Abstract
We analyzed the effects of castration on epididymal white adipose tissue (WAT) in C57BL/6J mice which were fed a regular or high-fat diet. Fourteen days following surgical castration profound effects on WAT tissue such as reductions in WAT wet weight and WAT/body weight ratio, induction of lipolysis and morphologic changes characterized by smaller adipocytes, and increased stromal cell compartment were documented in both dietary groups. Castrated animals had decreased serum leptin levels independent of diet but diet-dependent decreases in serum adiponectin and resistin. The castrated high-fat group had dramatically lower serum triglyceride levels. Immunohistochemical analysis revealed higher staining for smooth muscle actin, macrophage marker Mac-3, and Cxcl5 in the castrated than in the control mice in both dietary groups. We also detected increased fatty-acid synthase expression in the stromal compartment of WAT in the regular-diet group. Castration also reduces the expression of androgen receptor in WAT in the regular-diet group. We conclude that that castration reduces tissue mass and affects biologic function of WAT in mice.
1. Introduction
White adipose tissue (WAT) is a loose connective tissue that is crucial in the regulation of whole-body fatty-acid homeostasis. WAT is composed of adipocytes and other cells found in the stromal-vascular fraction, including macrophages, fibroblasts, pericytes, blood cells, endothelial cells, poorly differentiated mesenchymal cells, and preadipocytes (Fruhbeck, 2008). This dynamic, multifunctional endocrine tissue can secrete a large number of biologically active molecules, collectively called adipokines, which include hormones, growth factors, enzymes, cytokines, complement factors, and matrix proteins. For most of these molecules, WAT also expresses receptors that mediate extensive cross-talk both locally and systemically in response to specific external stimuli or metabolic changes (Fruhbeck, 2008, Galic et al., 2010). There is also an increasing body of evidence that a specific fat depot, the epididymal fat pad, produces a locally acting factor responsible for maintaining spermatogenesis in rodents (Chu et al., 2010, Hansel, 2010).
Storage of excessive fatty acids in an expanded adipose tissue mass characterizes obesity, which has reached epidemic proportions in the United States, where 35.1% of adults are now classified as obese (Catenacci et al., 2009). This epidemic is especially problematic because obesity is closely associated with the development of insulin resistance in peripheral tissues, such as skeletal muscle, and in the liver; moreover, it is an independent risk factor for the development of type 2 diabetes mellitus as well as myocardial infarction, stroke, and certain cancers (Galic, Oakhill, 2010). In fact, a high body mass index (BMI) is associated with increased risk of several common and less-common malignances in a sex- and site-specific manner. An association has also been reported between obesity and metabolic syndrome and not only with increased risk for the development of cancer but also for the progression of certain types of cancer (Fair and Montgomery, 2009, Roberts et al., 2010).
Additionally, adipose tissue is a major site for inflammation because the visceral adipose tissue depot contains more macrophages and releases more inflammatory cytokines, such as monocyte chemotactin protein 1 (MCP1)/CCL2, plasminogen activator inhibitor 1, and interleukin (IL) 6, IL-8, and IL-10, than subcutaneous adipose tissue does. In turn, inflammation in adipose tissue further increases the risk of obesity-related diseases and may be associated with the progression of cancer and its consequent mortality (Tran and Kahn, 2010).
The metabolism of adipose tissue is known to be affected by gonadal steroids such as testosterone. For example, testosterone deficiency, which can be caused by hypogonadism, aging, central obesity, or androgen-deprivation therapy in patients with prostate cancer, is associated with insulin resistance, type-2 diabetes, the metabolic syndrome, and cardiovascular disease in general (Bain, 2010).
In mouse models, it has been demonstrated that cells from WAT are recruited by experimentally induced tumors and promote cancer progression (Zhang et al., 2009). Also, although surgical castration of mice results in increased glucose uptake into adipose tissue (Tran and Kahn, 2010), the effect of testosterone deficiency on adipose tissue has not been studied extensively.
Therefore, we undertook this study to identify the sustained effects of castration on WAT in adult male mice. We used C57BL/6J mice, which are commonly used for studies involving a high-fat diet (HFD) (Collins et al., 2004). We used a regular-diet and HFD approaches to assess differences in the responses to castration as the result of WAT deposition adjacent to the epididymis.
Our study results describe the changes in tissue mass, morphologic characteristics, induction of lipolysis, expression of genes coding for cytokines, and serum concentrations of adipokines that occurred in the epididymal WAT of adult mice fed a regular diet or a HFD after surgical castration.
2. Materials and methods
2.1. Animals and treatments
C57BL/6J mice, 5 and 10 weeks old, were purchased from MD Anderson Cancer Center’s Department of Experimental Radiation Oncology. Mice were housed under specific pathogen–free conditions in facilities accredited by the American Association for Accreditation of Laboratory Animal Care, and all experiments were conducted in accordance with the principles and procedures outlined in the NIH’s Guide for the Care and Use of Laboratory Animals. For 7 weeks before and during the 2-week course of the experiments, the 5-week-old mice (n = 45) were fed a HFD (D12492; Research Diets, Inc., New Brunswick, NJ) containing 60 kcal% fat, 20 kcal% carbohydrate, and 20 kcal% protein, whereas the 10-week-old mice (n = 30) were fed a regular diet (Purina Conventional Rodent Chow 5001, St. Louis, MO) for 2 weeks. When all the mice were 12 weeks old, those in each dietary group were randomly allocated to three subgroups (n = 10-15 mice each), one of which underwent sham surgery, and the other two, surgical castration. We analyzed two independent groups (n = 30 per group) of mice fed with a regular diet and combined data for further analysis.
For the surgeries, mice were anesthetized with 2–4% isoflurane. After the surgical site was shaved, an incision was made in the scrotum. In the sham-surgery group, the incision was then simply closed with sutures. For castration of the other two groups, the incision was made in the tunica of the first testicle, and the testis was pulled out and removed. The procedure was repeated on the contralateral side. The scrotal incisions were then closed with sutures. The sham-operated animals and half of the castrated ones then received a subcutaneously implanted empty pellet on their back, creating control and castrated + empty pellet (EP) groups, respectively. The second half of the castrated animals received implanted testosterone pellets, creating a castrated + testosterone pellet (TP) group. Both testosterone (25 mg testosterone, 21-days release time) and placebo pellets were purchased from Innovative Research of America (Sarasota, FL).
