Abstract
There is robust evidence for a protective role of interpersonal factors such as social support on alcohol relapse, but research on the mechanisms that social factors may be acting on to effectively protect individuals against relapse is lacking. Prairie voles are highly social, monogamous rodents that freely self-administer ethanol in high amounts, and are a useful model for understanding social influences on alcohol drinking. Here we investigated whether prairie voles can be used to model social influences on relapse using the alcohol deprivation effect, in which animals show a transient increase in ethanol drinking following deprivation. In Experiment I, subjects were housed alone during four weeks of 24-hour access to 10% ethanol in a two-bottle choice test. Ethanol was then removed from the cage for 72 hours. Animals remained in isolation or were then housed with a familiar same-sex social partner, and ethanol access was resumed. Animals that remained isolated showed an increase in ethanol intake relative to pre-deprivation baseline, indicative of relapse-like behavior. However, animals that were socially housed did not show an increase in ethanol intake, and this was independent of whether the social partner also had access to ethanol. Experiment II replicated the alcohol deprivation effect in a separate cohort of isolated animals. These findings demonstrate that prairie voles display an alcohol deprivation effect and suggest a ‘social buffering’ effect of relapse-like behavior in the prairie vole. This behavioral paradigm provides a novel approach for investigating the behavioral and neurobiological underpinnings of social influences on alcohol relapse.
Keywords: prairie vole, alcohol, relapse, alcohol deprivation effect, ethanol, social support
Introduction
Rates of relapse from alcohol use disorders are estimated as high as 80% (Dawson et al. 2007). It is critical to understand the biological mechanisms underlying relapse-related behaviors and to identify target treatments for improving rates of remission in alcoholics. There is robust evidence for a protective role of interpersonal factors such as social support, marital status, and marital quality against alcohol relapse (Garmendia et al. 2008; Walter et al. 2006). However, research on the mechanisms that social factors may be acting on to effectively protect individuals against relapse is lacking (Hunter-Reel et al. 2009).
Prairie voles (Microtus ochrogaster) are highly social and, unlike other traditional rodent models, show specific social attachments for both same-sex (DeVries et al. 1997) and opposite-sex partners (Carter & Getz 1993). These animals freely self-administer ethanol in high amounts, and will do so without training on a sucrose-fading procedure (Anacker et al. 2011a). Prairie voles also display drinking patterns under social conditions that contrast with what is typically seen in other animal models. Specifically, voles that are housed in same-sex pairs show higher basal levels of both alcohol consumption and preference compared to animals housed in isolation (Anacker et al. 2011a; Hostetler et al. 2012). This is in contrast to the isolation-induced increases in drinking observed in many other rodents (as reviewed in Anacker & Ryabinin 2010), and is more similar to social facilitation of drinking that is observed in humans (de Castro, 1990). On the other hand, under certain social conditions, the drinking behavior of one animal can exert a direct and persistent effect to reduce drinking in a social partner (Anacker et al. 2011b). Thus, it is becoming increasingly clear that the details of social context are important factors in the ethanol drinking behavior of voles. This is relevant for modeling human behavior, in which the influence of a social partner can be highly specific to the social relationship and context.
Relapse has been modeled in mice and rats by the alcohol deprivation effect (ADE), in which alcohol-exposed subjects show elevated intake of alcohol following abstinence (Sinclair, 1968; Spanagel & Holter 1999). Specifically, animals show a transient (<48 hour) increase in alcohol drinking following deprivation. However, mice and rats do not show specific social attachments, and the effects of social influences on the ADE have not been studied in rodents. The aims of this study were two-fold: (1) determine whether prairie voles demonstrate an ADE, and (2) investigate whether the ADE is influenced by the social environment. Specifically, we hypothesized that the presence of a familiar social partner would ‘buffer’ against expression of the ADE. We also investigated whether the drinking behavior of a social partner would affect the ADE, and expected that a non-drinking (“abstinent”) partner would be more effective at social buffering of relapse-like behavior than a drinking partner.
