Abstract
Context:
Adding sodium (Na+) to drinks improves rehydration and ad libitum fluid consumption. Clinicians (∼25%) use pickle juice (PJ) to treat cramping. Scientists warn against PJ ingestion, fearing it will cause rapid plasma volume restoration and thereby decrease thirst and delay rehydration. Advice about drinking PJ has been developed but never tested.
Objective:
To determine if drinking small volumes of PJ, hypertonic saline (HS), or deionized water (DIW) affects ad libitum DIW ingestion, plasma variables, or perceptual indicators.
Design:
Crossover study.
Setting:
Laboratory.
Patients or Other Participants:
Fifteen, euhydrated (urine specific gravity ≤ 1.01) men (age = 22 ± 2 years, height = 178 ± 6 cm, mass = 82.9 ± 8.4 kg).
Intervention(s):
Participants completed 3 testing days (≥72 hours between days). After a 30-minute rest, a blood sample was collected. Participants completed 60 minutes of hard exercise (temperature = 36 ± 2°C, relative humidity = 16 ± 1%). Postexercise, they rested for 30 minutes; had a blood sample collected; rated thirst, fullness, and nausea; and ingested 83 ± 8 mL of PJ, HS, or DIW. They rated drink palatability (100-mm visual analog scale) and were allowed to drink DIW ad libitum for 60 minutes. Blood samples and thirst, fullness, and nausea ratings (100-mm visual analog scales) were collected at 15, 30, 45, and 60 minutes posttreatment drink ingestion.
Main Outcome Measure(s):
Ad libitum DIW volume, percentage change in plasma volume, plasma osmolality (OSMp,) plasma sodium concentration ([Na+]p), and thirst, fullness, nausea, and palatability ratings.
Results:
Participants consumed more DIW ad libitum after HS (708.03 ± 371.03 mL) than after DIW (532.99 ± 337.14 mL, P < .05). Ad libitum DIW ingested after PJ (700.35 ± 366.15 mL) was similar to that after HS and DIW (P > .05). Plasma sodium concentration, OSMp, percentage change in plasma volume, thirst, fullness, and nausea did not differ among treatment drinks over time (P > .05). Deionized water (73 ± 14 mm) was more palatable than HS (17 ± 13 mm) or PJ (26 ± 16 mm, P < .05).
Conclusions:
The rationale behind advice about drinking PJ is questionable. Participants drank more, not less, after PJ ingestion, and plasma variables and perceptual indicators were similar after PJ and DIW ingestion. Pickle juice did not inhibit short-term rehydration.
Key Words: acetic acid, electrolytes, rehydration, sodium, vinegar
Key Points
Drinking small volumes of pickle juice did not deter drinking or adversely affect some drinking factors.
Guidelines about drinking pickle juice are based on false assertions.
To ensure full rehydration, athletes should schedule their drinking rather than drink ad libitum.
Adding sodium (Na+) to drinks improves rehydration1,2 and ad libitum fluid consumption in hypohydrated individuals.3 Furthermore, drinks containing Na+ can more effectively restore plasma volume and plasma sodium concentration ([Na+]p) than drinks without Na+.4 To aid in rehydration and minimize electrolyte loss, the National Athletic Trainers' Association (NATA) recommends athletes replace 150% of their fluid losses and add 0.3 to 0.7 g of Na+ to every liter of the rehydration beverage.5 Some scientists6 and clinicians7 have experimented with atypical high [Na+] fluids (eg, chicken noodle soup and pickle juice).
Pickle juice, a salty ([Na+] ranging from 415.2 to 978.5 mmol·L−1), acidic brine8–10 has been advocated as a treatment for exercise-associated muscle cramps (EAMCs).7,11,12 In fact, 25% (92 of 370) of athletic trainers studied used or had used pickle juice as a treatment for EAMCs.12 Most athletic trainers who provide pickle juice to athletes to treat EAMC give small volumes (<200 mL).12 Although experimental data on pickle juice's effectiveness for treating EAMCs are limited,7 pickle juice has been shown to reduce electrically induced muscle-cramp duration in 3% hypohydrated males without altering [Na+]p.9
Some clinicians13 have expressed concern about athletes ingesting pickle juice because of its high Na+ content. They13 fear that drinking pickle juice will increase [Na+]p and cause a rapid plasma volume restoration, thereby decreasing thirst and delaying rehydration. This led to the creation of advice about drinking pickle juice13 and the recommendation that athletes drink according to a prescribed, rather than ad libitum, drinking schedule.14 However, these guidelines13 are theoretical, based on mathematical equations rather than scientific evidence, and fail to consider [Na+]p during rehydration. These fluid-ingestion estimates must therefore be considered “target volumes.” Data comparing actual ad libitum fluid ingestion after pickle-juice consumption with the drinking volume advised are nonexistent. Moreover, observations of ad libitum fluid ingestion after pickle-juice consumption are sparse.14 However, the experimental design prevents valid conclusions because these authors14 provided different fluids for participants to ingest ad libitum, tested fluids with different [Na+], and did not control the temperature of the fluids ingested. As a result, the validity of their14 ad libitum fluid-ingestion volumes is questionable because flavoring,15 [Na+],3 and temperature16 affect fluid ingestion.
