Abstract
Numerous studies have demonstrated a sexual dimorphism of the human corpus callosum. However, the question remains if sex differences in brain size, which typically is larger in men than in women, or biological sex per se account for the apparent sex differences in callosal morphology. Comparing callosal dimensions between men and women matched for overall brain size may clarify the true contribution of biological sex, as any observed group difference should indicate pure sex effects. We thus examined callosal morphology in 24 male and 24 female brains carefully matched for overall size. In addition, we selected 24 extremely large male brains and 24 extremely small female brains to explore if observed sex effects might vary depending on the degree to which male and female groups differed in brain size. Using the individual T1-weighted brain images (n=96), we delineated the corpus callosum at midline and applied a well-validated surface-based mesh-modeling approach to compare callosal thickness at 100 equidistant points between groups determined by brain size and sex. The corpus callosum was always thicker in men than in women. However, this callosal sex difference was strongly determined by the cerebral sex difference overall. That is, the larger the discrepancy in brain size between men and women, the more pronounced the sex difference in callosal thickness, with hardly any callosal differences remaining between brain-size matched men and women. Altogether, these findings suggest that individual differences in brain size account for apparent sex differences in the anatomy of the corpus callosum.
Keywords: Brain, Corpus Callosum, Gender, MRI, Sex
Introduction
A pioneering study in the early eighties reported a larger and more bulbous callosal splenium in women than in men (DeLacoste-Utamsing and Holloway, 1982). Given the novelty of this finding then, and its possible relevance for understanding apparent sex differences in cognition, emotion, and behavior, the sexual dimorphism of the human corpus callosum has been a continued object of exploration ever since. Some studies were able to replicate the direction of this initially reported sex effect (females > males), and most of them adjusted for the smaller brain size in women (see meta-analyses by Driesen and Raz, 1995). However, evidence seems to accumulate that unadjusted callosal size is larger in males, and sex differences may disappear when statistically correcting for brain size (see meta-analysis by Bishop and Wahlsten, 1997). The outcomes of one of our earlier studies addressing the sexual dimorphism of the corpus callosum (Luders et al., 2006b) are in close agreement with the latter conclusion. To clarify whether callosal measurements and related sex effects are affected by brain size adjustments, we had conducted comparisons with respect to unscaled callosal measures (i.e., unadjusted for brain size), obtained from brain images standardized using 6-parameter (rigid-body) transformations. In addition, we had examined scaled callosal measures (i.e., adjusted for brain size), obtained from brain images standardized using 12-parameter (affine) transformations. When we compared unscaled callosal size, men had significantly larger callosal dimensions than women. However, when we compared scaled callosal size, there were no significant differences between men and women, and we concluded that any sex differences in callosal size are largely accounted for by sex differences in brain size (Luders et al., 2006b).
Nevertheless, given that allometric relationships between brain size and callosal size have been proposed as well as demonstrated (Ringo et al., 1994; Steinmetz et al., 1996; Jancke et al., 1997; Jancke et al., 1999; Leonard et al., 2008; Bruner et al., 2012), there is still some uncertainty (and controversy) with respect to the appropriate procedure to properly account for individual brain size (Bishop and Wahlsten, 1997; Bermudez and Zatorre, 2001; Smith, 2005; Barnes et al., 2010). An ideal solution for studying the sexual dimorphism of the corpus callosum, independently of brain size, would be to extract callosal measures from male and female brains of equal size. An early study of Jancke and colleagues used an elegant approach, where they divided their sample of 120 subjects into forebrain volume (FBV) quintiles, with 24 brains per quintile (Jancke et al., 1997). For each quintile, FBV and callosal measures were compared between men and women. With one exception1, no significant gender differences were observed, and the authors drew the following conclusion based on the aforementioned analysis as well as other analyses conducted in that study: “We suggest that the previously described gender differences in CC anatomy may be better explained by an underlying effect of brain size, with larger brains having relatively smaller callosa” (Jancke et al., 1997). Another, more recent study has conducted callosal analyses in men and women who were truly group-matched for intracranial volume (Sullivan et al., 2001). Interestingly, it was revealed that men, with comparable brain size to women, still had larger midsagittal callosal areas suggesting that “sexual dimorphism in the corpus callosum is not a simple artifact of sex differences in brain size” (Sullivan et al., 2001).
