Abstract
Background
Soluble intercellular adhesion molecule-1 (sICAM-1) is associated with endothelial dysfunction and clinical cardiovascular disease. We investigated the relationship of subclinical atherosclerosis with sICAM-1 concentration.
Methods
sICAM-1 concentration was assayed at year 15 of the Coronary Artery Risk Development in Young Adults (CARDIA) Study (black and white men and women, average age 40 years). We assessed progression of coronary artery calcification through year 20 (CAC, n=2378), and both carotid artery stenosis (n=2432) and intima media thickness at year 20 (IMT, n = 2240).
Results
Median sICAM-1 was 145.9 ng/ml. Among a subgroup with advanced atherosclerotic plaque (either CAC or stenosis), IMT was 0.010 (95% confidence interval (CI) 0.003–0.017 mm) higher per standard deviation of sICAM-1 (44 ng/ml) in a model adjusted for age, race, sex, clinic, smoking, exercise, body size, education, blood pressure, antihypertensive medication, plasma lipids, and cholesterol lowering medication. With the same adjustment, the odds ratios (OR) for the presence of year 20 carotid artery stenosis per SD of sICAM-1 was 1.12 (CI 1.01–1.25, p<0.04), while for occurrence of CAC progression the OR was 1.16 (CI 1.04–1.31, p<0.01). The associations with CAC and carotid stenosis were strongest in the top 20th of the sICAM-1 distribution.
Conclusion
sICAM-1 concentration may be an early biomarker that indicates changes in the artery wall that accompany atherosclerosis, as well as the presence of advanced plaque in the coronary and carotid arteries. This finding holds in people with low total burden of atherosclerosis, decades prior to the development of clinical CVD.
Introduction
Intercellular adhesion molecule-1 (ICAM-1) is an endothelial adhesion molecule that stimulates leukocyte adhesion and transmigration into the vascular subendothelial space1. This activity is a key step in progression from early to more advanced atheroma2. Circulating soluble ICAM-1 (sICAM-1) is the result of cleavage of membrane-bound ICAM-1. Its concentration in serum/plasma has been associated with cardiovascular disease (CVD) in prospective epidemiologic studies, including the Women’s Health Study3, ARIC Study4, Physicians Health Study5, and the British Regional Heart Study6 of mature adults.
However, whether elevations in serum sICAM-1 concentration early in life are associated with subclinical cardiovascular disease is not well established. Establishing these associations is important in order to understand whether sICAM-1 is associated with early development of atherosclerosis, as opposed to such elevations promoting the development of cardiovascular disease only at a late stage. Several studies have examined the relationship between sICAM-1 and subclincal atherosclerosis. These studies found mixed results. In a random sample of Multinational MONItoring of trends and determinants in CArdiovascular disease (MONICA) subjects7, sICAM-1 was independently associated with the risk of having carotid atherosclerotic plaque, but not with carotid intima-media thickness (IMT). This pattern of association of sICAM-1 with plaque but not with IMT was replicated in a population-based French sample with low cardiovascular risk8. On the other hand, in subjects with CVD, sICAM-1 was associated with both IMT9,10 and change in IMT11. In children with hypertension and obesity12 and in familial combined hyperlipidemia pedigrees13, sICAM-1 was positively correlated with IMT. Additional studies reported that sICAM-1 was positively associated with the presence of coronary artery calcified plaque (CAC), an established measure of subclinical atherosclerosis and CVD events; however, the association was attenuated after adjusting for traditional cardiovascular risk factors14–17. Inconsistencies among these associations may reflect differential pathophysiology indicated by the different subclinical markers.
To extend our understanding of the relationship between circulating sICAM-1 and subclinical atherosclerosis, we investigated the relationship of sICAM-1 concentration with CAC, carotid atherosclerotic plaque and IMT in a healthy, young-adult population, the Coronary Artery Risk Development in Young Adults (CARDIA) Study. We hypothesized that sICAM-1 is positively associated with atherosclerotic plaque, whether calcified in the coronary arteries or a stenotic lesion of any type in the carotid artery, and also with the greater common carotid IMT (which rarely includes plaque18).
Methods
Subjects
This study was part of the Young Adult Longitudinal Study of Antioxidants (YALTA), an ancillary study to CARDIA, which tracks the evolution of cardiovascular disease risk beginning at ages 18–30 years in 1985–8619. CARDIA recruited a population-based sample of 5,115 black and white men and women in Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA; we focus on the reexaminations at years 15 and 20 (retention rates among surviving participants 74% and 72%, respectively). The study was approved by the Institutional Review Board at each participating center and all participants signed informed consent.
Measurements
Demographic, and lifestyle information were collected via questionnaire, and measurements were taken of height and weight to calculate body mass index (BMI, kg/m2). Resting systolic and diastolic blood pressures were measured using averages of the second and third random zero sphygmomanometer measurements. Physical activity was assessed using an interviewer administered questionnaire which measured the frequency of 13 different exercise activities during the past 12 months20,21. The total exercise score was in exercise units (a sum across 13 activities of frequency times intensity; duration of exercise activities was not asked).