Therefore, the purpose of our study was to answer the following question: Does ingesting small volumes (1 mL·kg−1 body mass) of pickle juice, hypertonic saline, or deionized water (DIW) immediately before ad libitum DIW consumption affect ad libitum fluid consumed; changes in plasma volume, [Na+]p; plasma osmolality (OSMp); perceptions of thirst, fullness, and nausea; or palatability in hypohydrated males? We hypothesized that ad libitum fluid ingestion, changes in plasma volume, [Na+]p, OSMp, and perceptual indicators (ie, thirst, fullness, and nausea) would be greater following pickle juice and hypertonic saline consumption than after DIW. We also hypothesized that palatability would be lower after pickle-juice or hypertonic saline ingestion than after DIW.
METHODS
Experimental Design
A 3 × 6 factorial, crossover, repeated-measures design guided data collection. The independent variables were treatment drink (pickle juice strained from whole kosher dill pickles, [Pinnacle Foods Corp, Cherry Hill, NJ], hypertonic saline, or DIW) and time (−105 and −0.5 minutes pre-ingestione and 15, 30, 45, and 60 minutes postingestion). The hypertonic saline solution contained a [Na+] similar to that of pickle juice and was added as a treatment drink in this study to allow us to determine if ingredients in pickle juice (eg, acetic acid, carbohydrates) other than its Na+ content affect ad libitum DIW ingestion. The dependent variables were volume of fluid consumed ad libitum (mL), hematocrit (%), hemoglobin concentration (g·dL−1), OSMp (mOsm·kg−1 H2O), and [Na+]p (mmol·L−1). Perceptual indicators were quantified using 100-mm visual analog scales (eg, 0 = no thirst, fullness, or nausea, 100 = extreme thirst, fullness, or nausea). Palatability of the treatment drinks was assessed using a single 100-mm visual analog scale (eg, 0 = unpalatable, 100 = palatable). Thirst and palatability were measured to test the hypothesis that pickle juice impedes thirst13 and is unpalatable.17 Nausea and fullness were measured to characterize gastrointestinal distress. Urine specific gravity was used to characterize hydration status before exercise; body mass measures pre-exercise and postexercise were used to determine percentage of hypohydration.
Participants
Sample size was estimated a priori from other scientists'6 ad libitum fluid-ingestion data. We estimated that 15 participants would be needed to achieve significance at an α level of .05 with 80% power. A convenience sample of 15 healthy men (age = 22 ± 2 years, height = 178 ± 6 cm, mass = 82.9 ± 8.4 kg) completed this study.
Volunteers were excluded if they (1) were female, (2) had a history of heat-related illness (eg, heat exhaustion, heat stroke, heat syncope) in the 6 months before data collection, (3) had a lower extremity injury or surgery in the 12 months before data collection, (4) self-reported a history of heart disease, asthma, diabetes, high blood pressure or cholesterol, (5) self-reported a history of chest pain, dizziness, fainting, blackouts, or unreasonable breathlessness during exercise, (6) had any food allergies, (7) smoked, (8) were not between the ages of 18 and 30 years, (9) did not meet current American College of Sports Medicine guidelines18 for physical activity, or (10) were heat acclimated.19 The study's procedures were approved by our university's institutional review board before data collection, and all participants provided written consent.
Procedures
Twenty-four hours before each testing session, participants were instructed to drink water consistently, avoid alcohol and caffeine, and refrain from strenuous exercise. They were instructed to fast for 12 hours before testing, drinking only water.
On each testing day, participants reported to a laboratory and voided their bladders completely. Urine was collected and analyzed with a refractometer (SUR-Ne; Atago USA Inc, Bellevue, WA) to ensure participants were euhydrated (urine specific gravity < 1.02).20 Each participant inserted a rectal thermistor (YSI; Advanced Instruments Inc, Norwood, MA) and attached a heart-rate monitor (Polar Electric Inc, Lake Success, NY). Next, a venous catheter was inserted into a superficial forearm vein. Each participant was weighed nude to the nearest tenth of a kilogram (DA-150, Denver Instrument, Bohemia, NY), and changed into sweatshirt and sweatpants. Next, he was seated and asked to minimize movement as much as possible for 30 minutes to allow for body fluid compartment equilibration.21
After equilibration, a 5-mL blood sample was collected (−105 minutes pre-ingestion). Participants then exercised in an environmental chamber (temperature = 36 ± 2°C, relative humidity = 16 ± 1%) at an intensity that kept their heart rate between 80% and 90% of their age-predicted maximal heart rate. This was considered hard exercise.22 They alternated between treadmill running and cycling on a standard cycle ergometer (Monark 818E, Stockholm, Sweden) every 15 minutes for 60 minutes. Exercise was terminated if rectal temperature exceeded 39°C, signs or symptoms of heat illness (eg, dizziness, nausea, confusion) arose, or the participant requested to stop. No fluid was administered during exercise. After exercise, participants biked at a self-selected pace for 5 minutes to cool down. After the cool down, they exited the environmental chamber, towel dried, voided their bladders completely, and were weighed nude. After changing into dry clothing, they were seated in a climate-controlled room (temperature = 21 ± 2°C), and instructed to minimize movement as much as possible for 30 minutes.