To our knowledge, no other study has compared callosal features in equally sized male and female brains. The sparseness of such analyses is probably attributable to the difficulty of stumbling on men and women with similar brain size, as intracranial volumes usually differ greatly between the two sexes. Fortunately, the recent establishment of databases encompassing thousands of brain images offers a unique opportunity to select from a vast pool of subjects. In this study we used the International Consortium for Brain Mapping (ICBM) database (Mazziotta et al., 2009) to select 24 male brains and 24 female brains, matched for size. We applied a well-validated anatomical surface-based mesh-modeling approach to compare men and women with respect to callosal thickness (Luders et al., 2006a). Any significant differences would indicate pure sex effects independent of brain size. Moreover, we complemented the aforementioned matched sample by an extreme sample that included 24 extra large [XL] male brains and 24 extra small [XS] female brains. We hypothesized that, if brain size had a significant impact on callosal size, observed sex effects should vary depending on the degree to which male and female subgroups differed in brain size.
Methods
Image Acquisition and Subject Selection
All brain images were acquired on the same site (UCLA) and on the same scanner (a Siemens Sonata 1.5-T MRI system) using a 3D T1-weighted sequence (MPRAGE) with the following parameters: TR = 1900 ms; TE = 4.38 ms; flip angle = 15°; 160 contiguous 1 mm sagittal slices; FOV = 256 mm × 256 mm; matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm. All subjects gave informed consent according to institutional guidelines by the University of Los Angeles, California (UCLA) Institutional Review Board. The initial sample, obtained from the ICBM database of normal adults (http://www.loni.ucla.edu/ICBM/), included 153 healthy subjects (72 men / 81 women) ranging between 18 and 82 years. Subjects with any medical disorders that could affect brain structure or function as well as subjects with anatomical abnormalities in their MRI scans had been excluded from this database (Mazziotta et al., 2009). All subjects older than 70 years were additionally excluded from the present study to minimize confounding effects of age-related callosal atrophy. The brain images of the remaining 145 subjects (72 men / 73 women) were processed as detailed below in order to determine brain size - more specifically, total intracranial volume (TIV).
Determining Brain Size
In order to calculate TIV, images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html), as detailed elsewhere (Luders et al., 2009). This procedure resulted in three main tissue compartments in each subject’s native space: gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The tissue volumes (in ml) were determined by counting the voxels representing GM, WM, and CSF. Subsequently, TIV (in ml) was calculated by adding the GM, WM, and CSF volumes. Based on the individual TIVs, two samples of subjects were created as detailed below – without any overlap between samples to ensure independence of data.
Creating Samples of Interest
Sample I, referred to as the matched sample, consisted of 48 subjects (24 men / 24 women) carefully matched for TIV. The maximum difference of TIV within a matched pair was 5.16 ml. Age ranged between 21–61 years (matched men) and 19–69 years (matched women). There was no significant difference in mean age between matched men and matched women. A summary of group-specific TIV and age is provided in Table 1 (left columns). The matched sample included 5 left-handers (2 men / 3 women); handedness information was obtained using the ICBM Demographic and Neurocognitive Inventory (http://ric.uthscsa.edu:9000/icbm_dani/).
Table 1.
Group-specific descriptive statistics (TIV and age)
| Matched Sample (n=48) | Extreme Sample (n=48) | |||
|---|---|---|---|---|
| 24 matched men | 24 matched women | 24 extreme [XL] men | 24 extreme [XS] women | |
| TIV | 1406.57 ± 101.69 | 1406.62 ± 101.41 | 1623.74 ± 68.94 | 1221.58 ± 62.99 |
| Age | 42.96 ± 12.31 | 43.88 ± 14.74 | 39.33 ± 14.79 | 45.75 ± 13.64 |
TIV (total intracranial volume) is shown in ml (mean ± standard deviation).
Age is shown in years (mean ± standard deviation).