Overnight fasting blood samples were collected and processed within 90 minutes of blood collection and stored at −70°C. Soluble ICAM-1 concentrations were measured at the Molecular Epidemiology and Biomarker Research Laboratory in the University of Minnesota, using serum samples diluted 1:400 fold from exam year 15. The ELISA assay was performed according to the manufacturer’s recommendations (R&D Systems; Cat No DY720). This assay was not affected by ICAM-1 single nucleotide polymorphism (SNP) rs5491, which occurs primarily in blacks and blocks sICAM-1 detection by some antibodies. The limit of detection was 31.0 pg/mL and the coefficient of variation was 9.4%. The correlation of 287 pairs of blinded quality control samples was 0.88. Plasma total cholesterol, HDL-cholesterol, and triglycerides were measured enzymatically within six weeks of collection at the Northwest Lipid Research Laboratory in the University of Washington, Seattle, WA. High-density lipoprotein cholesterol (HDL-C) was determined after precipitation of low-density lipoprotein (LDL)-containing lipoproteins with dextran sulfate/magnesium chloride. The test-retest correlation, in 448 blind duplicate samples, was 0.98–0.99 for total cholesterol, HDL-C, and triglycerides. C-reactive protein (CRP) assays of exam year 15 samples were performed with a BNII nephelometer from Dade Behring at the University of Vermont. It has a particle enhanced immunoepholometric system with a range of 0.175 – 1100 mg/L, intrassay coefficients of variation (CV) of 2.3–4.4% and inter-assay CV of 2.1–5.7%. We estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration formula22.
CAC was measured in years 15 and 20 in Oakland, CA and Chicago, IL by electron beam CT (Imatron, Inc.) and in Birmingham, AL and Minneapolis, MN by multidetector CT (General Electric Lightspeed in Birmingham and Siemens S4+ Volume Zoom in Minneapolis) in all willing participants who attended the respective examination. Pregnant women and subjects who exceeded the weight restriction for the scanner were ineligible. The CT scanning protocol included a hydroxyapatite calibration phantom to monitor image brightness and noise. The calcium score (also known as the Agatston score) calculated for each artery (left main, left anterior descending, left circumflex, and right coronary artery) was summed to obtain the total calcium score used in all analyses using FDA approved and commercially available software by experienced analysts (Calcium Score, TeraRecon Inc. San Mateo, CA). The minimum calcified focus was defined as 4 adjacent pixels comprising an area of at least 1.87 mm2. Scans were performed in duplicate; discordant scan pairs (both within and between examination years) were adjudicated side-by-side by an experienced radiologist and in those judged falsely positive the Agatston score was reset to zero or excluded as appropriate.
Carotid artery ultrasound was performed at the year 20 CARDIA examination by trained technicians at each field center using standard procedures, using GE Logiq 700 and later analyzed at a central reading center (New England Medical Center). The maximum IMT was defined for the common carotid artery and for the bulb/internal carotid artery as the mean of the maximal IMT of the near and far wall on both the left and right sides. Stenosis was assessed as any diminution of the lumen (almost all 1–25% narrowing) in any arterial segment according to visual examination separately for the left and the right carotid arteries. Stenosis indicates development of sufficient atherosclerotic plaque of any type to result in luminal narrowing (also known as inward remodeling). Any such atherosclerotic plaque was included as part of the wall thickness. Presence or absence of calcium in plaque could not be assessed by the ultrasound method.
Statistical Analysis
The overall outcome variable in this study was subclinical atherosclerosis as measured in carotid and coronary arteries by ultrasound and CT, respectively, structured to reflect 4 stages of atherosclerosis. The first stage was assessed by the level of common carotid artery IMT in the absence of known ongoing advanced atherosclerotic process. Such advanced process would be indicated by any visualizable carotid artery stenosis (which generally occurs in the bulb or internal carotid segment18) or CAC. This earliest stage is assumed to represent slow remodeling. The next stage, considered to be faster remodeling, was assessed by the level of common carotid artery IMT in people who had a known ongoing atherosclerotic process. The third stage, advanced atherosclerotic plaque, was stenosis anywhere in the carotid artery. The fourth stage, plaque organization, was a nonzero Agatston score, indicating calcified plaque. We focused on occurrence of CAC progression as a dichotomy (any incident CAC or increased Agatston score between years 15 and 20). Among participants with any change in Agatston score between years 15 and 20, 95% progressed to a higher score at year 20.
This analysis was based on subjects who participated in the year 15 and year 20 examinations that occurred between June, 2000 and June, 2001 and between June, 2005 and June, 2006, respectively, and who had available fasting serum samples. After excluding 4 participants with year 15 serum creatinine ≥2 mg/dl, there were n=2587 participants with no missing covariate data and any of the outcome variables measured; these formed the basis for Table 1. Analysis of common carotid artery IMT (n=2240) was done stratified on absence or presence of CAC at year 20 or carotid artery stenosis (stages 1 and 2); n=2432 for occurrence of carotid artery stenosis (stage 3); and n=2378 for the occurrence of CAC progression (stage 4).
Table 1.