After this equilibration period, a 5-mL blood sample was collected (−0.5 minutes pre-ingestion). Participants rated their perceived thirst, nausea, and fullness by making marks on separate 100-mm visual analog scales. They then had 30 seconds to consume 1 mL·kg−1 body mass (pre-exercise body mass) of chilled (5 ± 1°C) pickle juice, hypertonic saline, or DIW. Immediately after consuming the treatment drink, participants rated the treatment drink's palatability. They were then given preweighed opaque water bottles containing room-temperature (21 ± 2°C) DIW and were instructed to drink as they pleased during the 60-minute rehydration period.
At 15, 30, 45, and 60 minutes posttreatment drink ingestion, 5-mL blood samples were collected, and participants rated their perceived thirst, fullness, and nausea. After the last blood sample, they were weighed nude, and the venous catheter, rectal thermistor, and heart-rate monitor were removed before they were excused. Participants were randomly assigned a treatment drink order on the first day of testing, and treatment drink order was counterbalanced a priori. Testing days were separated by at least 72 hours, and each participant was tested at approximately the same time of day.
To minimize bias, participants were told the purpose of the study was to determine the effect of the treatment drinks on core temperature, heart rate, and blood variables after exercise in the heat. To minimize visual analog scale bias, they were not allowed to see their prior visual analog scale ratings. They were told not to spray themselves with the DIW or spit during the rehydration period.
Blood Analysis
For each 5-mL blood sample, 1 mL of whole blood was used to measure hematocrit and hemoglobin concentration (in triplicate). The remaining 4 mL of blood was sealed in a 6-mL lithium heparin Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) and placed on ice until the final blood sample was drawn.
To determine hematocrit, blood was drawn into heparinized microcapillary tubes and centrifuged at 3000 rpm for 5 minutes and measured using a microcapillary reader (IEC 2201; Damon/IEC, Needham Heights, MA). The cyanomethemoglobin (6500; Eagle Diagnostics, Cedar Hill, TX) procedure was used to determine hemoglobin concentration. Changes in plasma volume were calculated from hemoglobin concentration and hematocrit measurements using the Dill and Costill equation.23
The remaining blood was centrifuged at 3000 rpm for 15 minutes at 3°C. Plasma was drawn off the packed red blood cells and analyzed in duplicate for OSMp using freezing-point depression osmometry (3D3; Advanced Instruments Inc, Norwood, MA). Plasma [Na+] was measured in duplicate with an ion-selective electrode system (model 16; NOVA Biomedical, Waltham, MA).
Treatment Drink Analysis
Sodium concentration, potassium concentration, chloride concentration, glucose concentration, pH, specific gravity, and osmolality of pickle juice, hypertonic saline, and DIW were analyzed in duplicate and averaged (Table 1). Electrolyte and glucose concentrations were measured with an ion-selective electrode analyzer. Drink pH was measured using a pH meter (AB 15; Fischer Scientific, Pittsburgh, PA). Specific gravity was measured using a refractometer. Osmolality was measured using freezing-point depression osmometry.
Table 1. .
Composition of Treatment Drinksa (Mean ± SD)
| Characteristic |
Treatment Drink |
||
| Pickle Juice |
Hypertonic Saline |
Deionized Water |
|
| OSM, mOsm·kg−1 H2O | 853 ± 3 | 727 ± 2 | 0 ± 0 |
| [Na+], mmol·L−1 | 395 ± 0 | 403 ± 4 | 0 ± 0 |
| [K+], mmol·L−1 | 30 ± 0 | 0 ± 0 | 0 ± 0 |
| [Cl−], mmol·L−1 | 305 ± 0 | 390 ± 0 | 0 ± 0 |
| [Glucose], mmol·L−1 | 28.3 ± 0.4 | 0 ± 0 | 0 ± 0 |
| [Glucose], g·dL−1 | 0.5 ± 0.1 | 0 ± 0 | 0 ± 0 |
| pH | 3.82 ± 0.01 | 5.89 ± 0.04 | 5.86 ± 0.11 |
| Specific gravity | 1.020 ± 0.0 | 1.012 ± 0.0 | 1.000 ± 0.0 |
Abbreviations: [Cl−], chloride concentration; [Glucose], glucose concentration; [K+], potassium concentration; [Na+], sodium concentration; OSM, osmolality.
Analyses done in duplicate.
Statistical Design
Means and standard deviations for all dependent variables were calculated and used for statistical analysis. Differences in ad libitum fluid ingested, plasma variables, and perceptual indicators of thirst, fullness, and nausea between treatment drinks and over time were analyzed with separate repeated-measures analyses of variance (NCSS 2007, Kaysville, UT). Geisser-Greenhouse adjustments to P values were made when sphericity was violated. When we calculated significant F values, we used Tukey-Kramer post hoc tests to identify differences between drinks at each time point. Significance was set at P < .05.
RESULTS
Participants were similarly euhydrated before exercise (F2,28 = 2.8, P = .08), were similarly hypohydrated at the end of exercise (F2,28 = 1.9, P = .17), and lost similar volumes of fluid before ingesting the treatment drink (F2,28 = 1.7, P = .20). Therefore, their hypohydration and fluid-lost data were combined (Table 2). Treatment-drink volume and Na+ ingested are also found in Table 2.
Table 2. .
