehydration. Med Sci Sports Exerc. 1976;8(4):239–243. [PubMed] [Google Scholar]
- 8.Ebert TR, Martin DT, Bullock N, et al. Influence of hydration status on thermoregulation and cycling hill climbing. Med Sci Sports Exerc. 2007;39(2):323–329. doi: 10.1249/01.mss.0000247000.86847.de. [DOI] [PubMed] [Google Scholar]
- 9.Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of blood volume on forearm venous volume and cardiac stroke volume during exercise. J Appl Physiol. 1983;55(3):884–890. doi: 10.1152/jappl.1983.55.3.884. [DOI] [PubMed] [Google Scholar]
- 10.Montain SJ, Coyle EF. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol. 1992;73(4):1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- 11.Nadel ER, Fortney SM, Wenger CB. Effect of hydration state on circulatory and thermal regulations. J Appl Physiol. 1980;49(4):715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- 12.Sawka MN, Coyle EF. Influence of body water and blood volume on thermoregulation and exercise performance in the heat. Exerc Sport Sci Rev. 1999;27:167–218. [PubMed] [Google Scholar]
- 13.Sawka MN, Gonzalez RR, Young AJ, Dennis RC, Valeri CR, Pandolf KB. Control of thermoregulatory sweating during exercise in the heat. Am J Physiol. 1989;257(2 pt 2):R311–R316. doi: 10.1152/ajpregu.1989.257.2.R311. [DOI] [PubMed] [Google Scholar]
- 14.Sawka MN, Young AJ, Francesconi RP, Muza SR, Pandolf KB. Thermoregulatory and blood responses during exercise at graded hypohydration levels. J Appl Physiol. 1985;59(5):1394–1401. doi: 10.1152/jappl.1985.59.5.1394. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine. LE Armstrong, Casa DJ, et al. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 16.Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol. 1981;51(6):1594–1600. doi: 10.1152/jappl.1981.51.6.1594. [DOI] [PubMed] [Google Scholar]
- 17.Nose H, Takamata A. Integrative regulations of body temperature and body fluid in humans exercising in a hot environment. Int J Biometeorol. 1997;40(1):42–49. doi: 10.1007/BF02439410. [DOI] [PubMed] [Google Scholar]
- 18.Casa DJ, Maresh CM, Armstrong LE, et al. Intravenous versus oral rehydration during a brief period: stress hormone responses to subsequent exhaustive exercise in the heat. Int J Sport Nutr Exerc Metab. 2000;10(4):361–374. doi: 10.1123/ijsnem.10.4.361. [DOI] [PubMed] [Google Scholar]
- 19.Castellani JW, Maresh CM, Armstrong LE, et al. Endocrine responses during exercise-heat stress: effects of prior isotonic and hypotonic intravenous rehydration. Eur J Appl Physiol. 1998;77(3):242–248. doi: 10.1007/s004210050328. [DOI] [PubMed] [Google Scholar]
- 20.Costill DL, Sparks KE. Rapid fluid replacement following thermal dehydration. J Appl Physiol. 1973;34(3):299–303. doi: 10.1152/jappl.1973.34.3.299. [DOI] [PubMed] [Google Scholar]
- 21.Kenefick RW, O'Moore KM, Mahood NV, Castellani JW. Rapid IV versus oral rehydration: responses to subsequent exercise heat stress. Med Sci Sports Exerc. 2006;38(12):2125–2131. doi: 10.1249/01.mss.0000235358.39555.80. [DOI] [PubMed] [Google Scholar]
- 22.Maresh CM, Whittlesey MJ, Armstrong LE, et al. Effect of hydration state on testosterone and cortisol responses to training-intensity exercise in collegiate runners. Int J Sports Med. 2006;27(10):765–770. doi: 10.1055/s-2005-872932. [DOI] [PubMed] [Google Scholar]
- 23.Casa DJ, Maresh CM, Armstrong LE, et al. Intravenous versus oral rehydration during a brief period: responses to subsequent exercise in the heat. Med Sci Sports Exerc. 2000;32(1):124–133. doi: 10.1097/00005768-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Maresh CM, Gabaree-Boulant CL, Armstrong LE, et al. Effect of hydration status on thirst, drinking, and related hormonal responses during low-intensity exercise in the heat. J Appl Physiol. 2004;97(1):39–44. doi: 10.1152/japplphysiol.00956.2003. [DOI] [PubMed] [Google Scholar]
- 25.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 26.Baker LB, Lang JA, Kenney WL. Change in body mass accurately and reliably predicts change in body water after endurance exercise. Eur J Appl Physiol. 2009;105(6):959–967. doi: 10.1007/s00421-009-0982-0. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19(3):531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 28.Greenleaf JE, Sargent F., II Voluntary dehydration in man. J Appl Physiol. 1971;20(4):719–724. doi: 10.1152/jappl.1965.20.4.719. [DOI] [PubMed] [Google Scholar]
- 29.Maresh CM, Herrara-Soto A, Armstrong LE, et al. Perceptual responses in the heat after brief intravenous versus oral rehydration. Med Sci Sports Exerc. 2001;33(6):1039–1045. doi: 10.1097/00005768-200106000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Maughan RJ, Leiper JB, Shirreffs SM. Factors influencing the restoration of fluid and electrolyte balance after exercise in the heat. Br J Sports Med. 1997;31(3):175–182. doi: 10.1136/bjsm.31.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard RW, Sandick BL, Matthew WT, et al. Voluntary dehydration and allesthesia for water. J Appl Physiol. 1984;57(3):868–873. doi: 10.1152/jappl.1984.57.3.868. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JB, Grandjean PW, Pizza FX, Starling RD, Holtz RW. The effect of volume on rehydration and gastric emptying following exercise-induced dehydration. Med Sci Sports Exerc. 1994;26(9):1135–1143. [PubMed] [Google Scholar]
- 33.Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ. Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Med Sci Sports Exerc. 1996;28(10):1260–1271. doi: 10.1097/00005768-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Noakes TD. Hydration in the marathon: using thirst to gauge safe fluid replacement. Sports Med. 2007;37(4–5):463–466. doi: 10.2165/00007256-200737040-00050. [DOI] [PubMed] [Google Scholar]
- 35.Godek SF, Bartolozzi AR, Godek JJ. Sweat rate and fluid turnover in American football players compared with runners in a hot and humid environment. Br J Sports Med. 2005;39(4):205–211. doi: 10.1136/bjsm.2004.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laursen PB, Suriano R, Quod MJ, et al. Core temperature and hydration status during an Ironman triathlon. Br J Sports Med. 2006;40(4):320–325. doi: 10.1136/bjsm.2005.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]