Participant characteristics by year 15 sICAM-1 concentration
| Year 15 sICAM-1 Percentile Categories | |||||||
|---|---|---|---|---|---|---|---|
| sICAM-1 (ng/ml) | Total | <10 | 10–49 | 50–89 | 90–94 | ≥95 | P-trend |
| N | 2587 | 258 | 1035 | 1036 | 129 | 129 | |
| Median | 145.9 | 103.8 | 129.9 | 164.7 | 214.9 | 254.4 | |
| Range and cutpoints | 55.8, 820.8 | 55.8, 110.8 | 110.9, 145.9 | 146.0, 205.7 | 205.8, 229.6 | 229.8, 820.8 | |
| Demographic Year 15 | |||||||
| Age, years | 40.4 (3.6) | 40.7 (3.5) | 40.3 (3.5) | 40.3 (3.6) | 40.8 (3.6) | 40.7 (3.5) | 0.79 |
| Gender, % female | 55 | 66 | 55 | 52 | 52 | 57 | 0.13 |
| Race, % white | 57 | 73 | 67 | 49 | 40 | 36 | <0.001 |
| Education, years | 15.1 (2.5) | 15.9 (2.5) | 15.6 (2.4) | 14.7 (2.5) | 14.0 (2.4) | 13.6 (2.1) | <0.001 |
| Smoking, % current | 20 | 7 | 10 | 25 | 36 | 64 | <0.001 |
| Smoking, % former | 26 | 36 | 26 | 25 | 25 | 16 | <0.001 |
| Exercise total intensity score, EU | 353.9 (281.3) | 395.4 (304.6) | 380.5 (277.6) | 334.4 (280.3) | 270.1 (224.3) | 297.4 (286.4) | <0.001 |
| Anthropometric Year 15 | |||||||
| Body mass index, kg/m2 | 28.5 (6.2) | 25.5 (4.9) | 27.1 (5.4) | 29.8 (6.3) | 32.1 (7.8) | 30.6 (7.6) | <0.001 |
| Waist Girth, cm | 88.9 (14.1) | 80.6 (11.9) | 86.0 (13.1) | 92.1 (13.4) | 97.4 (15.3) | 95.4 (16.1) | <0.001 |
| Systolic blood pressure, mm Hg | 112.8 (14.4) | 108.7 (12.3) | 110.4 (12.7) | 114.9 (15.2) | 117.5 (15.3) | 118.3 (18.4) | <0.001 |
| Diastolic blood pressure, mm Hg | 74.3 (11.2) | 71.0 (10.6) | 72.7 (10.5) | 75.7 (11.2) | 78.6 (11.8) | 78.3 (14.3) | <0.001 |
| Antihypertensive Medication, % | 6.8 | 2.3 | 4.3 | 9.7 | 10.1 | 10.1 | <0.001 |
| Plasma cholesterol, mg/dL | 185.1 (34.7) | 179.2 (31.9) | 184.6 (33.3) | 186.7 (34.4) | 187.1 (39.1) | 185.5 (46.1) | 0.07 |
| High density lipoprotein cholesterol, mg/dL | 50.6 (14.5) | 57.1 (14.8) | 52.6 (14.7) | 48.1 (13.5) | 46.8 (13.0) | 44.7 (13.4) | <0.001 |
| Triglycerides, mg/dL | 104.8 (94.6) | 85.7 (109.3) | 97.9 (101.9) | 111.3 (83.5) | 122.9 (77.0) | 128.0 (91.7) | <0.001 |
| Cholesterol lowering medication, % | 3 | 1 | 2 | 2 | 3 | 10 | 0.004 |
| C-Reactive Protein, µg/ml | 1.3 (0.5–3.6) | 0.7 (0.2–15.8) | 0.9 (0.2, 106.0) | 1.8 (0.2, 78.5) | 3.8 (0.2, 40.4) | 4.4 (0.2–82.5) | <0.001 |
| Estimated Glomerular Filtration Rate, ml/min/1.73m2 | 103.2 (15.9) | 101.8 (14.1) | 101.7 (15.0) | 104.1 (16.8) | 106.5 (16.5) | 108.7 (16.3) | <0.001 |
| Coronary Artery Calcified Plaque (CAC) | |||||||
| Prevalence year 15, % | 10 | 6 | 9 | 10 | 13 | 23 | <0.001 |
| Amount†, AU‡ | 19.3 (5.6–62.1) | 19.8 (7.3–72.6) | 18.6 (5.6–43.7) | 16.8 (5.3–63.3) | 8.2 (4.9–60.6) | 35.8 (8.2–161.2) | 0.22 |
| Prevalence year 20, % | 19 | 12 | 16 | 20 | 25 | 34 | <0.001 |
| Amount†, AU | 24.8 (7.7–88.8) | 15.9 (6.1–82.9) | 26.6 (7.7–71.7) | 20.2 (7.0–86.2) | 22.5 (5.6–61.9) | 63.9 (18.7–200.0) | 0.02 |
| Presence of CAC Progression (Year 15–20), % | 18 | 12 | 16 | 20 | 24 | 33 | <0.001 |
| Amount†, AU | 19.1 (6.8–59.6) | 13.4 (5.1–66.3) | 18.5 (7.6–45.9) | 16.4 (6.6–68.3) | 19.4 (5.6–43.0) | 50.8 (12.4–184.8) | 0.01 |
| Common Carotid Artery Year 20 | |||||||
| Stenosis prevalence, % | 18 | 13 | 17 | 19 | 20 | 33 | <0.001 |
| Intima media thickness, mm | 0.80 (0.13) | 0.77 (0.10) | 0.78 (0.12) | 0.82 (0.13) | 0.84 (0.12) | 0.86 (0.14) | <0.001 |
Table entries are percentage, mean (SD), or median (25th–75th percentile), p value is a test of trend in linear regression
EU: Exercise Units
Non-zero values
AU: Agatston Units
sICAM-1 was modeled both as a continuous variable and in categories. We hypothesized that sICAM-1 concentration is associated with stenosis, CAC progression and IMT. Because our study population comprised young adults with presumably low sICAM-1 concentrations, it was possible that if there were a threshold effect of sICAM-1 on atherogenesis, such effect would only be observed among participants with the highest sICAM-1 concentrations; therefore unequal size categories were used to reflect sICAM-1 concentrations that would have different biological importance. Categories were formed by initially creating quintiles, then subdividing the lowest and highest quintiles. Thus, the lowest 2 categories were participants below the 10th percentile and within the 10th–19th percentile, the next 3 categories were quintiles 2–4 (20th–39th percentile, 40th–59th percentile, and 60th–79th percentile), the 6th category comprised participants in the 80th–89th percentile, the 7th category included participants in the 90th to 94th percentiles, and the 8th category consisted of participants with sICAM-1 at the 95th percentile or above. Middle categories were grouped for simplicity in Table 1. Tests for trend were based on continuous sICAM-1.