Hydration Data, Treatment-Drink Volume and Na+ Consumed (Mean ± SD)
| Pickle Juice |
Hypertonic Saline |
Deionized Water |
|
| Pre-exercise Usg | 1.008 ± 0.004 | 1.007 ± 0.004 | 1.01 ± 0.004 |
| Volume of fluid lost, L | 1.65 ± 0.43 | 1.67 ± 0.37 | 1.51 ± 0.44 |
| Hypohydration, % | 1.99 ± 0.48 | 2.04 ± 0.52 | 1.83 ± 0.53 |
| Treatment drink volume, mL | 83 ± 9 | 83 ± 8 | 83 ± 8 |
| Na+ consumed, g | 0.75 ± 0.08 | 0.77 ± 008 | 0 ± 0 |
Abbreviations: Na+, sodium; Usg, urine specific gravity.
Ad Libitum Fluid Consumed
Participants ingested different volumes of DIW ad libitum based on the treatment drink consumed each testing day (F2,28 = 4.2, P = .03). They consumed more DIW ad libitum postingestion of hypertonic saline (708 ± 371 mL) than when DIW was the treatment drink (533 ± 337 mL, P < .05). They consumed similar volumes of DIW ad libitum during the pickle juice (700 ± 366 mL) and hypertonic saline trials (P > .05). Moreover, no difference was noted in the volume of DIW consumed ad libitum between pickle juice and DIW (P > .05).
Plasma Variables
No interaction between time and treatment drink (F10,140 = 1.7, P = .09; Figure 1), or drink effect occurred for [Na+]p (F2,28 = 2.1, P = .14). However, [Na+]p did change over time (F5,70 = 30.4, P < .001). Pre-exercise [Na+]p (−105 minutes) was lower than at all other times (P < .05). Plasma [Na+] was higher at −0.5 and 15 minutes than at 30, 45, and 60 minutes posttreatment drink ingestion (P < .05).
Figure 1. .
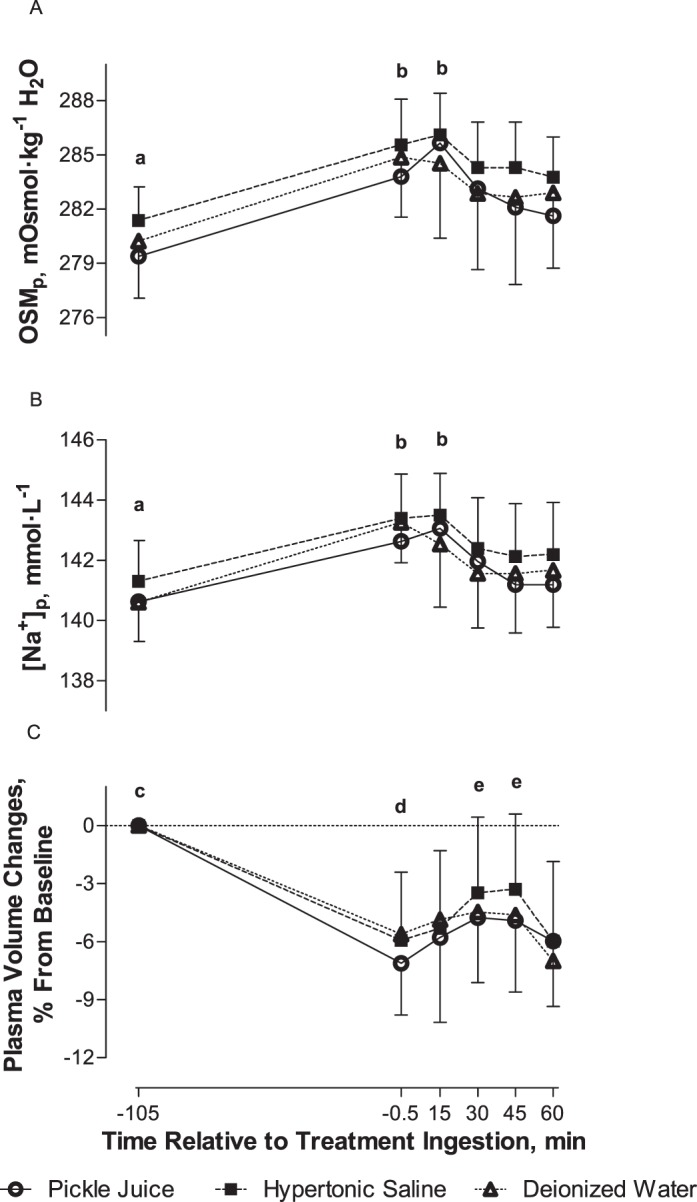
A, Plasma osmolality (OSMp), B, plasma sodium concentration ([Na+]p), and C, percentage change in plasma volume pre-ingestion and postingestion of pickle juice, hypertonic saline, or deionized water (mean ± SD). a Indicates −105 min < all other times. b Indicates −0.5 and 15 min > 30, 45, and 60 min. c Indicates −105 min > all other times. d Indicates −0.5 min < 30 and 45 min. e Indicates 30 and 45 min > 60 min. Significance set at P < .05 (n = 15).
Plasma osmolality did not differ among treatment drinks over time (F10,140 = 1.6, P = .18; Figure 1), or vary by drink (F2,28 = 1.8, P = .19). However, OSMp changed over time (F5,70 = 34.5, P < .001). Pre-exercise OSMp (−105 minutes) was lower than at all other times (P < .05). Plasma osmolality was higher at −0.5 and 15 minutes than at 30, 45, and 60 minutes posttreatment drink ingestion (P < .05).