Methods
The subjects used in this study were from a breeding colony housed at the Portland Veterans Affairs Medical Center Veterinary Medical Unit. Animals were weaned at 21 days and housed in same-sex sibling groups in cages (27 cm x 27 cm x 13 cm) under controlled temperature, humidity, and 14L:10D light conditions. Food (LabDiet Hi-Fiber Rabbit chow, cracked corn, and oats) and water were available ad libitum throughout the experiments. All subjects had access to cotton nestlets throughout the experiments. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Portland Veterans Affairs Medical Center. Male (n=36) and female (n=35) subjects were tested as adults (110–190 days of age at start of testing). Different subjects were used for each experiment.
Experiment I
All subjects were housed alone in small shoebox cages (27 cm x 16.5 cm x 13 cm) during initial ethanol access and deprivation. Animals had 24 hour access to 10% ethanol and tap water in a 2-bottle choice test for 4 weeks (as previously described: Anacker et al. 2011a). On the morning of Day 29, ethanol bottles were removed for a 72-hour deprivation period. On Day 32, two groups of animals were placed in mesh-divided housing with a familiar same-sex social partner (Anacker et al. 2011a). Keeping the animals separated allowed individual monitoring of fluid consumption, and the mesh allowed animals to maintain contact and interaction. In one group, the social partners also had access to ethanol (“with drinking partners”; n=20) and partners in the second group had access to water only (“with abstinent partners”; n=20). A third group remained isolated in their home cage (“isolated”; n=19). In social housing conditions, bottles were placed directly on either side of the mesh. Ethanol access was resumed immediately after pairing for an additional 48 hours.
Ethanol consumption (g/kg) and ethanol preference (volume ethanol/total fluid consumed) were each analyzed via repeated measures ANOVA with time (baseline, Day 32, and Day 33) as within subject factor and housing (isolated, with drinking partner, or with abstinent partner) as the between subjects factor. A pre-deprivation baseline was calculated from the average of the final 6 days of initial access (Days 23–28; Gilpin et al. 2003). Sex was not a significant factor and was dropped from all models. Post-hoc comparisons between baseline and each Day 32 and Day 33 were performed via paired t-tests. To examine whether significant changes in ethanol intake could be explained by changes in total fluid intake (total volume of fluid consumed/weight), total fluid consumption was examined via paired t-tests. Significance was set at p<0.05.
Experiment II
The second experiment aimed to replicate the ADE in isolated animals seen in Experiment I and to examine ethanol drinking over a longer post-deprivation period. Animals were isolated and had access to 10% ethanol in a 2-bottle choice test as described in Experiment I. In contrast to Experiment I, all 12 subjects remained isolated throughout the study and ethanol access was maintained and monitored for 7 days following deprivation.
For each day post-deprivation, ethanol consumption and preference were analyzed via paired t-tests comparisons to baseline. Total fluid consumption was analyzed between baseline and Day 32 via paired t-tests. Significance was set at p<0.05.
Results
Experiment I
Daily ethanol intake and preference during Experiment I are presented in Figure 1. The baseline daily g/kg intake (average of Days 23–28) for each group was: isolated: 8.67±1.3; with dinking partner: 9.34 ±1.3; with abstinent partner: 8.5±1.3. There was a significant interaction between time and housing on ethanol intake (F4,112=3.91, p=0.005; Fig. 1a), but not preference (F4,112=0.85, p=0.49; Fig. 1b). Post-hoc comparisons indicated a significant increase in ethanol intake relative to baseline on the first day following deprivation (Day 32) in isolated animals (t=−2.37, df=18, p=0.03), but no change in animals housed with a social partner (with drinking partner: t=1.28, df=19, p=0.21; with abstinent partner: t=0.36, df=19, p=0.72).
Figure 1.