Linear regression models were used to model association of sICAM-1 with the common carotid artery IMT, including a product term for the presence or absence of advanced atherosclerotic plaque. Logistic regression methods were used to predict dichotomous variables indicating CAC progression and carotid artery stenosis. Two models were considered: minimally adjusted and multivariable adjusted. The minimal model included age, sex, race and examination center. Further adjustments for year 15 values of years of education, smoking status (never, former, current), physical activity, BMI, waist circumference, blood pressure, antihypertensive medication use, total cholesterol, triglycerides, HDL cholesterol, and cholesterol lowering medication use were included in the multivariable model.
A sensitivity analysis included CRP and eGFR as explanatory covariates on the theory that the associations observed for sICAM-1 would be partly explainable by inflammation and kidney function. All analyses were performed using SAS Version 9.2 (SAS Institute Inc. Cary NC).
Results
The mean sICAM-1 concentration was 153.8 ng/ml (standard deviation (SD)= 44.2 ng/ml). The mean sICAM-1 concentration in men (155.2 ng/ml, SD=41.4) was similar to that in women (152.6 ng/ml, SD=46.5, p=0.139); blacks had a greater sICAM-1 concentration (165.1 ng/ml, SD=49.6) than whites (145.5 ng/ml, SD=37.8, p<0.001). Table 1 shows the median 145.9 ng/ml and the interquartile range 125.8 to 171.7 ng/ml; considerable right skewness was indicated by the 95th percentile of 229.6 and maximum of 820.8 ng/ml. Soluble ICAM-1 was associated with many traditional CVD risk factors including a strong positive association with current smoking. Other positive associations were with black race, blood pressure, and antihypertensive medication use, BMI, waist circumference, and total cholesterol, triglycerides, and cholesterol lowering medication use. Inverse associations were with exercise and HDL-cholesterol.
Carotid artery stenosis occurred in 18.4% of participants. Carotid artery stenosis and common segment IMT both increased in a graded fashion across sICAM-1 categories in unadjusted analyses. CAC was present in 10.1% of subjects in year 15 (n=260, median Agatston score 19.3) and in 18.7% at year 20 (n=449, median Agatston score 24.8, 75th percentile 88). The prevalence of CAC at years 15 and 20 trended upward with increasing sICAM-1 concentration. As a result, occurrence of CAC progression between years 15 and 20 increased from those below the 10th sICAM-1 percentile (12% of participants) to those above the 95th sICAM-1 percentile (33% of participants), although amount of Agatston score progression was clearly higher only above the 95th sICAM-1 percentile. The strength and direction of the associations with demographic and clinical characteristics were similar for CAC progression to those associations previously reported for year 15 CAC23.
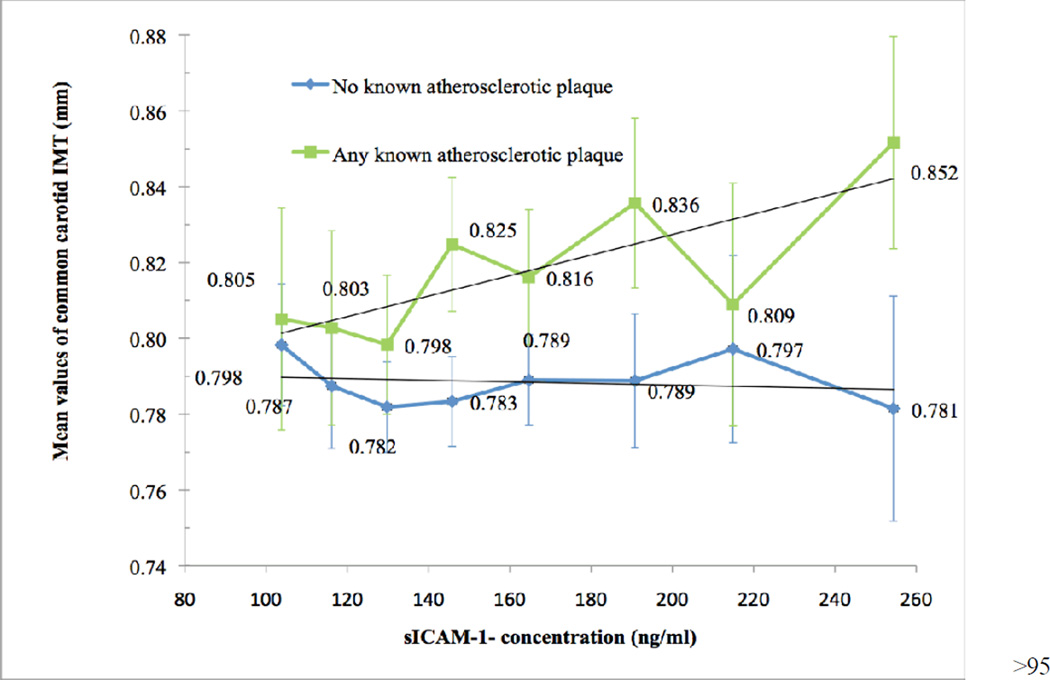
Soluble ICAM-1 concentrations were not associated with common carotid artery IMT in adjusted analyses (0.004 mm (95% CI: −0.001–0.009 mm) per SD of sICAM-1). To pursue the model of 4 atherosclerotic stages, we stratified the sample by presence of advanced plaque in either the carotid or the coronary arteries (prevalence in either location: 31.9%). In the fully adjusted model (Figure 1), the subgroup with any advanced atherosclerotic plaque showed an increase in common carotid artery IMT of 0.010 mm per SD of sICAM-1 (95% CI 0.003–0.017 mm), while the corresponding coefficient in those with no advanced plaque was close to zero (p for interaction between atherosclerosis subgroups = 0.009). Among those with any advanced plaque detected, the mean IMT generally increases above the 40th percentile of sICAM-1 and the increase was substantial above the 95th percentile of IMT.