Percentage change in plasma volume did not differ among treatment drinks over time (F10,140 = 0.7, P = .74; Figure 1) or vary among treatment drinks (F2,28 = 0.4, P = .65). However, a change in plasma volume occurred over time (F5,70 = 35.7, P < .001). Plasma volume decreased after 65 minutes of exercise and never returned to pre-exercise levels (P < .05). Changes in plasma volume were also smaller at −0.5 minutes than at 30 and 45 minutes posttreatment drink ingestion (P < .05). Changes in plasma volume were greater at 30 and 45 minutes posttreatment drink ingestion than at 60 minutes (P < .05).
Perceptual Indicators
Palatability differed among treatment drinks (F2,28 = 64.1, P < .001). Deionized water was more palatable (73 ± 14 mm) than hypertonic saline (17 ± 13 mm) or pickle juice (26 ± 16 mm, P < .05). Palatability did not differ between pickle juice and hypertonic saline (P > .05).
Thirst did not differ among treatment drinks over time (F8,112 = 1.13, P = .35; Figure 2) or vary among treatment drinks (F2,28 = 0.69, P = .51). However, thirst changed over time (F4,56 = 29.88, P < .001). Thirst was greater at −0.5 minutes than at all other times (P < .05). Thirst was also greater at 15 minutes than at 45 or 60 minutes posttreatment drink ingestion (P < .05).
Figure 2. .
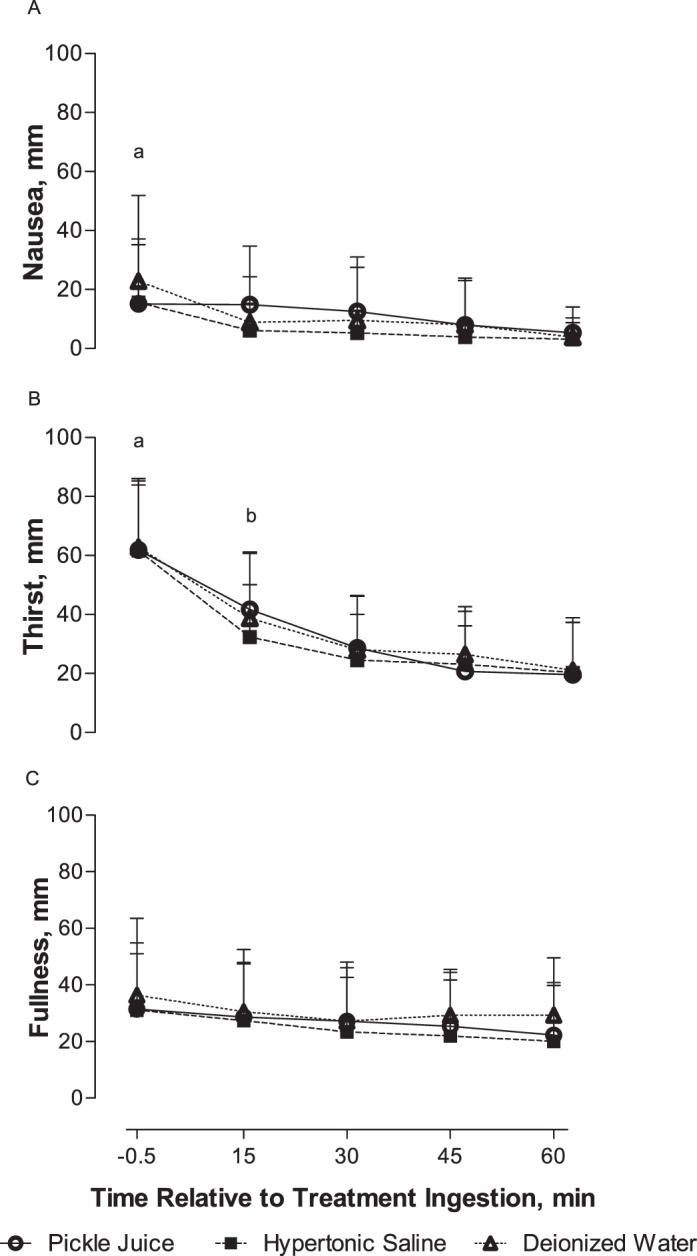
A, Nausea, B, thirst, and C, fullness over pre-ingestion and postingestion of pickle juice, hypertonic saline, or deionized water (mean ± SD). a Indicates −0.5 min > all other times. b Indicates 15 min > 45 and 60 min. Significance set at P < .05 (n = 15).
No interaction between treatment drinks and time (F8,112 = 1.19, P = .32; Figure 2) or drink effect occurred for nausea (F2,28 = 1.78, P = .18). However, a change in nausea occurred over time (F4,56 = 10.35, P = .001). Nausea was worse immediately before treatment-drink ingestion (−0.5 min) than at all other times (P < .05).
Fullness did not differ among treatment drinks over time (F8,112 = 0.45, P = .89; Figure 2), between drinks (F2,28 = 1.15, P = .33), or over time (F4,56 = 3.39, P = .052).