2.2. Blood and tissue sampling and processing
All mice from both dietary groups were euthanized by using the CO2 inhalation method 2 weeks after having undergone the surgical and pellet-implantation procedures. Their body weight and length were recorded. Blood samples were collected from the posterior vena cava, allowed to clot overnight at 4°C, and then centrifuged for 20 minutes at 2,000 g. The resulting serum was stored frozen at −80°C.
Further, the epididymal WAT pads were collected from each mouse, as were the anterior, ventral, and dorsolateral lobes of the prostate, an androgen-dependent organ used as a control, and the wet weights of all those tissues were measured. WAT tissue was processed as follows: one half of WAT was snap-frozen in liquid nitrogen and stored at −80°C for further analysis of protein expression and RNA extraction. The second half of WAT was fixed in paraformaldehyde, embedded in paraffin, and cut into 5-μm sections for subsequent histologic and immunohistochemical (IHC) analyses.
2.3. Histological analysis of WAT
The effects of castration on the size of adipocytes were quantitatively evaluated using images, acquired at 20× magnification, of hemotoxylin and eosin (H&E)–stained WAT sections. The analysis was performed with a Nikon Eclipse 90i image analysis system and the NIS-Elements AR software 3.0 (both from Nikon Instruments, Inc., Melville, NY). One hundred randomly selected adipocytes from each section were outlined using the manual function of the system, and the areas of individual adipocyte profiles were recorded. To analyze the size distribution of adipocytes, the histogram function in Excel (Microsoft Corporation, Redmont, WA) was used and graphs were created.
2.4. Western blotting
A portion of each WAT specimen was homogenized in ice-cold radioimmunoprecipitation assay buffer by using a battery-powered handheld homogenizer (Sigma- Aldrich, St. Louis, MO). The resulting suspension was then spun in a bench centrifuge at 16,000 g for 10 minutes. The supernatant was collected and cleared by passage through a Vivaclear Mini clarifying filter (Sartorius Stedim Biotech, Aubagne, France). Protein concentration was measured by using a protein assay kit from Bio-Rad Laboratories (Hercules, CA). Conventional Western blotting was performed using the following primary antibodies to visualize proteins: hormone-sensitive lipase (Hsl), phospho–hormone-sensitive lipase (pHslSer660), adipose triglyceride lipase (Atgl), and fatty acid synthase (Fasn) all from Cell Signaling Technology, Beverly, MA). Gapdh (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as a loading control. Images were quantified with UN-SCAN-IT gel software Version 6.1 (Silk Scientific, Inc., Orem, UT).
2.5. Enzyme-linked immunosorbent assay (ELISA)
Serum testosterone concentrations were measured by using an ELISA kit (Calbiotech, Spring Valley, CA) according to the manufacturer’s instructions. Serum leptin, adiponectin, and resistin concentrations were measured by using an ELISA kit for a respective cytokine (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions.
2.6. Measurement of serum glucose and triglyceride (TG) levels
Serum glucose levels were measured using a glucose (GO) assay kit (Sigma-Aldrich). The manufacturer’s procedure was modified to minimize the use of serum: 1 μl of serum was mixed with 99 μl of distilled water, and 200 μl of assay reagent was added. The reaction mix in a 1.7-ml test tube was incubated for 30 minutes at 37°C. The reaction was stopped with 200 μl of 6 M sulfuric acid, and then 100 μl of the reaction mix was transferred to a 96-well plate. Absorbance at 540 nm was read by using a conventional plate reader.
Serum TG levels were measured by using a triglyceride assay kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s protocol.
2.7. Gene-expression analysis
Total RNA was isolated from frozen WAT specimens by using a standard extraction protocol with Trizol reagent (Invitrogen Corporation, Carlsbad, CA). An iScript™ cDNA Synthesis Kit from Bio-Rad was used to generate cDNA for further analysis by quantitative real-time polymerase chain reaction (qRT-PCR) testing with SYBR Green stain. We initially used a Mouse Chemokines & Receptors RT2 Profiler PCR Array (SA Biosciences, Frederick, MD) to detect genes that were affected by castration in epididymal WAT. Genes that were up-regulated after castration were further analyzed by using primers from PrimerBank (Spandidos et al., 2010).
2.8. IHC analysis
IHC with primary antibodies to the stromal marker smooth muscle α-actin (Acta2; 1:1,000 dilution, Sigma, cat# A5228), Cxcl5 (1:50, R&D Systems, cat# MAB433), the macrophage marker Mac-3 (1:50, BD Biosciences, Pharmingen, San Diego, CA, cat# 550292), Fasn (1:50; Cell Signaling Technology, cat# 3180), and androgen receptor Ar (1:50, Santa Cruz Biotechnology, cat#sc-816) was performed on paraffin-embedded sections of WAT pads using the Vectastain® ABC kit or ImmPRESS™ reagents (Vector Laboratories, Inc., Burlingame, CA) according to the manufacturer’s instructions. Specificity of the IHC staining was verified by incubating WAT sections with nonspecific rabbit or mouse immunoglobulin G in place of the primary antibody.
Acta2 and Fasn immunostaining was evaluated quantitatively using the Nikon Eclipse 90i with the NIS-Elements AR software. Fifteen to 20 images from each immunostained section of the WAT pad were randomly acquired at 10× magnification. The area of stroma that stained positively for Acta2 was detected by using a pixel classifier that recognizes brown DAB staining; the results were recorded as the percentage of the Acta2-positive stroma among a total tissue area.
To quantify the Cxcl5 and Mac-3 IHC staining results, we used the manual function to count the stromal cells that stained positively on 20 images acquired randomly at 20× magnification and recorded the total number of positively labeled cells for each antibody.
2.9. Statistical analysis
Statistical analysis was performed in Microsoft Office Excel using two-tailed Student’s t testing. The level of significance was set at p <0.05. Values are expressed as mean ± SE.
3. Results
3.1. Castration induces changes in epididymal WAT
3.1.1. Regular-diet group
Analysis of epididymal WAT (20 mice in each group) wet weights revealed that in the castration + EP (castration) group of mice, the weight was lower than that in the sham-surgery control group (control) by 43% (p < 0.001), and in the castration + TP group, lower by 16% (p = 0.01) (Fig. 1A). The WAT wet weight/body weight ratios (Fig. 1B) were also lower, by 40% in the castration group and 22% in the castration + TP group, than in the control group (p < 0.001 for both comparisons). Together, these results indicate that testosterone supplementation after surgical castration partially offsets the effect of castration.