Fig. 1. Adjusted association of sICAM-1 concentration at year 15 with common carotid artery IMT at year 20 by presence of atherosclerotic plaque (carotid artery stenosis or CAC).
Shown are 95% confidence bars and fitted lines. Data fully adjusted for age, sex, race, exam center, and year-15 variables: smoking status, exercise level, BMI, waist circumference, education (years), systolic blood pressure, diastolic blood pressure, antihypertensive medication use, plasma cholesterol, HDL cholesterol, triglycerides, and cholesterol-lowering medication use.
A significant association was found between sICAM-1 concentration and both presence of carotid artery stenosis and CAC progression (Table 2). Every SD increase in sICAM-1 was associated in the linear fit model with a fully-adjusted OR for carotid artery stenosis of 1.12 (95% CI: 1.01–1.25). Most of this association appeared to be driven by sICAM-1 concentrations above the 95th percentile, which had a fully adjusted OR of 1.95 (95% CI: 1.07–3.58) compared to those below the 10th percentile. Every SD increase in sICAM-1 was associated in the linear fit model with an odds ratio of 1.16 (95% CI: 1.03–1.30) increase in the fully adjusted odds ratio of CAC progression between year 15 and 20. Statistically significant increase for individual sICAM-1 category was only found above the 95th percentile (OR: 1.90, 95% CI: 1.00–3.64). While, a threshold model may be visually appealing, some ambiguity is present, in that adding an indicator for being above the 95th percentile to the fully adjusted linear fit model does not yield a statistically significant improvement in the fit for either carotid artery stenosis or CAC progression.
Table 2.
Presence of carotid artery stenosis at year 20 and Coronary Artery Calcification (CAC) Progression between year 15 and 20 predicted by sICAM-1.
| Minimally Adjusted† |
Fully adjusted‡ | |||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| A. Presence of Carotid Artery Stenosis at year 20 | ||||
| sICAM-1: Per 44.2 ng/ml (n = 2432) | 1.23 (1.11–1.36) | <0.001 | 1.12 (1.01–1.25) | 0.03 |
| sICAM-1: Categories | ||||
| <10 Percentile (reference) | 1 | 1 | ||
| 10–19 vs. <10 Percentile | 1.44 (0.87–2.39) | 0.19 | 1.38 (0.82–2.30) | 0.26 |
| 20–39 vs. <10 Percentile | 1.39 (0.88–2.18) | 0.14 | 1.29 (0.82–2.05) | 0.25 |
| 40–59 vs. <10 Percentile | 1.57 (1.00–2.46) | 0.06 | 1.34 (0.84–2.12) | 0.22 |
| 60–79 vs. <10 Percentile | 1.32 (0.84–2.08) | 0.23 | 1.08 (0.67–1.72) | 0.75 |
| 80–89 vs. <10 Percentile | 1.66 (1.01–2.75) | 0.05 | 1.21 (0.71–2.06) | 0.45 |
| 90–94 vs. <10 Percentile | 1.42 (0.78–2.59) | 0.33 | 1.00 (0.53–1.89) | 0.86 |
| ≥95 vs. <10 Percentile | 3.03 (1.73–5.30) | <0.001 | 1.95 (1.07–3.58) | 0.03 |
| B. Occurrence of CAC progression between year 15 and 20 | ||||
| sICAM-1: Per 44.2 ng/ml (n = 2378) | 1.35 (1.21–1.50) | <0.001 | 1.16 (1.03–1.30) | 0.01 |
| sICAM-1: Categories | ||||
| <10 Percentile (reference) | 1 | 1 | ||
| 10–19 vs. <10 Percentile | 1.28 (0.75–2.21) | 0.37 | 1.20 (0.69–2.10) | 0.52 |
| 20–39 vs. <10 Percentile | 1.23 (0.76–2.00) | 0.39 | 1.06 (0.65–1.75) | 0.81 |
| 40–59 vs. <10 Percentile | 1.58 (0.99–2.54) | 0.07 | 1.21 (0.74–1.98) | 0.45 |
| 60–79 vs. <10 Percentile | 1.62 (1.00–2.61) | 0.06 | 1.09 (0.66–1.80) | 0.73 |
| 80–89 vs. <10 Percentile | 2.15 (1.27–3.65) | 0.004 | 1.30 (0.74–2.28) | 0.36 |
| 90–94 vs. <10 Percentile | 2.26 (1.23–4.15) | 0.009 | 1.30 (0.68–2.47) | 0.44 |
| ≥95 vs. <10 Percentile | 3.95 (2.20–7.12) | <0.001 | 1.94 (1.00–3.71) | 0.05 |
Minimal Adjustment: year 15 age, sex, race, exam center.