DISCUSSION
Our data do not support the concern that drinking a high-Na+ drink, such as pickle juice, increases [Na+]p or OSMp.13 We observed no differences in [Na+]p or OSMp among treatment drinks over 60 minutes postingestion. The fact that participants drank more DIW ad libitum postingestion of hypertonic saline (and likely pickle juice) cannot explain the lack of differences in [Na+]p or OSMp. Other scientists8,24 have observed similar results when only pickle juice was ingested. Miller et al8 gave euhydrated participants 1 mL·kg−1 body mass of pickle juice (∼86 mL) and monitored [Na+]p and OSMp over 60 minutes. No changes in [Na+]p or OSMp occurred at any time postingestion despite participants consuming 0.8 g of Na+.8 These observations are not unique to pickle-juice ingestion. Johannsen et al6 had participants ingest 1.4 g of Na+ by drinking chicken noodle soup ([Na+] = 166 mmol·L−1) and also observed no changes in [Na+]p or OSMp within 45 minutes of ingestion. Thus, clinicians need not worry about increasing OSMp or [Na+]p if athletes drink small volumes of pickle juice.
The fear that a rapid restoration of plasma volume occurs after pickle-juice ingestion13 is also unsupported. In the current study, drinking pickle juice did not cause a greater increase in plasma volume than DIW. In fact, most studies8,10 examining changes in plasma volume after pickle-juice ingestion indicate plasma volume decreases. Miller et al10 observed a 5% decrease in plasma volume when euhydrated participants ingested a large bolus of pickle juice (550 mL) and only a mild plasma volume expansion (<1.3%) when euhydrated participants ingested a small volume (86 mL).8 Johannsen et al6 also observed a small (∼2%) but insignificant decrease in plasma volume in euhydrated participants at 45 minutes postingestion of chicken noodle soup. Therefore, changes in plasma volume appear to depend on the volume of the high-Na+ drink consumed and the participant's hydration status. If small volumes of pickle juice are consumed, the effect on plasma volume is negligible.
Interestingly, a small (∼2.5%) decrease in plasma volume was observed 60 minutes after treatment-drink ingestion. This decrease may be the result of isotonic fluid shifting from the extracellular fluid space to the intracellular fluid space25 or increased urine production. In our study, plasma volume gradually increased over the first 45 minutes of the rehydration period. The resulting decrease in OSMp may have caused fluid to move into the intracellular fluid space,25 thus decreasing plasma volume. Anecdotally, our participants appeared to consume more DIW at the onset of the ad libitum rehydration period. Similarly, Nose et al3 witnessed 2% hypohydrated participants rapidly consumed water ad libitum in the first 30 minutes of its availability and slowed ad libitum consumption thereafter. Moreover, they3 observed a small (<1%) decrease in plasma volume between the 30- and 60-minute blood samples when participants rehydrated ad libitum with water. Therefore, if more DIW was ingested at the beginning of the rehydration period, most of the fluid would have emptied from the stomach within 30 minutes,10 urine production may have increased, and fluid would have been free to shift between body fluid compartments. Because the small (∼2.5%) decrease in plasma volume in our study was seen with all treatment drinks, it is unlikely to be a consequence of the content of the treatment drink.
Our observations also indicate that drinking pickle juice did not decrease thirst as speculated.13 Perceived thirst did not differ among treatment drinks at any point during the 60-minute rehydration period. Scientists26,27 have observed that the act of drinking, not a decrease in OSMp or [Na+]p, is responsible for the rapid satiety of thirst. Additionally, the Na+ content of the fluid consumed appears to have a minimal effect on thirst.28 Phillips et al28 infused ∼335 mL of a hypertonic (450 mmol·L−1) saline solution into participants' stomachs and had them rate their perceived thirst. Although an increase in thirst was observed immediately postinfusion, thirst had returned to pre-infusion levels within 5 minutes of ad libitum drinking. These changes occurred well before changes in OSMp or [Na+]p.28 Therefore, the volume of fluid ingested, rather than its content, affected perceived thirst.
The most prominent concern by some clinicians, that ad libitum drinking would be inhibited after pickle-juice ingestion,13 was also not supported. We observed that participants consumed more, not less, DIW ad libitum postingestion of small volumes of hypertonic saline than after DIW. A total of 8 mL more DIW was consumed after hypertonic saline ingestion than after pickle-juice ingestion, whereas 167 mL more DIW was consumed after pickle juice than after DIW. The difference in DIW consumed ad libitum between pickle juice and DIW was not statistically different, but this volume is clinically significant and shows that participants' drinking behaviors more resembled those of the hypertonic saline trial than the DIW trial. Authors24 observed that ingesting small volumes of pickle juice in fluid-restricted, 3% hypohydrated participants increased [Na+]p by 0.4 mmol·L−1. To return these participants' [Na+]p to a hypothetical normal (eg, 140 mmol·L−1), they would need to ingest 62 mL more DIW if they ingested pickle juice than if they drank nothing. The current investigation confirms that participants were capable of ingesting enough fluid ad libitum after pickle-juice ingestion to return their [Na+]p to normal. Our participants consumed more DIW ad libitum with the incorporation of these treatment drinks, yet they did not consume enough to meet the NATA's5 guidelines for fluid replacement. The NATA5 recommends replacing 150% of fluid losses and adding 0.3 to 0.7 g of Na+ to every liter of the rehydration beverage to offset Na+ lost via exercise-induced sweating. Our participants lost 1600 mL of fluid; therefore, to comply with these recommendations, they would have needed to consume between 1000 and 2500 mL of DIW ad libitum. Participants ingested 700 ± 366 mL after pickle juice, 708 ± 371 mL after hypertonic saline, and 533 ± 337 mL after DIW. As a result, they only replaced 43% of their losses when pickle juice and hypertonic saline were consumed and 33% when DIW was consumed. Thus, they were still 1.3 ± 0.7% hypohydrated at the end of the 60-minute rehydration period. Therefore, regardless of whether pickle juice is ingested, ad libitum rehydration is unlikely to fully rehydrate athletes within 60 minutes.