Fig. 1.
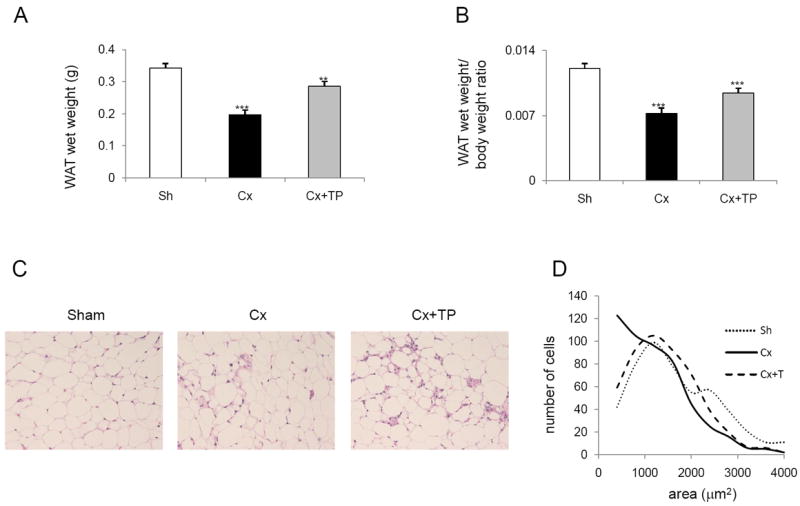
The regular-diet group: (A) WAT wet weight, (B) WAT wet weight/body weight ratio, (C) hematoxylin and eosin staining of WAT, and (D) the adipocyte size distribution. (A) C57BL/6J mice fed regular diet were sham-operated, castrated, and castrated and treated with testosterone pellets as described in Materials and Methods. At the end of the experiment, mice were euthanized and their WAT wet weights and body weights were recorded. Columns, WAT wet weight; bars, SE; **p < 0.01; ***p < 0.001. (B) The WAT wet weight/body weight ratio was calculated. Columns, WAT wet weight/body weight ratio; bars, SE; ***p < 0.001. (C) Hematoxylin and eosin staining of WAT from sham-operated, castrated, and castrated + TP mice was performed as described in Materials and Methods. Original magnification, 20×. (D) Line-graphs represent the adipocyte size distribution which was acquired by the image analyses. For each experimental group, 500 adipocytes were randomly selected from 5 specimens, and the areas of individual adipocytes were measured. Histogram function was used; bins were selected as 400 μm2 increments.
As expected, castration resulted in significantly reduced wet weights of all prostatic lobes (Fig. 1S, Supplementary data). To detect changes in the size of adipocytes, we evaluated the H&E-stained WAT sections from 5 mice in each group. The adipocytes in the castration group appeared smaller than those in the control and castration + TP groups did (Fig. 1C). Indeed, quantitative image analysis (Fig. 1D) revealed that the average area of the adipocytes was less in the castration group (1020 ± 116 μm2; p = 0.027) than it was in the control (1522 ± 145 μm2) and the castration + TP (1262 ± 67 μm2) groups.
We also detected a change in the stroma that was characterized by more fibroblast-like stromal cells and macrophages in the WAT from the castrated group than there were in that from the control and castration + TP groups. Some small adipocytes were surrounded by stromal cells.
3.1.2. High-fat diet group
The HFD group had higher body weights, epididymal WAT wet weight/body weight ratios, and BMIs than the regular-diet group had (Fig. 2S, Supplementary data). The wet weights of the anterior, ventral, and dorsolateral prostate lobes, used as controls, are shown in Fig. 1S, Supplementary data.
Analysis of epididymal WAT (15 animals in each group) wet weight revealed that the castration group had reduced WAT wet weight by 53% (p < 0.001), and castration + TP group by 18% (p = 0.3) compared to the control group (Fig. 2A). The castration group had reduced WAT wet weight/body weight ratio by 47% (p < 0.001), and the castration + TP group by 16% (p = 0.28) compared to the control group (Fig. 2B).
Fig. 2.
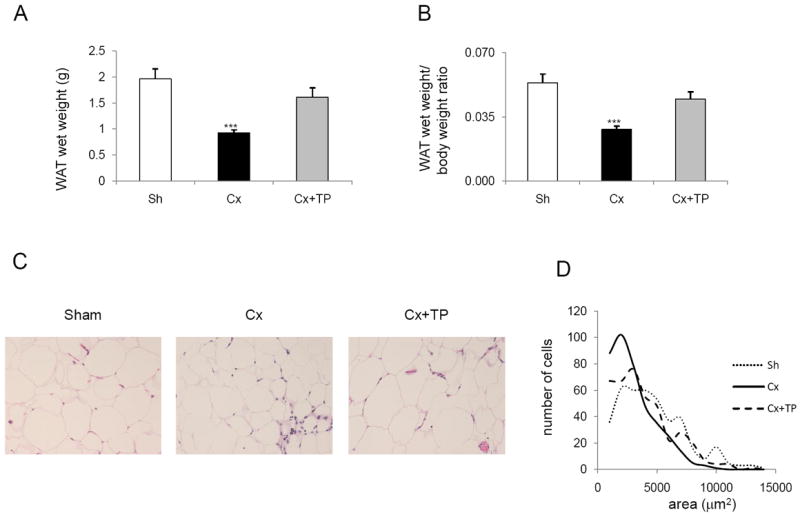
The high-fat diet group: (A) WAT wet weight, (B) WAT wet weight/body weight ratio, (C) hematoxylin and eosin staining of WAT, and (D) the adipocyte size distribution. (A) C57BL/6J mice fed with high-fat diet were sham-operated, castrated, and castrated and treated with testosterone pellets as described in Materials and Methods. At the end of the experiment, mice were euthanized and their WAT wet weights and body weights were recorded. Columns, WAT wet weight; bars, SE; **p < 0.01; ***p < 0.001. (B) The WAT wet weight/body weight ratio was calculated. Columns, WAT wet weight/body weight ratio; bars, SE; ***p < 0.001. (C) Hematoxylin and eosin staining of WAT from sham-operated, castrated, and castrated + TP mice was performed as described in Materials and Methods. Original magnification, 10×. (D) Line-graphs represent the adipocyte size distribution which was acquired by the image analyses. For each experimental group 500 adipocytes were randomly selected from 5 specimens and the areas of individual adipocytes were measured. Histogram function was used; bins were selected as 1,000 μm2 increments).