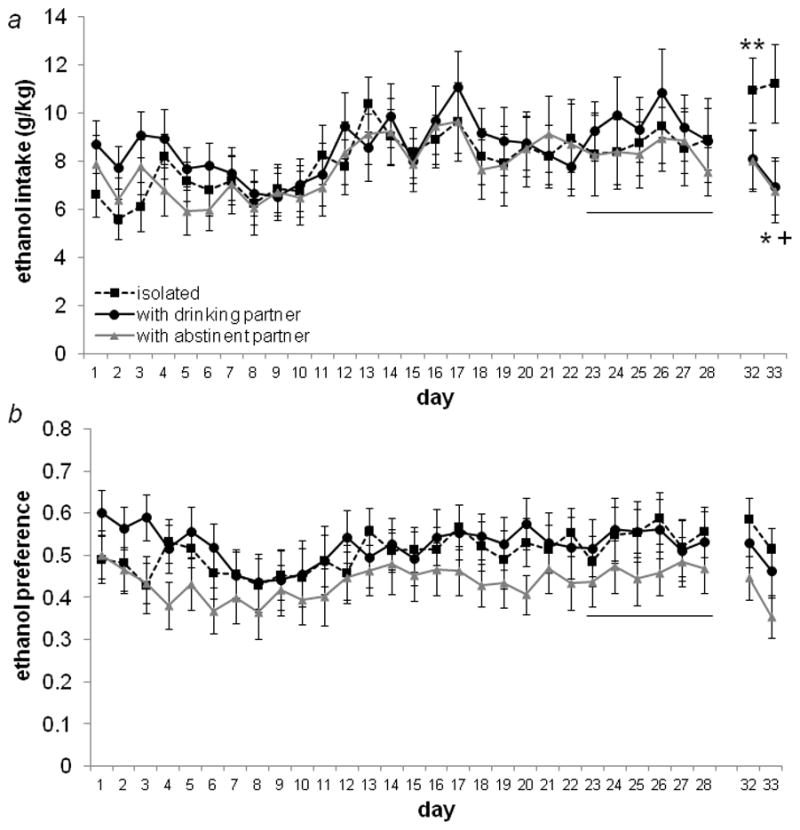
Daily ethanol intake (a) and preference (b) in Experiment I. Following 28 days of 24 hour access to 10% ethanol in a two-bottle choice test, ethanol was removed for 72 hours. Before ethanol access was resumed on Day 32, animals either remained isolated (“isolated”) or were placed in mesh-divided social housing with a familiar same-sex social partner that was also drinking alcohol (“with drinking partner”) or water only (“with abstinent partner”). A pre-deprivation baseline was calculated from the average of the final 6 days of initial access (Days 23–28, underlined). Relative to baseline, ethanol intake was significantly increased in animals that remained isolated following deprivation (**p=0.03), indicative of an alcohol deprivation effect. Animals that were housed with a social partner following deprivation showed no change in either ethanol consumption (a) or preference (b). On the second day post-deprivation (Day 33), animals housed with a social partner had reduced ethanol intake (with abstinent partner: *p=0.05; with drinking partner, trend only: +p=0.057).
This increase in ethanol intake in isolated animals is transient, as ethanol consumption on Day 33 does not differ from baseline (t=−1.89, df=18, p=0.08). Interestingly, both socially housed groups decreased ethanol intake on Day 33 relative to baseline (with drinking partner, trend only: t=2.03, df=19, p=0.057; with abstinent partner: t=2.10, df=19, p=0.05). None of the significant changes in ethanol intake could be explained by changes in overall total fluid consumption (p≥0.13 for all comparisons).
Experiment II
On the first day following deprivation (Day 32), both ethanol intake (t=−3.32, df=11, p=0.007; Fig. 2a) and preference (t=−3.419, df=11, p=0.006; Fig. 2b) were significantly increased. However, neither measure differed from pre-deprivation baseline at any other time point (p≥0.17 for all comparisons). Total fluid consumption did not differ from baseline on Day 32 (t=−0.28, df=11, p=0.79).
Figure 2.