Full Adjustment: Minimal adjustment + year 15 variables: smoking status, exercise level, body mass index, waist circumference, education (years), systolic blood pressure, diastolic blood pressure, antihypertensive medication use, plasma cholesterol, high density lipoprotein cholesterol, triglycerides, and cholesterol lowering medication use.
The age, race, and sex adjusted correlations of year 15 CRP and eGFR with sICAM-1 were 0.25 and 0.05, respectively. In a model to study whether inflammation or kidney function mediated the associations with sICAM-1, we found that further adjustment of the models predicting common carotid IMT, carotid artery stenosis or CAC progression for year 15 CRP concentration and eGFR did not substantively alter the findings (data not shown). Additionally there was no interaction between sICAM-1 and CRP on CAC progression, stenosis or IMT.
Discussion
In a prospective study of healthy blacks and whites, circulating levels of sICAM-1 above the 40th percentile were associated significantly and in a graded fashion with common carotid artery IMT in subjects with advanced plaque (faster remodeling). This association of a marker of endothelial dysfunction with changes in the arterial intima, which consists primarily of endothelial cells, links sICAM-1 with early events in the atherosclerotic process. Furthermore, higher values of sICAM-1 were associated with carotid artery stenosis (atherosclerosis) and CAC progression (plaque organization). These associations were independent of traditional CVD risk factors and of C-reactive protein. Thus, the study indicates an early (mean age=40) and independent involvement of sICAM-1 in the development of atherosclerosis. Interestingly, no association was found with the slower remodeling of the common carotid artery IMT in subjects with neither carotid artery stenosis nor CAC. The pattern of sICAM-1 association with carotid artery stenosis, but not IMT is consistent with two prior studies7–8 (mean age <50 years with low risk of cardiovascular disease). Also concordant with our study, the presence of carotid plaque was associated with elevated sICAM-1 concentration in healthy 58 year old men24. It is notable that the association in the current study occurred in the context of relatively low burdens of advanced plaque, specifically carotid artery stenosis in 18.4% of subjects at year 20 and CAC in 18.7%, and total calcium score at year 20 less than 88 in 75% of those participants with a nonzero Agatston score. These finding are consistent with a model of early atherosclerosis as described in the Statistical Analysis section, wherein CAC and carotid artery stenosis are preceded by an increase in common carotid IMT, particularly in those who display evidence that the atherosclerotic process has already started.
Associations of sICAM-1 with carotid artery IMT may depend on CVD status, since several clinical studies have found such associations in patients with a high risk of CVD. In 30 people who had both hypertension and diabetes25 elevated levels of sICAM-1 correlated with high carotid artery IMT. In a sample of hypercholesterolemic subjects, sICAM-1 was associated with an elevated carotid artery IMT26. Thus, these studies found an association of sICAM-1 with subclinical atherosclerosis, which was not found in the low risk populations described above. The association with IMT may also reflect that participants in these studies were older than in studies of low risk populations that did not find an association with IMT. Our findings are in agreement with these studies in the sense that sICAM-1 was associated with common carotid artery IMT only in those participants in whom carotid or coronary artery advanced plaque also was present.
An association of sICAM-1 with subclinical CVD is consistent with the biological role of ICAM-1. ICAM-1 is expressed on the surface of endothelial cells in response to tissue damage and inflammation1, and possibly additional environmental factors27–28. It facilitates the capture and movement of monocytes and leukocytes into the subendothelial space of blood vessels1, 29–30. Uptake of these cells produces an increase in oxidative damage with the formation of oxidized LDL particles and induction of several atherogenic factors, which induce the formation of atherosclerotic plaque2. The essential nature of ICAM-1 in the pathogenesis of atherosclerosis is indicated by knockout mice30. Knockout of the ICAM-1 gene prevents the development of atherosclerosis. It should be noted that sICAM-1 is a proteolytic product of ICAM-1 and an indicator of endothelial dysfunction. The sICAM-1 concentrations in blood are a reflection of ICAM-1 expression on the endothelial cell surface and are a good indicator of tissue ICAM-1 concentrations.
Our findings differ from those of a few previous studies in that the strong minimally adjusted association of sICAM-1 with CAC remained significant after adjustment for traditional risk factors and C-reactive protein. The association of sICAM-1 and CAC did not remain significant in fully-adjusted models of four studies14–17. However, these studies were limited by small sample sizes14,17, and low sICAM-1 assay precision16 Tang et al.15 studied 2246 white family members in the NHLBI Family Heart Study, mean age 55 years. Unadjusted or minimally adjusted associations of sICAM-1 with CAC are concordant with our findings, but we could not identify obvious differences between our study and these other studies that would explain why sICAM-1 remained significant after full adjustment in our study.
It is noteworthy that the increased risk of CAC progression in our study was largely found in those with sICAM-1 concentrations above the 80th percentile, and even more so above the 95th percentile, although our statistical tests could not clearly distinguish a linear from a threshold model. If a threshold model were correct, another factor that induced exceptionally high sICAM-1 could be important. For example, genetic state could be important, since several SNP variants in the ICAM gene have been associated with elevated sICAM-1 concentration14, 32–34.
An association between inflammation and expression of sICAM-1 is well-established1, 35–37. The induction of sICAM-1 as part of an inflammatory response has been demonstrated in animal models and clinical studies30. In many studies of older adults and CVD patients, inflammation may be a primary factor in the regulation of ICAM-1 and associations with sICAM-1 can be attenuated by adjustment for CVD risk factors, such as C-reactive protein. However, in the current study, the association between sICAM-1 and the subclinical measures was not influenced by C-reactive protein levels. This lack of influence by C-reactive protein may reflect the young age and relative absence of CVD in this cohort of young healthy adults. Furthermore, it may indicate the importance of sICAM-1 outside the classical inflammatory response that occurs in individuals with high plaque burden prior to clinical events.