The analysis of H&E-stained WAT sections revealed that the size of adipocytes was reduced in the castration group compared to the control group (Fig. 2C). Reduced size of adipocytes in the castrated group was confirmed by the quantitative image analysis (Fig. 2D). The average area of the adipocytes was 2555 ± 257 μm2 (p = 0.003) after castration compared to 4166 ± 228 μm2 in the control group. The presence of testosterone (i.e., castration + TP group) prevented the reduction of the size of adipocytes only partially as the adipocyte average area was 3388 ± 268 μm2.
Thus, castration induces statistically significant WAT weight and the WAT wet weight/body weight ratio reduction independently of diet, but testosterone treatment partially offsets the effect of castration on WAT wet weight. Castration also induces morphologic changes in adipocytes: cells become smaller and have a larger stromal compartment. In general, castration led to reduced adipocyte size, and this effect was reversed in part by testosterone in the regular-fat diet group but not in the HFD group.
3.2. Castration-induced lipolysis
We hypothesized that castration induces lipolysis in WAT on the basis of our observations of reduced WAT wet weight and reduced size of adipocytes. To validate this hypothesis, we performed Western blotting to analyze protein levels of Hsl, pHslSer660, and Atgl in WAT from both dietary groups after experimental treatments. Western blotting (Fig. 3) revealed reduced pHslSer660 levels by 46% (n = 10, p = 0.004) in WAT from the regular-diet group after castration. Atgl protein levels were reduced as well, but the reduction was not statistically significant. The protein level of Hsl was increased by 22% in the presence of testosterone; the pHslSer660 levels and Atgl levels were similar to those detected in the castration group but without statistical significance.
Fig. 3.
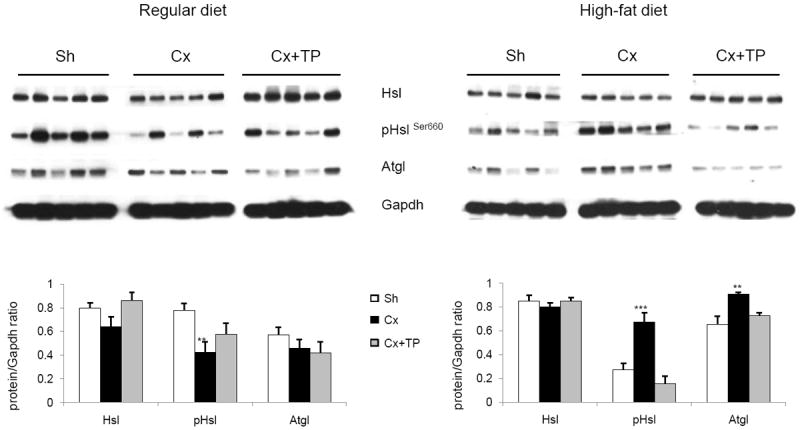
Western blot analysis of hormone-sensitive lipase (Hsl), phosphorylated hormone sensitive lipase on serine 660 (pHslSer660), adipose tissue triglyceride lipase (Atgl) protein levels in WAT from mice fed with regular diet and high-fat diet. Western blotting was performed as described in Materials and Methods. Gapdh was used as a loading control. Images were quantified using UN-SCAN-IT gel 6.1 software to calculate protein/Gapdh ratio. Columns, protein/Gaphd ratio; bars, SE; *p < 0.05; **p < 0.01; ***p < 0.001.
In contrast, we detected an increase of almost 2.5 times the pHslSer660 levels (n = 5, p = 0.003) and 40% increased Atgl protein levels (n = 5, p = 0.006) in WAT from the HFD group after castration, suggesting that lipolysis is ongoing 14 days after castration. Differences detected in pHslSer660 and Atgl levels were offset by the presence of testosterone (Fig. 3).
Thus, it appears that castration-induced lipolysis in WAT of HFD-fed animals is more robust and/or sustained 14 days after castration than in animals fed a regular diet.
3.3. Serum adipokine levels
We further analyzed serum leptin, adiponectin, and resistin levels from mice from both dietary groups using commercial ELISA kits (Table 1). We detected reduced leptin levels in serum from the regular-diet group after castration by 72% (n = 8, p < 0.001) compared to the control group (n = 8). Castrated animals treated with TP also had reduced serum leptin levels by 46% (n = 8, p = 0.007) compared to the control group.
Table 1.
Serum levels of adipokines, triglycerides (TG), and glucose
| RD | HFD | |||||
|---|---|---|---|---|---|---|
| Sh | Cx | Cx+TP | Sh | Cx | Cx+TP | |
|
| ||||||
| Leptin (pg/ml) | 2.5 ± 0.3 | 1.1 ± 0.2 ** | 1.7 ± 0.3 | 24.3 ± 1.5 | 9.4 ± 0.9 *** | 21.8 ± 2.0 |
| Resistin (pg/ml) | 22.4 ± 0.5 | 31.2 ± 1.1 *** | 19.5 ± 1.0 | 30.6 ± 1.8 | 30.2 ± 2.2 | 28.7 ± 1.0 |
| Adiponectin (μg/ml) | 13.9 ± 0.1 | 15.6 ± 0.1 ** | 10.4 ± 0.2 *** | 13.6 ± 0.5 | 14.0 ± 0.3 | 12.9 ± 0.9 |
| TG (mg/ml) | 12.6 ± 0.8 | 10.4 ± 0.3* | 11.2 ± 0.8 | 18.1 ± 2.5 | 6.3 ± 0.1 ** | 9.3 ± 1.0 * |
| Glucose (mg/ml) | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 3.0 ± 0.1 | 3.6 ± 0.2 * | 3.4 ± 0.1* |
Values are expressed as mean ± SE.
p < 0.05;
p < 0.01;
p < 0.001
We detected increased adiponectin levels in serum from castrated mice by 13% (n = 8, p = 0.0039) compared to the control group. Castrated mice with TP had reduced serum adiponectin levels by 25% (n = 8, p = 0.0004) compared to the control group. Similarly, castration resulted in increased serum resistin levels by about 28% (n = 8, p < 0.0001) compared to the control group. Castrated mice with TP had serum resistin levels comparable with the control group.