Sample II, referred to as the extreme sample, also consisted of 48 subjects (24 men / 24 women) and represented the male subjects with the largest TIVs (extreme [XL] men) as well as the female subjects with the smallest TIVs (extreme [XS] women). Age ranged between 18–69 years (extreme [XL] men) and 19–65 years (extreme [XS] women). There was no significant difference in mean age between XL men and XS women. Please see Table 1 (right columns). The extreme sample included 7 left-handers (4 men / 3 women).
Analyzing Callosal Thickness
First, automated radio-frequency bias field corrections were applied to correct image volumes for intensity drifts caused by magnetic field inhomogeneities (Shattuck et al., 2001). Then, linear (6-parameter rigid-body) transformations were applied (Woods et al., 1998) to correct image volumes for differences in head position and orientation, while preserving the brain’s native dimensions. Using the bias-corrected and aligned image volumes, the corpus callosum was outlined automatically, as previously described (Luders et al., 2010). This resulted in two midsagittal callosal segments (i.e., the upper and the lower callosal boundary) for each subject. Each callosal segment was overlaid onto the MR image from which it had been extracted and visually inspected to ensure that automatically generated callosal outlines precisely followed the natural course and boundaries of the corpus callosum.
As detailed elsewhere (Luders et al., 2006a; Luders et al., 2007), to obtain highly localized measures of callosal thickness, we used anatomical surface-based mesh-modeling methods (Thompson et al., 1996a; Thompson et al., 1996b). That is, the upper and lower callosal boundaries were re-sampled at regular intervals to render the discrete points comprising the boundaries spatially uniform. Then, a new segment (the medial core) was automatically created by calculating a spatial average 2D curve from 100 equidistant surface points representing the upper and lower callosal boundaries. Finally, the distances between 100 surface points of the medial core and the 100 corresponding surface points of both the upper and the lower callosal boundaries were computed. These regional distances (in mm) indicate callosal thickness at 100 locations distributed evenly over the callosal surface.
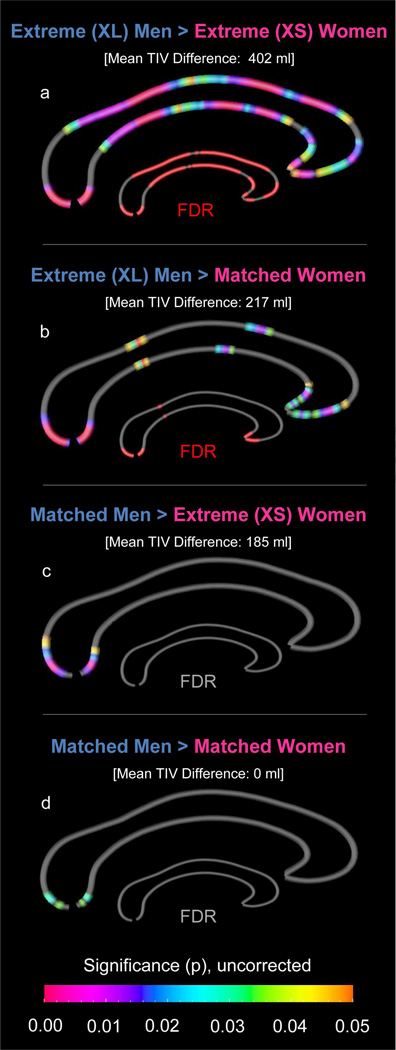
The four subgroups (n=24, each) were compared with respect to point-wise callosal thickness, while removing the variance associated with age. More specifically, we compared (a) extreme [XL] men and extreme [XS] women (mean TIV difference: 402 ml); (b) extreme [XL] men and matched women (mean TIV difference: 217 ml); (c) matched men and extreme [XS] women (mean TIV difference: 185 ml); and (d) matched men and matched women (mean TIV difference: 0 ml). Significance values, uncorrected at p=0.05 as well as corrected using False Discovery Rate (FDR) (Benjamini and Hochberg, 1995) at q=0.05, were projected onto the group-averaged callosal surface models.
Results
As illustrated in Figure 1, when comparing the four subgroups defined by sex and brain size, the corpus callosum was always thicker in men than in women. However, this apparent sex effect was clearly driven by brain size. That is, the larger the sex difference in brain size, the more pronounced the sex difference in callosal thickness (i.e., the smaller the p-value and the more extended the effect).