This investigation has several strengths. CARDIA is a large population-based study with ample sample size in two racial groups. It is unique in that the subjects were recruited at a relatively young age allowing for the evaluation of risk factors at the earliest stages of atherosclerosis and it has had routine follow-up exams, approximately every five years. The study also has limitations. Levels of sICAM-1 were only measured during the year 15 examination; thus limiting our ability to assess the importance of associations in those younger than the minimum age at year 15 (33 years) and the effect of changes in sICAM concentration on subclinical disease.
In conclusion, these data extend our understanding by showing that moderate and higher sICAM-1 concentrations were significantly associated with common carotid artery IMT when a known atherosclerotic process was observed elsewhere in the subject, while individuals with the highest levels of sICAM-1 had increased occurrence of both carotid artery stenosis and CAC, independent of traditional risk factors in a relatively young healthy population. However, in individuals without evidence of advanced atherosclerotic plaque, no relation was present between sICAM-1 and common carotid artery IMT. These results suggest that sICAM-1 concentrations may be an early biomarker that indicates disturbance of the endothelium and the presence of advanced plaque in the coronary and carotid arteries even in young adults with low total burden of atherosclerosis and decades prior to the development of clinical CVD.
Acknowledgment
Supported by the National Heart, Lung, and Blood Institute of the United States (YALTA: NIH 1RO1-HL53560-01A1 and CARDIA: Field centers NO1-HC-48047 through 48050, Coordinating Center N01-HC-95095, and reading subcontracts from the Coordinating Center to Harbor-UCLA Research Education Institute, Computed Tomography Reading Center, N01-HC-05187, Wake Forest University Health Sciences, Computed Tomography Reading Center, HHSN268200425205C, and New England Medical Center Hospitals, Inc., Ultrasound Reading Center, HHSN268200425204C.
References
- 1.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96(12):4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 6.Malik I, Danesh J, Whincup P, Bhatia V, Papacosta O, Walker M, Lennon L, Thomson A, Haskard D. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358(9286):971–976. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- 7.Bongard V, Elias A, Bal dit Sollier C, Ruidavets J, Boccalon H, Drouet L, Ferrieres J. Soluble intercellular adhesion molecule-1 is associated with carotid and femoral atherosclerosis but not with intima-media thickness in a population-based sample. Atherosclerosis. 2002;164(2):297–304. doi: 10.1016/s0021-9150(02)00071-0. [DOI] [PubMed] [Google Scholar]
- 8.Amar J, Fauvel J, Drouet L, Ruidavets JB, Perret B, Chamontin B, Boccalon H, Ferrieres J. Interleukin 6 is associated with subclinical atherosclerosis: a link with soluble intercellular adhesion molecule 1. Journal of hypertension. 2006;24(6):1083–1088. doi: 10.1097/01.hjh.0000226198.44181.0c. [DOI] [PubMed] [Google Scholar]
- 9.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, Rifai N, Libby P, Creager MA, Ridker PM. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18(11):1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 10.Papagianni A, Kalovoulos M, Kirmizis D, Vainas A, Belechri AM, Alexopoulos E, Memmos D. Carotid atherosclerosis is associated with inflammation and endothelial cell adhesion molecules in chronic haemodialysis patients. Nephrol Dial Transplant. 2003;18(1):113–119. doi: 10.1093/ndt/18.1.113. [DOI] [PubMed] [Google Scholar]
- 11.Kondo K, Kitagawa K, Nagai Y, Yamagami H, Hashimoto H, Hougaku H, Hori M. Associations of soluble intercellular adhesion molecule-1 with carotid atherosclerosis progression. Atherosclerosis. 2005;179(1):155–160. doi: 10.1016/j.atherosclerosis.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Glowinska-Olszewska B, Tolwinska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocrinol Metab. 2007;20(10):1125–1136. [PubMed] [Google Scholar]
- 13.Karasek D, Vaverkova H, Halenka M, Budikova M, Novotny D. Soluble cell adhesion molecules s-VCAM-1 and s-ICAM-1 in subjects with familial combined hyperlipidemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):101–108. doi: 10.5507/bp.2005.012. [DOI] [PubMed] [Google Scholar]
- 14.Reilly MP, Wolfe ML, Dykhouse J, Reddy K, Localio AR, Rader DJ. Intercellular adhesion molecule 1 (ICAM-1) gene variant is associated with coronary artery calcification independent of soluble ICAM-1 levels. J Investig Med. 2004;52(8):515–522. doi: 10.1136/jim-52-08-23. [DOI] [PubMed] [Google Scholar]