The HFD group had reduced serum leptin levels after castration by 61% (n = 8, p < 0.001) compared to the control group (n = 8). Castrated animals treated with TP (n = 8) had serum leptin levels similar to the control group.
We did not detect castration-induced changes in serum adiponectin and resistin levels in castrated male mice compared to the controls.
Thus, mice fed a regular diet had reduced serum leptin and increased serum adiponectin and resistin levels after castration. The effect of testosterone was only partial as the serum leptin and adiponectin levels were lower compared to the control group. Castration had no effect on serum adiponectin and resistin levels in the HFD group.
3.4. Serum TG and glucose levels
We measured TG levels in serum collected from the control, castrated, and castrated + TP mice fed with a regular diet and HFD (Table 1). As expected, controls in the HFD group had higher serum TG levels than the controls in the regular-diet group had (p = 0.03). Castration of the regular-diet group resulted in a small but significant reduction in serum TG levels (p = 0.018) that was marginally offset by testosterone. However, castrated animals in the HFD group had three times lower serum TG levels than the control group had (p = 0.002). Castrated animals treated with testosterone had about two times lower serum TG levels than the control group had (p = 0.013), suggesting that testosterone did not offset the effect of castration.
We also measured serum glucose levels (Table 1). There were no differences in serum glucose levels in the regular-diet group. Control mice in the HFD group had significantly higher serum glucose levels than the control mice from the regular-diet group had (p = 0.004). Castrated mice in the HFD group had increased serum glucose levels by 22% compared to the controls (p = 0.027). Increased serum glucose levels by about 14% were also detected in the castration + TP group compared to the controls (p = 0.017).
Thus, our data demonstrate that castration has a small but significant reduction of serum TG levels in the regular diet group and a dramatic reduction of serum TG in the HFD group. This effect was not offset by testosterone replacement in the HFD group. Serum glucose levels were affected only in the HFD group. Testosterone did not offset the castration effect.
3.5. Castration induces cytokines in epididymal WAT
It has been reported that in humans, visceral WAT generates and releases inflammatory cytokines, including CCL2, PAI-1, IL-6, IL-8, and IL-10, under specific conditions (Sengenes et al., 2007). We asked whether castration induces changes in the expression of genes coding for cytokines in epididymal WAT. qRT-PCR revealed that numerous cytokine genes are induced in WAT after castration. The most up-regulated genes were Cxcl5, Cxcl2, and Il4 in WAT from the regular-diet group after castration (Table 2). Genes coding for Cxcl5, Il1α, and Mmp2 were the most up-regulated in WAT from the HFD group after castration (Table 3). We also detected reduced leptin mRNA after castration (Tables 2 and 3). In both dietary groups, mRNA levels of genes coding for cytokines were lower compared to controls after testosterone treatment (Tables 2 and 3). We also compared mRNA levels of cytokines in control mice from the regular-diet and HFD groups. We detected increased expression of genes coding for Cxcl1 (59.3 ± 31.7), Ccl7 (22.5 ± 4.3), Ccl2 (15.5 ± 5.3), Cxcl2 (12.3 ± 3.1), Tnf (11.8 ± 2.6), and Ccl3 (10.0 ± 0.7) genes; numbers in brackets are fold ± SE.
Table 2.
Changes in the cytokine expression in epididymal WAT 14d after castration, regular diet
| Gene | Gene ID | Official name | Cx | Cx+TP | ||
|---|---|---|---|---|---|---|
| Fold | SE | Fold | SE | |||
| Cxcl5 | 20311 | chemokine (C-X-C motif) ligand 5 | 56.2 | 31.5 | 4.0 | 2.6 |
| Cxcl2 | 20310 | chemokine (C-X-C motif) ligand 2 | 22.3 | 15.3 | 6.0 | 1.7 |
| Il4 | 16189 | interleukin 4 | 15.9 | 7.6 | 4.1 | 2.4 |
| Ccl3 | 20302 | chemokine (C-C motif) ligand 3 | 14.1 | 3.3 | 3.4 | 1.2 |
| Xcl1 | 16963 | chemokine (C motif) ligand 1 | 9.7 | 5.2 | 1.7 | 0.0 |
| Il10 | 16153 | interleukin 10 | 7.8 | 2.8 | -1.2 | 1.3 |
| Ccl2 | 20296 | chemokine (C-C motif) ligand 2 | 7.3 | 4.3 | 2.3 | 1.0 |
| Tnf | 21926 | tumor necrosis factor alpha | 6.8 | 2.6 | 1.8 | 0.2 |
| Il1a | 16175 | interleukin 1 alpha | 6.5 | 2.5 | 2.2 | 0.6 |
| Ccl4 | 20303 | chemokine (C-C motif) ligand 4 | 6.3 | 1.4 | -1.0 | 1.0 |
| Cxcl1 | 14825 | chemokine (C-X-C motif) ligand 1 | 6.0 | 2.1 | 1.4 | 0.3 |
| Il6 | 16193 | interleukin 6 | 5.5 | 1.6 | 1.8 | 0.4 |
| Ccl7 | 20306 | chemokine (C-C motif) ligand 7 | 5.2 | 3.1 | 0.7 | 1.4 |
| Mmp2 | 17390 | matrix metallopeptidase 2 | 4.3 | 0.5 | 2.1 | 0.5 |
| Tnfrsf1a | 21937 | tumor necrosis factor receptor superfamily, member 1a | 3.1 | 0.5 | 1.6 | 0.4 |
| Lep | 16846 | leptin | -3.2 | 0.5 | -0.1 | 1.2 |
Table 3.