Figure 1.
Sex differences between the four sub-samples (24 matched men, 24 matched women 24 extreme [XL] men, 24 extreme [XS] women). The color bar encodes the uncorrected significance (p≤0.05). The smaller callosal maps indicate in red where significant sex differences survived FDR-corrections for multiple comparisons (q≤0.05).
More specifically, when comparing extreme [XL] men and extreme [XS] women (mean TIV difference: 402 ml; Figure 1a), the corpus callosum in men was thicker than in women almost entirely, with the exception of two regions near the posterior and anterior callosal bends (where no significant differences were detected). Sex differences remained largely significant when applying standard corrections for multiple comparisons (critical p=0.036 at q=0.05), as indicated in the smaller FDR-corrected callosal map (in red).
When comparing extreme [XL] men to the women from the matched sample (mean TIV difference: 217 ml; Figure 1b), the corpus callosum was thicker in men within posterior splenium, isthmus, anterior midbody, and rostrum2. Sex differences remained significant within the posterior splenium, isthmus and rostrum (albeit less pronounced) when applying corrections for multiple comparisons (critical p=0.004 at q=0.05).
When comparing the men from the matched sample to extreme [XS] women (mean TIV difference: 185 ml; Figure 1c), the corpus callosum was thicker in men only within the posterior splenium; however, findings did not survive FDR corrections. Similarly, when comparing men and women from the matched sample (mean TIV difference: 0 ml; Figure 1d), the corpus callosum was thicker in men but the significance cluster was restricted to an even smaller cluster within the posterior splenium. Again, this effect did not survive FDR corrections. Regardless of whether corrections for multiple comparisons were applied or not, no callosal region was significantly thicker in women compared to men.
Discussion
Using a well-validated mesh-modeling technique capturing callosal dimensions with an extremely high regional specificity, we investigated the impact of brain size and biological sex on callosal thickness. As expected, the corpus callosum was thicker in men than in women. However, the magnitude of this sex difference seems to be strongly determined by the cerebral sex difference overall. That is, the larger the discrepancy in brain size between men and women, the more pronounced the sex difference in callosal thickness. To our knowledge, this is one of the first studies exploring callosal anatomy in a unique dataset including male and female brains pair-wise matched for brain size and using a distinctive mapping technique providing callosal thickness measures at 100 equidistant surface points. Notably, only one previous study – focusing on midsagittal callosal area – compared 27 men and 22 women who were matched as groups for mean and range in intracranial volume (Sullivan et al., 2001).
Correspondence with Previous Findings
The outcomes of our study suggest that brain size, not sex, is the main denominator of apparent sex differences in callosal morphology, specifically callosal thickness3. While this is in close agreement with a number of studies as discussed further below, it seems to conflict (at least, partially) with findings by Sullivan and colleagues (Sullivan et al., 2001). There, larger midsagittal callosal areas were observed in male brains, regardless of whether one focused on the size-matched subsample or the entire sample and also regardless of whether analyses were conducted on raw callosal measures or size-adjusted measures – achieved by covarying for intracranial volume or residualization. Note though, when expressing callosal measures as a ratio of intracranial volume, even Sullivan and colleagues detected similar sized corpora callosa in men and women, in agreement with our current findings. The partial discrepancy is still somewhat puzzling but potentially due to entirely different approaches in both studies with respect to (I) measuring callosal attributes (i.e., total callosal area vs. point-wise callosal thickness), (II) determining intracranial volume (i.e., extrapolating x-, y-, z-distances from three brain slices vs. obtaining tissue volumes from the entire brain); and (III) matching the male and female subgroups for brain size (i.e., group-wise matching vs. pair-wise matching).