The greater volume of DIW consumed posthypertonic saline (and likely pickle-juice) ingestion was not due to an increase in thirst, [Na+]p, OSMp, or changes in plasma volume as previously discussed. Although Na+ affects ad libitum fluid ingestion,3,6 the Na+ content of the treatment drinks cannot fully explain why our participants drank more DIW ad libitum with pickle juice and hypertonic saline than with DIW. We hypothesize that participants drank more DIW because the palatability of pickle juice and hypertonic saline was low. Some clinicians13,17 have expressed concern over the palatability of pickle juice, and not surprisingly, participants favored DIW over pickle juice and hypertonic saline. Thus, they may have ingested more DIW to remove the unpleasant taste from their mouths. Anecdotally, they appeared to drink more DIW immediately after consumption of pickle juice and hypertonic saline than in the latter portions of the rehydration period. The effect of flavoring on ad libitum drinking is well established.15,16 After exercise, humans have an increased preference for sweet29 and salty30 drinks, whereas their preference for bitter-tasting drinks29 remains unchanged. Despite humans having an increased preference for Na+ after exercise,30 the mild fluid (and likely Na+) losses in the current study were likely not great enough to alter participants' perceived palatability of pickle juice.
Some scientists31 have suggested that pickle-juice ingestion may cause nausea. Participants complained of mild nausea and fullness during the rehydration period that was not exacerbated by any treatment drink. Miller et al10 observed mild (4 mm on a 100-mm scale, 100 being extremely nauseous) nausea when euhydrated participants ingested a large volume (∼550 mL) of pickle juice. Moreover, Phillips et al28 observed no differences in fullness after infusion of ∼335 mL of hypertonic (450 mmol·L−1) and isotonic (150 mmol·L−1) saline into the stomach. Thus, drinking small volumes of pickle juice postexercise does not cause significant feelings of nausea or fullness.
Finally, we acknowledge 3 limitations to our study. First, simulating “real-life” drinking situations in a laboratory environment is difficult. To ensure valid measures of [Na+]p, OSMp, and plasma volume, our participants were seated in the same position for 60 minutes with unlimited access to water. In athletic settings, the pace of play often dictates water breaks. Less fluid may be ingested in an athletic setting because athletes may have fewer opportunities to drink. Second, our rehydration period only lasted 60 minutes. Complete rehydration takes much longer (>12 hours) and should include food.20 Finally, our participants were given room-temperature water to drink. Participants prefer and drink more fluid ad libitum when cool fluids are provided postexercise.32 Therefore, providing cool fluids after pickle-juice ingestion may result in consumption of more fluid ad libitum. Future researchers may examine this assertion, the effect of pickle-juice ingestion before exercise on ad libitum drinking during and postexercise, and the effects of pickle-juice ingestion on rehydration for longer than 60 minutes.
In conclusion, consuming small volumes of pickle juice did not decrease ad libitum DIW ingestion. The concern of some clinicians13 that the high Na+ content of pickle juice would cause a rapid increase in [Na+]p, OSMp, and plasma volume and a subsequent decrease in thirst is also unsupported. Although less palatable than DIW, pickle juice did not exacerbate nausea or fullness. Pickle juice also did not deter drinking; however, total body fluid replacement did not occur within 60 minutes postexercise. Although advice on drinking pickle juice13 is based on false assertions, the advice itself will likely ensure that athletes rehydrate more completely. To ensure total rehydration, athletes should drink according to a schedule, regardless of whether pickle juice is consumed, and follow the NATA's recommendations5 for fluid replacement.