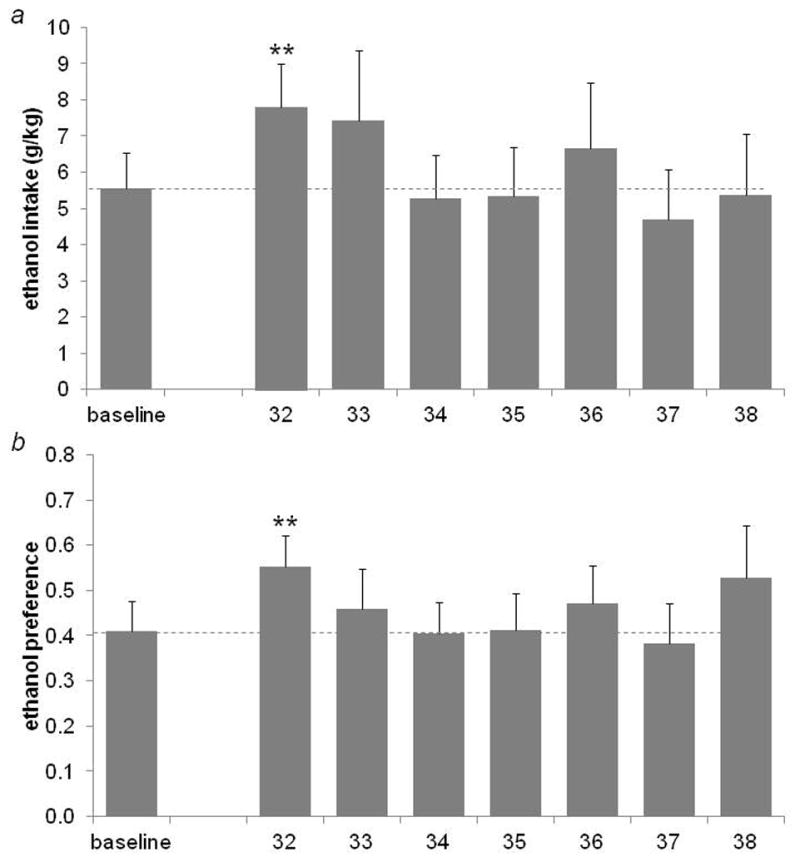
Ethanol intake (a) and preference (b) for pre-deprivation baseline and post-deprivation days in Experiment II. Both intake and preference were significantly increased on the first day following deprivation (**p<0.007), but did not differ from baseline on any other post-deprivation days (p>0.17 for all comparisons). Dotted line represents the baseline mean.
Discussion
We demonstrated for the first time that prairie voles display an ADE, indicative of relapse-like behavior. In two experiments we found that four weeks of self-administration followed by a brief (72 hour) forced abstinence period is sufficient to induce relapse-like alcohol drinking in the prairie vole. Isolated voles increased both ethanol intake (26% and 40% in Experiment I and II, respectively; Figs. 1a, 2a) and preference (35%, but this increase was significant only in Experiment II; Fig. 2b). Increased preference was only observed in Experiment II, suggesting that this is a less robust measure of the ADE in voles. These effects are transient and only observed on the first day following deprivation. The observed increases in intake and preference are smaller than what is typically reported in rats and mice, and this may be due to a number of reasons. First, many rodent studies on the ADE use inbred or selectively bred strains with high drinking. In contrast, the voles used in this study are from an outbred colony demonstrating a high but varied degree of alcohol intake. Many studies of the ADE use longer procedures involving repeated and intermittent deprivations or initially enhance the incentive value of alcohol intake with the use of sweetener, which may contribute to a larger effect size. We would expect that a similar approach in voles would yield a greater ADE. Finally, it is possible that these increases would be even greater if fluid consumption was measured over a shorter period (1–4 hours) following resumed ethanol access and should be addressed in future research.