Changes in the cytokine expression in epididymal WAT 14d after castration, high-fat diet
| Gene | Gene ID | Official name | Cx | Cx+TP | ||
|---|---|---|---|---|---|---|
| Fold | SE | Fold | SE | |||
| Cxcl5 | 20311 | chemokine (C-X-C motif) ligand 5 | 59.8 | 17.7 | 7.7 | 4.4 |
| Il1a | 16175 | interleukin 1 alpha | 6.1 | 2.3 | 0.4 | 1.0 |
| Mmp2 | 17390 | matrix metallopeptidase 2 | 6 | 0.8 | 1.0 | 1.3 |
| Il12 | 16159 | interleukin 12 | 5.7 | 1.1 | 0.8 | 1.0 |
| Il4 | 16189 | interleukin 4 | 5.4 | 1.6 | -0.3 | 0.8 |
| Ccl3 | 20302 | chemokine (C-C motif) ligand 3 | 4.8 | 1.6 | 3.7 | 1.1 |
| Il10 | 16153 | interleukin 10 | 4.5 | 0.8 | 0.6 | 0.7 |
| Tnf | 21926 | tumor necrosis factor alpha | 3.3 | 0.6 | -0.1 | 0.8 |
| Ccl4 | 20303 | chemokine (C-C motif) ligand 4 | 2.4 | 0.3 | 1.5 | 0.5 |
| Lep | 16846 | leptin | -4.4 | 1.7 | -0.4 | 1.5 |
Therefore, these experiments revealed that castration leads to changes in the expression of specific cytokine genes in epididymal WAT. Cxcl5 was the most-induced cytokine in both dietary groups. Further, there is a set of cytokines which are produced by WAT in the control HFD group compared to the control regular-diet group.
3.6. IHC staining of WAT
The H&E results suggested that castration induced morphologic changes in WAT associated with increased stromal compartment and macrophage infiltration (Fig. 1C, 2C). We performed IHC of paraffin-embedded WAT sections from the regular-diet (Fig. 4A) and HFD (Fig. 4B) groups. We used Acta2 as a stromal marker and Mac-3 as a macrophage marker. We also labeled sections with Cxcl5 and Fasn antibodies.
Fig. 4.
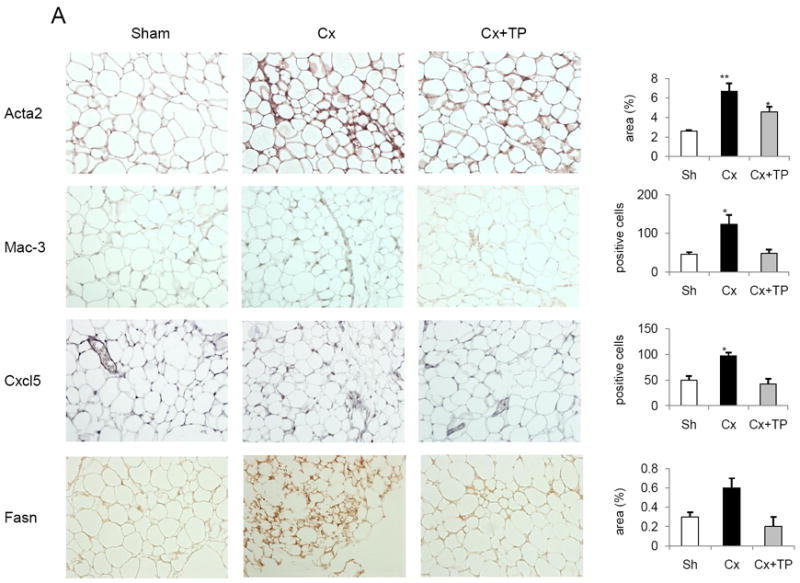
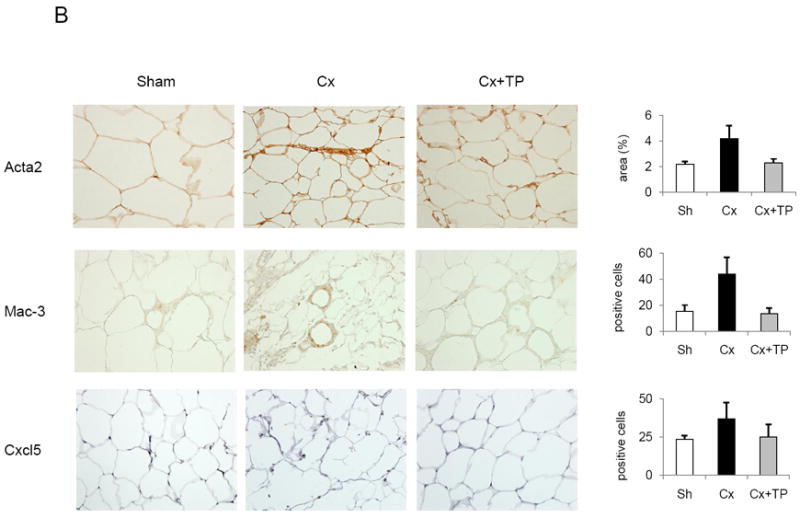
Immunohistochemical (IHC) staining of paraffin-embedded WAT from mice fed a regular diet (A) or a high-fat diet (B). (A, B) Antibodies against smooth muscle actin (Acta2), Mac-3, Cxcl5, and Fas were used as described in Materials and Methods. Images were depicted at 20× magnification. Column graphs represent results from the semiquantitative analysis of the IHC images. Sh, sham-operated; Cx, castration; Cx + TP, castration + testosterone pellet. Bars, SE; *p < 0.05; **p < 0.01.
Fig. 4A shows stained sections and results of the quantitative analysis of WAT from mice on regular diet. Castration resulted in a significant increase of Acta2, Mac-3, and Cxcl5 protein expression. Testosterone offset castration-induced increase of Mac-3 and Cxcl5 proteins. Cxcl5 staining pattern suggests that Cxcl5 is most likely expressed by WAT macrophages.
We further hypothesized that increased presence of stromal cells in WAT is a result of the tissue regenerative process characterized by the presence of differentiating pre-adipocytes with the increased Fasn expression. IHC for Fasn confirmed that there is a subset of cells in WAT with increased Fasn staining. Increased Fasn staining was mostly localized to the stromal compartment of WAT after castration (Fig. 4A).
Fig. 4B shows stained sections and results of the quantitative analysis of WAT from the HFD group. Increased Acta2, Mac-3, and Cxcl5 proteins were detected, although the increases did not reach the level of statistical significance. Testosterone offset the castration-induced increase of Mac-3 and Cxcl5 proteins. We did not detect subset of WAT with increased Fasn staining in the HFD group (data not shown).