- 15.Tang W, Pankow JS, Carr JJ, Tracy RP, Bielinski SJ, North KE, Hopkins PN, Kraja AT, Arnett DK. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord. 2007;7:30. doi: 10.1186/1471-2261-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohatgi A, Owens AW, Khera A, Ayers CR, Banks K, Das SR, Berry JD, McGuire DK, de Lemos JA. Differential associations between soluble cellular adhesion molecules and atherosclerosis in the Dallas Heart Study: a distinct role for soluble endothelial cell-selective adhesion molecule. Arterioscler Thromb Vasc Biol. 2009;29(10):1684–1690. doi: 10.1161/ATVBAHA.109.190553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao AH, Wasko MC, Krishnaswami S, Wagner J, Edmundowicz D, Shaw P, Cunningham AL, Danchenko N, Sutton-Tyrrell K, Tracy RP, Kuller LH, Manzi S. C-reactive protein and coronary artery calcium in asymptomatic women with systemic lupus erythematosus or rheumatoid arthritis. Am J Cardiol. 2008;102(6):755–760. doi: 10.1016/j.amjcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zureik M, Temmar M, Adamopoulos C, Bureau JM, Courbon D, Thomas F, Bean K, Touboul PJ, Ducimetière P, Benetos A. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens. 2002 Jan;20(1):85–93. doi: 10.1097/00004872-200201000-00013. PubMed PMID: 11791030. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs DR, Hahn LP, Haskell WL, et al. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007 May 22;49(20):2013–2020. doi: 10.1016/j.jacc.2007.03.009. Epub 2007 May 4. PubMed PMID: 17512357. [DOI] [PubMed] [Google Scholar]
- 24.Hulthe J, Wikstrand J, Mattsson-Hultén L, Fagerberg B. Circulating ICAM-1 (intercellular cell-adhesion molecule 1) is associated with early stages of atherosclerosis development and with inflammatory cytokines in healthy 58-year-old men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond) 2002 Aug;103(2):123–129. doi: 10.1042/cs1030123. [DOI] [PubMed] [Google Scholar]
- 25.Rubio-Guerra AF, Vargas-Robles H, Serrano AM, Vargas-Ayala G, Rodriguez-Lopez L, Escalante-Acosta BA. Correlation between the levels of circulating adhesion molecules and atherosclerosis in hypertensive type-2 diabetic patients. Clin Exp Hypertens. 2010;32(5):308–310. doi: 10.3109/10641960903443533. [DOI] [PubMed] [Google Scholar]
- 26.Wiklund O, Hulthe J, Bondjers G, Hurt-Camejo E. Cell adhesion molecules and secretory type II phospholipase A2 in relation to carotid atherosclerosis in patients with hypercholesterolaemia. J Intern Med. 2001 May;249(5):441–449. doi: 10.1046/j.1365-2796.2001.00827.x. [DOI] [PubMed] [Google Scholar]
- 27.Sjögren P, Cederholm T, Heimbürger M, Stenvinkel P, Vedin I, Palmblad J, Hellenius ML. Simple advice on lifestyle habits and long-term changes in biomarkers of inflammation and vascular adhesion in healthy middle-aged men. Eur J Clin Nutr. 2010 Dec;64(12):1450–1456. doi: 10.1038/ejcn.2010.182. [DOI] [PubMed] [Google Scholar]
- 28.Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpí-Sardà M, Llorach R, Lamuela-Raventós RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. 2009 Nov;90(5):1144–1150. doi: 10.3945/ajcn.2009.27716. Epub 2009 Sep 23. [DOI] [PubMed] [Google Scholar]
- 29.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [CrossRef][Medline] [Order article via Infotrieve] [DOI] [PubMed] [Google Scholar]
- 30.Laudanna C, Alon R. Right on the spot: chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb Haemost. 2006;95(1):5–11. [PubMed] [Google Scholar]
- 31.Bullard DC. Adhesion molecules in inflammatory diseases: insights from knockout mice. Immunol Res. 2002;26(1–3):27–33. doi: 10.1385/IR:26:1-3:027. [DOI] [PubMed] [Google Scholar]
- 32.Zee RY, Cheng S, Erlich HA, Lindpaintner K, Rifai N, Buring JE, Ridker PM. Intercellular adhesion molecule 1 (ICAM1) Lys56Met and Gly241Arg gene variants, plasma-soluble ICAM1 concentrations, and risk of incident cardiovascular events in 23,014 initially healthy white women. Stroke. 2007 Dec;38(12):3152–3157. doi: 10.1161/STROKEAHA.107.490219. [DOI] [PubMed] [Google Scholar]
- 33.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF, Jr, Vasan RS, Mitchell GF, Benjamin EJ. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008 Jun;51(6):1651–1657. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert MA, Pare G, Morris A, Rose L, Buring J, Ridker PM, Zee RY. Candidate genetic variants in the fibrinogen, methylenetetrahydrofolate reductase, and intercellular adhesion molecule-1 genes and plasma levels of fibrinogen, homocysteine, and intercellular adhesion molecule-1 among various race/ethnic groups: data from the Women's Genome Health Study. Am Heart J. 2009 Apr;157(4) doi: 10.1016/j.ahj.2008.12.012. 777-83.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bijanzadeh M, Ramachandra NB, Mahesh PA, Savitha MR, Vijayakumar GS, Kumar P, Manjunath BS, Jayaraj BS. Soluble intercellular adhesion molecule-1 and E-selectin in patients with asthma exacerbation. Lung. 2009 Sep-Oct;187(5):315–320. doi: 10.1007/s00408-009-9171-5. Epub 2009 Aug 23. [DOI] [PubMed] [Google Scholar]
- 36.Navarro-Hernández RE, Oregon-Romero E, Vázquez-Del Mercado M, Rangel-Villalobos H, Palafox-Sánchez CA, Muñoz-Valle JF. Expression of ICAM1 and VCAM1 serum levels in rheumatoid arthritis clinical activity. Association with genetic polymorphisms. Dis Markers. 2009;26(3):119–126. doi: 10.3233/DMA-2009-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluz J, Kopeć W, Jakobsche-Policht U, Adamiec R. Circulating endothelial cells, endothelial apoptosis and soluble markers of endothelial dysfunction in patients with systemic lupus erythematosus-related vasculitis. Int Angiol. 2009 Jun;28(3):192–201. [PubMed] [Google Scholar]