In general, our current findings closely resonate with conclusions drawn based on a meta-analysis of 49 studies published between 1982–1994, suggesting that unadjusted callosal size is larger in men, but that sex differences disappear when statistically correcting for brain size (Bishop and Wahlsten, 1997). They further agree with findings and implications of the early study by Jancke and colleagues (Jancke et al., 1997), who were among the first to suggest that previously described sex differences in callosal anatomy may be driven by an underlying effect of brain size. The present findings are also in agreement with outcomes from more recent studies suggesting that “sex did not contribute unique variance to the relationship between relative corpus callosum size and cerebral volume” (Leonard et al., 2008) and that observed differences in callosal size and shape between men and women “result from size variation, not from sex-related characters” (Bruner et al., 2012). Last but not least, the current findings agree with our own prior observations in an independent sample (30 men / 30 women) using the same mesh-modeling approach (Luders et al., 2006b). In that study, we observed thicker corpora callosa in male brains when analyzing data in their native dimensions (unscaled). These sex differences, however, were no longer evident when analyzing brain-sized corrected (scaled) data. Moreover, in both of our studies, regardless of brain size adjustments (via scaling / via matching), no callosal region was significantly thicker in women compared to men. While this contrasts with the initially reported larger splenial dimensions in female brains (DeLacoste-Utamsing and Holloway, 1982)4, it corroborates more recent outcomes from various other studies (i.e., studies not included in the aforementioned meta-analysis by Bishop and Wahlsten) revealing either larger callosal dimensions in male brains or no callosal sex differences whatsoever (Giedd et al., 1999; Luders et al., 2003; Lee et al., 2003; Westerhausen et al., 2004; Hwang et al., 2004; Ng et al., 2005).
Outcomes of the current study, although explicitly focused on the corpus callosum, also appear to be in line with findings from other studies examining non-callosal attributes. For example, brain volume, rather than sex per se, was found to be the main denominator for apparent sex differences in global tissue volumes or the size of selected pre-defined structures (Luders et al., 2002; Leonard et al., 2008; Luders et al., 2009). Nevertheless, it is important to acknowledge, that brain size may not fully account for observed sex differences in all instances. For example, anatomical differences between male and female brains in local gray matter volume (e.g., within the right caudate, left superior temporal gyrus, left superior frontal gyrus) as well as local cortical thickness (e.g., within frontal, temporal, and parietal cortices) seem to exist independently of brain size (Sowell et al., 2007; Luders et al., 2009).
Summary and Outlook
Altogether, our findings suggest that individual differences in brain size account for apparent sex differences in the anatomy of the corpus callosum. Of course, we admit that a truly vast sample might still pick up minor sex differences in size-matched brains, but they are not evident in our sample, which is reasonably well-powered and around the size of many others in the literature. Brain size matching, as applied in the present study, is not intended to replace more traditional analyses that include men and women with typical brain dimensions (i.e., larger male brains / smaller female brains). Such analyses will continue to provide important clues about cerebral differences between men and women, especially if appropriate strategies are used to account for individual variations in brain size. Nevertheless, future studies may expand this current line of work by utilizing brain repositories containing data from children and/or adolescents in order to examine callosal features in brain size-matched boys and girls. Further insights are also to be gained by complementing indicators of callosal macro-structure with descriptors of callosal micro-structure through analyzing diffusion-weighted data, as already demonstrated in men and women with sex-typical brain dimensions (Westerhausen et al., 2003; Westerhausen et al., 2004; Liu et al., 2010; Westerhausen et al., 2011).
Acknowledgments
For generous support the authors thank the Brain Mapping Medical Research Organization, the Robson Family and Northstar Fund, and the following Foundations: Brain Mapping Support, Pierson-Lovelace, Ahmanson, Tamkin, William M. & Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community, Jennifer Jones-Simon, and Capital Group Companies. This study was additionally supported by the NIH (P41 EB015922; RR12169; MH092301) as well as by grants from the Human Brain Project (P20-MHDA52176; 5P01-EB001955).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For the third quintile, the area and ratio measures of the total corpus callosum were found to be larger in women than in men. However, as argued by the authors, “a sampling error may well account for the result” because the third quintile contained 19 male brains but only five female brains (Jancke et al., 1997).
Our callosal analyses were not based on traditional parcellation schemes because the statistical maps offer better localization of the effects. However, for the sake of clarity, we will describe our findings by referring to well-known vertical callosal segments (Witelson, 1989). There, the splenium represents the posterior fifth, the isthmus two fifteenths, the posterior midbody and anterior midbody each one sixth. The remaining anterior third may be further subdivided in rostral body, genu, and rostrum.