IHC staining for Fasn was confirmed by Western blotting (Fig. 3S, Supplementary data). We detected increased Fasn protein levels in WAT after castration in the regular-diet group compared to the control group (Fasn/Gapdh ratio was 1.15 vs 0.98, n = 4, p = 0.008). A slight increase of Fasn protein levels (Fasn/Gapdh ratio was 1.05 vs 0.98, n = 4) was not statistically significant in the castration + TP regular-diet group. We did not detect changes in Fasn protein levels in the HFD group.
Thus, IHC staining confirmed that castration-induced WAT morphologic changes are accompanied by changes in protein expression. We detected increased expression of Acta2, Mac-3, Cxcl5, and Fasn.
3.7. Effect of castration on the expression of androgen receptor Ar in WAT
Initially, we used ELISA to measure serum testosterone levels in samples (n = 8 per treatment group) from both diet groups. The analysis confirmed that castrated animals in both diet groups had background levels of serum testosterone; 0.04 ± 0.02 ng/ml or 0.05 ± 0.03 ng/ml in the regular-diet and the HFD group, respectively. We detected lower levels of serum testosterone in the control HFD group (0.26 ± 0.13 ng/ml; p = 0.16) than in the regular-diet group (1.65 ± 0.57 ng/ml). Implantation of testosterone pellets resulted in serum testosterone levels of 23.63 ± 0.81 ng/ml or 17.59 ± 0.70 ng/ml in the regular-diet and the HFD castration +TP group, respectively.
To assess the effect of castration on the expression of Ar, we performed qRT-PCR and IHC staining of WAT sections from both diet groups. In the regular-diet group, castration resulted in reduced Ar mRNA by 4.1 ± 0.9 fold. A similar decrease was detected in the castration +TP group (4.7 ± 1.4 fold). IHC staining (Fig. 5) revealed that nuclear Ar was undetectable in WAT from the castrated regular-diet group. Fewer cells stained for nuclear Ar in WAT from the castration + TP group compared to the control group.
Fig. 5.

Immunohistochemical staining for androgen receptor (Ar) of paraffin-embedded WAT from mice fed a regular diet. Antibodies against Ar were used as described in Materials and Methods. Images were depicted at 20× magnification. Sh, sham-operated; Cx, castration; Cx + TP, castration + testosterone pellet.
In WAT from the HFD group, the Ar mRNA and protein levels are very low to undetectable (data now shown).
Thus, castration results in the reduced expression of Ar in WAT on both mRNA and protein levels. Ar mRNA and protein is not detectable in WAT from animals fed HFD.
4. Discussion
Our data demonstrate that surgical castration has profound effects on WAT. The WAT wet weight and the WAT wet weight/body weight ratio were lower in the castration + TP group and reached statistical significance in the regular-diet group. These results suggest that testosterone does not fully abrogate effects of castration in the regular-diet group. Morphologic changes after castration were associated with adipocyte size reduction, an increase in a stromal compartment, and macrophage infiltration.
Our data further indicate that lipolytic enzymes can be activated in a sustained fashion in response to castration, as observed in the HFD group. We also detected increased Atgl protein levels in the HFD group after castration. Our data further indicate that animals in the HFD group respond differently to the castration stimulus than animals in the regular-diet group, potentially as a consequence of a more robust and/or sustained lipolytic program. In our view, these data further suggest that the lipolytic process does not continue indefinitely and may subside at a certain set-point. It may take longer for the HFD group to reach such set-point as lipolysis is still ongoing 14 days after castration. Future studies that analyze pHslSer660 and Atgl protein levels at different time points after castration under various conditions may clarify this hypothesis.
Serum leptin levels were reduced in both dietary groups after castration, whereas serum resistin and adiponectin levels were increased in the regular-diet group after castration. Serum resistin and adiponectin levels were not affected by castration in the HFD group. Inoue at al. (Inoue et al., 2010) reported increased plasma adiponectin levels in castrated C57BL/6 mice independent of diet, and decreased plasma leptin levels in castrated mice on high carbohydrate diet. Xu at al. (Xu et al., 2005) measured increased serum adiponectin levels in castrated C57BL/6 mice.
Controls in the HFD group had higher body weight, the WAT wet weight/body weight ratio and BMI than the controls in regular-diet group at the time of sacrifice (Fig. 1S, Supplementary data). We also measured serum glucose and triglyceride (TG) levels. Our data demonstrate that castration has a small but significant effect in the regular diet group and a profound effect on serum TG levels in the HFD group. This effect was modified but not offset by testosterone replacement. Serum glucose levels were also affected only in the HFD group by castration and testosterone did not offset the castration effect. Our results suggest that castration alters the mechanism(s) regulating serum TG and glucose levels, selectively in the HFD group. The physiological mechanisms responsible for these metabolic changes need further investigation.
In addition to leptin, adiponectin, and resistin, adipose tissue expresses a wide range of factors, including pro-inflammatory cytokines and chemokines. The expression of IL-8, MCP-1/CCL2, and macrophage inflammatory protein is increased with adipocity in animals and humans (Sengenes, Miranville, 2007). We detected Cxcl5, Cxcl2, and Il4 in the regular-diet group and Cxcl5, Il1a, and Mmp2 in the high-fat–diet group among the most induced cytokines after castration. The mRNA levels of Cxcl1, Ccl7, Ccl2, Cxcl2, Tnf, and Ccl3 were higher in the control WAT from the HFD group than they were in the control regular-diet group. Chemokines and their corresponding receptors, including CCL2, CCL5, CXCL5, CXCL13, CXCL16, and CXCR5, have been shown to be involved in prostate cancer progression and organ-specific metastasis (Loberg et al., 2006, Sung et al., 2008, Singh et al., 2009). Further studies are needed to characterize cytokine-producing cells types in WAT.
We hypothesized that increased presence of stromal cells may be a result of the tissue regeneration process induced in WAT by castration. We detected increased Fasn staining in the stromal compartment of WAT from castrated mice, suggesting that the stromal compartment is involved in WAT remodeling after castration. Fasn is highly expressed in differentiating mouse 3T3-L1 pre-adipocytes (Paulauskis and Sul, 1988).
Interestingly, the stromal cells in WAT from the castration + TP regular-diet group did not stain for Fasn. This may suggest that castration induces the growth of stromal cells before testosterone released from the pellet reaches steady-state levels. Testosterone may then inhibit differentiation of stromal pre-adipocytes. It was reported that testosterone inhibits adipogenic differentiation of mouse 3T3-L1 pre-adipocytes (Singh et al., 2006).