REFERENCES
- 1.Ray ML, Bryan MW, Ruden TM, Baier SM, Sharp RL, King DS. Effect of sodium in a rehydration beverage when consumed as a fluid or meal. J Appl Physiol. 1998;85(4):1329–1336. doi: 10.1152/jappl.1998.85.4.1329. [DOI] [PubMed] [Google Scholar]
- 2.Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ. Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Med Sci Sports Exerc. 1996;28(10):1260–1271. doi: 10.1097/00005768-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Nose H, Mack GW, Shi XR, Nadel ER. Role of osmolality and plasma volume during rehydration in humans. J Appl Physiol. 1988;65(1):325–331. doi: 10.1152/jappl.1988.65.1.325. [DOI] [PubMed] [Google Scholar]
- 4.Merson SJ, Maughan RJ, Shirreffs SM. Rehydration with drinks differing in sodium concentration and recovery from moderate exercise-induced hypohydration in man. Eur J Appl Physiol. 2008;103(5):585–594. doi: 10.1007/s00421-008-0748-0. [DOI] [PubMed] [Google Scholar]
- 5.Casa DJ, Armstrong LE, Hillman SK, et al. National Athletic Trainers' Association position statement: fluid replacement for athletes. J Athl Train. 2000;35(2):212–224. [PMC free article] [PubMed] [Google Scholar]
- 6.Johannsen NM, Lind E, King DS, Sharp RL. Effect of preexercise electrolyte ingestion on fluid balance in men and women. Med Sci Sports Exerc. 2009;41(11):2017–2025. doi: 10.1249/MSS.0b013e3181a82940. [DOI] [PubMed] [Google Scholar]
- 7.Williams RB, Conway DP. Treatment of acute muscle cramps with pickle juice: a case report [abstract] J Athl Train. 2000;35(suppl 2):S24. [Google Scholar]
- 8.Miller KC, Mack G, Knight KL. Electrolyte and plasma changes after ingestion of pickle juice, water, and a common carbohydrate-electrolyte solution. J Athl Train. 2009;44(5):454–461. doi: 10.4085/1062-6050-44.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KC, Mack GW, Knight KL, et al. Reflex inhibition of electrically induced muscle cramps in hypohydrated humans. Med Sci Sports Exerc. 2010;42(5):953–961. doi: 10.1249/MSS.0b013e3181c0647e. [DOI] [PubMed] [Google Scholar]
- 10.Miller KC, Mack GW, Knight KL. Gastric emptying after pickle-juice ingestion in rested, euhydrated humans. J Athl Train. 2010;45(6):601–608. doi: 10.4085/1062-6050-45.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams R. Those devilish cramps. Train Condition. 2000 Dec;:23–28. [Google Scholar]
- 12.Miller KC, Knight KL, Williams RB. Athletic trainers' perceptions of pickle juice's effects on exercise associated muscle cramps. Athl Ther Today. 2008;13(5):31–34. [Google Scholar]
- 13.Dale RB, Leaver-Dunn D, Bishop P. A compositional analysis of a common acetic acid solution with practical implications for ingestion. J Athl Train. 2003;38(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Dale RB, Bishop PA, Richardson MR, et al. Proceedings of the 14th International Congress of the World Confederation for Physical Therapy. Barcelona, Spain: The effect of oral acetic acid solution administration upon ad libitum water ingestion. June 7–12, 2003. [Google Scholar]
- 15.Wilk B, Bar-Or O. Effect of drink flavor and NaCl on voluntary drinking and hydration in boys exercising in the heat. J Appl Physiol. 1996;80(4):1112–1117. doi: 10.1152/jappl.1996.80.4.1112. [DOI] [PubMed] [Google Scholar]
- 16.Szlyk PC, Sils IV, Francesconi RP, Hubbard RW, Armstrong LE. Effects of water temperature and flavoring on voluntary dehydration in men. Physiol Behav. 1989;45(3):639–647. doi: 10.1016/0031-9384(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 17.Bergeron MF. Sodium: the forgotten nutrient. Gatorade Sports Sci Inst Sport Sci Exch. 2000;13(3):1–4. [Google Scholar]
- 18.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 19.Binkley HM, Beckett J, Casa DJ, Kleiner DM, Plummer PE. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37(3):329–343. [PMC free article] [PubMed] [Google Scholar]
- 20.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 21.Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414–417. doi: 10.1152/jappl.1978.45.3.414. [DOI] [PubMed] [Google Scholar]
- 22.Balady GJ, Chaitman B, Driscoll D, et al. AHA/ACSM joint position statement: recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Med Sci Sports Exerc. 1998;30(6):1009–1018. [PubMed] [Google Scholar]
- 23.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 24.Electrolyte Miller KC. and plasma responses after pickle juice, mustard, and deionized water ingestion in dehydrated humans. J Athl Train. doi: 10.4085/1062-6050-49.2.23. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawka MN, Coyle EF. Influence of body water and blood volume on thermoregulation and exercise performance in the heat. Exerc Sport Sci Rev. 1999;27:167–218. [PubMed] [Google Scholar]
- 26.Figaro MK, Mack GW. Regulation of fluid intake in dehydrated humans: role of oropharyngeal stimulation. Am J Physiol. 1997;272(6 pt 2):R1740–R1746. doi: 10.1152/ajpregu.1997.272.6.R1740. [DOI] [PubMed] [Google Scholar]
- 27.Maresh CM, Herrera-Soto JA, Armstrong LE, et al. Perceptual responses in the heat after brief intravenous versus oral rehydration. Med Sci Sports Exerc. 2001;33(6):1039–1045. doi: 10.1097/00005768-200106000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ. Osmotic thirst and vasopressin release in humans: a double-blind crossover study. Am J Physiol. 1985;248(6 pt 2):R645–R650. doi: 10.1152/ajpregu.1985.248.6.R645. [DOI] [PubMed] [Google Scholar]
- 29.Horio T, Kawamura Y. Influence of physical exercise on human preferences for various taste solutions. Chem Senses. 1998;23(4):417–421. doi: 10.1093/chemse/23.4.417. [DOI] [PubMed] [Google Scholar]
- 30.Leshem M, Abutbul A, Eilon R. Exercise increases the preference for salt in humans. Appetite. 1999;32(2):251–260. doi: 10.1006/appe.1999.0228. [DOI] [PubMed] [Google Scholar]
- 31.Burns J, Clarkson PM. Why don't athletes drink enough during exercise, and what can be done about it? Gatorade Sports Sci Inst Sport Sci Exch. 2001;12(1):1–4. [Google Scholar]
- 32.Hubbard RW, Sandick BL, Matthew WT, et al. Voluntary dehydration and alliesthesia for water. J Appl Physiol. 1984;57(3):868–873. doi: 10.1152/jappl.1984.57.3.868. [DOI] [PubMed] [Google Scholar]