While brain size appears to drive the association between sex and callosal thickness, it is also possible that other factors determine both brain size and callosal morphology. For example, genes coding for brain and body size, hormonal levels, and hemispheric connectivity may co-locate in close proximity and be selected together, thus possibly driving the observed associations rather than brain size per se.
Interestingly though, in the current study, large parts of the splenium seem to show a lack of the significant sex difference that is detected elsewhere. Even when comparing the extra large (XL) male brains to the extra small (XS) female brains (i.e., where brain size differences were most pronounced), the region near the posterior bend (i.e., within the splenium) was free of any sexual dimorphism. That is, even though we were unable to replicate the initially reported direction of the sex effect (females > males), the splenium seems to be unique in that it did not follow the overall direction of the currently observed sex effect (males > females).
No Conflict of Interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Reference List
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. 1995;57:289–300. [Google Scholar]
- Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001;13:1121–1130. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci. Biobehav. Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Bruner E, de la Cuetara JM, Colom R, Martin-Loeches M. Gender-based differences in the shape of the human corpus callosum are associated with allometric variations. J. Anat. 2012;220:417–421. doi: 10.1111/j.1469-7580.2012.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216:1431–1432. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosum morphology: A meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ji EK, Lee EK, Kim YM, Shin dY, Cheon YH, Rhyu IJ. Gender differences in the corpus callosum of neonates. Neuroreport. 2004;15:1029–1032. doi: 10.1097/00001756-200404290-00019. [DOI] [PubMed] [Google Scholar]
- Jancke L, Preis S, Steinmetz H. The relation between forebrain volume and midsagittal size of the corpus callosum in children. Neuroreport. 1999;10:2981–2985. doi: 10.1097/00001756-199909290-00020. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb. Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Chen Y, Schlaug G. Corpus callosum: musician and gender effects. Neuroreport. 2003;14:205–209. doi: 10.1097/00001756-200302100-00009. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb. Cortex. 2008;18:2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Vidarsson L, Winter JD, Tran H, Kassner A. Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Res. 2010;1343:37–45. doi: 10.1016/j.brainres.2010.04.064. [DOI] [PubMed] [Google Scholar]
- Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, Petrides M, Caltagirone C. Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. Neuroreport. 2007;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J. Neurosci. 2009;29:14265–14270. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb. Cortex. 2006a;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006b;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb. Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Luders E, Steinmetz H, Jancke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13:2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Woods R, Iacoboni M, Sicotte N, Yaden K, Tran M, Bean C, Kaplan J, Toga AW. The myth of the normal, average human brain--the ICBM experience: (1) subject screening and eligibility. Neuroimage. 2009;44:914–922. doi: 10.1016/j.neuroimage.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WH, Chan YL, Au KS, Yeung KW, Kwan TF, To CY. Morphometry of the corpus callosum in Chinese children: relationship with gender and academic performance. Pediatr. Radiol. 2005;35:565–571. doi: 10.1007/s00247-004-1336-z. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb. Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Smith RJ. Relative size versus controlling for size. Interpretation of ratios on sexual dimorphism in the human corpus callosum. Current Anthropology. 2005;46:249–273. [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H, Staiger JF, Schlaug SG, Huang Y, Jancke L. Inverse relationship between brain size and callosal connectivity. Naturwissenschaften. 1996;83:221. doi: 10.1007/BF01143327. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol. Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J. Neurosci. 1996a;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996b;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kompus K, Dramsdahl M, Falkenberg LE, Gruner R, Hjelmervik H, Specht K, Plessen K, Hugdahl K. A critical re-examination of sexual dimorphism in the corpus callosum microstructure. Neuroimage. 2011;56:874–880. doi: 10.1016/j.neuroimage.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos SS, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res. Cogn Brain Res. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Walter C, Kreuder F, Wittling RA, Schweiger E, Wittling W. The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neurosci. Lett. 2003;351:99–102. doi: 10.1016/j.neulet.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J. Comput. Assist. Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]