It has been suggested that macrophages present in noninflamed tissue (i.e., resident macrophages) help maintain homeostasis and participate in tissue remodeling. In mice, these cells originate from circulating CCR2−CX3CR1hi monocytes. In contrast, CCR2+CX3CR1low monocytes migrate into inflamed tissue and differentiate into macrophages, which coordinate inflammatory responses by producing chemokines and clearing debris by phagocytosis (Lumeng et al., 2007). Future studies may explain the origin and provide an expression profile of macrophages that are found in WAT after castration.
Dieudonne at al. (Dieudonne et al., 1998) documented that human and rat preadipocytes and adipocytes express androgen receptor and suggested that androgens may contribute, through regulation of their own receptors, to the control of adipose tissue development. Yu et al. (Yu et al., 2008) generated adipose-specific Ar knockout mice by a conditional genetic knockout approach. Dhindsa at al. (Dhindsa et al., 2010) reported that obesity is probably the condition most frequently associated with subnormal free testosterone concentrations in males. Our analysis confirmed the expression of Ar in epididymal WAT from mice fed regular diet and that the consumption of high-fat diet may result in the reduction of the Ar expression and reduced serum testosterone levels. Kyprianou and Isaacs (Kyprianou and Isaacs, 1988) demonstrated that castration induced a series of temporally discrete biochemical events, including rapid loss of nuclear Ar receptor and programmed cell death, within the rat ventral prostate. We detected the loss of nuclear Ar receptor in WAT after castration but no apparent apoptosis (data not shown). Thus, mechanisms leading to reduced Ar levels in WAT after castration ought to be further studied.
Complex short-term effects of castration in rodents have not been studied in great detail. Published studies focus on long-term effects of castration (Koncarevic et al., 2010, Axell et al., 2006, Hastings and Hill, 1997, Vanderschueren et al., 2004) which are relevant to men undergoing androgen deprivation therapy. We hypothesize that we fortuitously selected a time point at which biochemical events which contribute to the effects of long-term androgen deprivation therapy in WAT are initiated.
In conclusion, our study demonstrated that castration has profound effects on mouse epididymal WAT. Future studies involving human WAT are needed to understand underlying biologic mechanisms during castration and to test the clinical relevance of these findings to the management of obesity, metabolic syndrome, and prostate cancer in humans.
Supplementary Material
Highlights.
We analyze the effects of sustained castration on epididymal white adipose tissue (WAT) in C57BL/6J mice which were fed a regular or high-fat diet.
Castration has profound effects on WAT morphology (smaller adipocytes and increased stromal cell compartment) and function.
Castration reduces WAT wet weight and WAT wet weight/body weight ratio.
Castration induces lipolysis with different kinetics between diet groups.
Serum leptin levels are reduced after castration independent of diet; changes in adiponectin and resistin were diet-dependent.
Castration induces cytokines in WAT.
Acknowledgments
The authors thank Karen F. Phillips, ELS, from the Department of Genitourinary Medical Oncology, MD Anderson Cancer Center, for editorial assistance.
Grants
This research is supported in part by the NIH grant R01CA050588, the DOD grant PC093932, and in part by the NIH through MD Anderson’s Cancer Center Support Grant, CA016672.
Footnotes
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology. 2010;151:5669–79. doi: 10.1210/en.2010-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansel W. The essentiality of the epididymal fat pad for spermatogenesis. Endocrinology. 2010;151:5565–7. doi: 10.1210/en.2010-1146. [DOI] [PubMed] [Google Scholar]
- 5.Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30:415–44. vii. doi: 10.1016/j.ccm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Fair AM, Montgomery K. Energy balance, physical activity, and cancer risk. Methods Mol Biol. 2009;472:57–88. doi: 10.1007/978-1-60327-492-0_3. [DOI] [PubMed] [Google Scholar]
- 7.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 8.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bain J. Testosterone and the aging male: to treat or not to treat? Maturitas. 2010;66:16–22. doi: 10.1016/j.maturitas.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–66. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–8. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–9. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengenes C, Miranville A, Lolmede K, Curat CA, Bouloumie A. The role of endothelial cells in inflamed adipose tissue. J Intern Med. 2007;262:415–21. doi: 10.1111/j.1365-2796.2007.01853.x. [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Zakikhani M, David S, Algire C, Blouin MJ, Pollak M. Effects of castration on insulin levels and glucose tolerance in the mouse differ from those in man. Prostate. 2010;70:1628–35. doi: 10.1002/pros.21198. [DOI] [PubMed] [Google Scholar]
- 15.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 16.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–86. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Singh R, Sharma PK, Singh UP, Rai SN, Chung LW, Cooper CR, Novakovic KR, Grizzle WE, Lillard JW., Jr Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett. 2009;283:29–35. doi: 10.1016/j.canlet.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulauskis JD, Sul HS. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J Biol Chem. 1988;263:7049–54. [PubMed] [Google Scholar]
- 20.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–54. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 22.Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998;274:C1645–52. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 23.Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, Yeh S, Chang C. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–8. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–62. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- 26.Koncarevic A, Cornwall-Brady M, Pullen A, Davies M, Sako D, Liu J, Kumar R, Tomkinson K, Baker T, Umiker B, Monnell T, Grinberg AV, Liharska K, Underwood KW, Ucran JA, Howard E, Barberio J, Spaits M, Pearsall S, Seehra J, Lachey J. A soluble activin receptor type IIb prevents the effects of androgen deprivation on body composition and bone health. Endocrinology. 2010;151:4289–300. doi: 10.1210/en.2010-0134. [DOI] [PubMed] [Google Scholar]
- 27.Axell AM, MacLean HE, Plant DR, Harcourt LJ, Davis JA, Jimenez M, Handelsman DJ, Lynch GS, Zajac JD. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab. 2006;291:E506–16. doi: 10.1152/ajpendo.00058.2006. [DOI] [PubMed] [Google Scholar]
- 28.Hastings IM, Hill WG. The effect of testosterone in mice divergently selected on fat content or body weight. Genet Res. 1997;70:135–41. doi: 10.1017/s0016672397002905. [DOI] [PubMed] [Google Scholar]
- 29.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