Importantly, our results show that the ADE is specific to animals that remain in isolation, as animals housed with a familiar social partner following deprivation do not have an ADE. Additionally, the ability of a social partner’s presence to block the ADE is independent of the partner’s drinking behavior. We have previously observed that when voles have initial access to ethanol in isolation, the presence of a social partner may actually reduce ethanol drinking (Anacker et al. 2011b). This reduction is specific to ethanol, and is not observed when animals consume sucrose, another highly rewarding fluid. In the present study, we found a similar effect of decreased ethanol intake in socially housed animals, although this was specific to the second post-deprivation day (Day 33). Overall the present data suggest a ‘social buffering’ effect of relapse-like behavior in the prairie vole. We speculate that if this model mimics the human condition in which a relapsing individual may be more likely to cut back his drinking in social condition than in isolation. This hypothesis is in need of further investigation in human subjects. It would also be informative to examine social influences on the ADE in animals that were socially housed throughout the initial ethanol access period. In this situation, the voles may associate alcohol with their social partners and therefore social housing may promote, rather than inhibit, drinking following deprivation. This would be consistent with the social facilitation of drinking seen in same-sex pairs under non-deprivation conditions (Anacker et al. 2011a; Hostetler et al. 2012). This might model the human condition in which the presence of former drinking partners may promote relapse. Methodologically, this behavioral paradigm provides a novel approach for investigating the behavioral and neurobiological underpinnings of social influences on alcohol relapse.
There are limitations to interpreting the findings in Experiment I. First, it is presumed that the subject recognizes the social partner as familiar, but this may not be the case. It is also possible that it is not necessary for a partner to be familiar: perhaps any social partner would be sufficient to ‘buffer’ against the ADE. A related possibility is that the social condition may be effective due to the novel environment, rather than the social partner. This could be addressed by including a control group of isolated animals that were exposed to a novel mesh-divided cage without a social partner, and should be considered in future studies. On the other hand, in our experience voles are more much behaviorally reactive to social partners than to novel objects, arguing against this possibility. Behavioral observations would also be informative for understanding why animals housed with a social partner are drinking less than their isolated counterparts. For example, it is possible that socially housed animals spend more time investigating the social partner and the mesh divider and this contributes to decreased drinking in this group relative to isolated voles. Investigating the activity budgets of animals in different social contexts and how this relates to alcohol drinking and relapse-like behavior would be particularly useful for translating this research to humans.
Although relapse-like behavior is one of the characteristics of dependence, it is too early to conclude that prairie voles display all hallmarks associated with alcohol dependence. Future work should explore the translational validity of the ADE procedure described here by examining whether alterations in dependence-related behaviors such as anxiety, depressive-like behavior, and stress responsivity are observed, and whether these behaviors are also moderated by social housing conditions. A possible neural mechanism for this effect of social environment on the ADE could involve the corticotropin-releasing factor (CRF) system. CRF and the urocortins have been identified as key modulators of alcohol dependence and relapse (Ciccocioppo et al. 2009), and social behaviors (Hostetler & Ryabinin 2013). There is also evidence from both non-human primates and humans that the CRF system modulates interactions of ethanol-related behavior and social environment (reviewed in Hostetler & Ryabinin 2013).
This is the first report of protective effects of social environment in the ADE. Given the powerful role of social relationships on relapse in humans, these findings highlight the prairie vole as an important animal model for understanding the protective influences of social relationships on alcohol relapse.
Acknowledgments
We gratefully acknowledge Allison Anacker, Jennifer Loftis and the Portland VA animal care staff for assistance on this project.
Role of Funding Source
This research was funded by NIH grant AA019893. The funding agency had no role in the design of the present study or in the analysis, interpretation, or writing of the data.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Contributors
The study was designed by AMH and AER. CMH performed and analyzed the study and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011a;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011b;35:1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7:473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de GG, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Social, circadian, nutritional, and subjective correlates of the spontaneous pattern of moderate alcohol intake of normal humans. Pharmacol Biochem Behav. 1990;35:923–931. doi: 10.1016/0091-3057(90)90380-z. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Canadian Journal of Zoology-Revue Canadienne de Zoologie. 1997;75:295–301. [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 2004;176:82–87. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- Garmendia ML, Alvarado ME, Montenegro M, Pino P. Social support as a protective factor of recurrence after drug addiction treatment. Rev Med Chil. 2008;136:169–178. [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27(5):787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Anacker AM, Loftis JM, Ryabinin AE. Social housing and alcohol drinking in male-female pairs of prairie voles (Microtus ochrogaster) Psychopharmacology (Berl) 2012;224:121–132. doi: 10.1007/s00213-012-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. The CRF system and social behavior: a review. Frontiers in Neuroscience. 2013 doi: 10.3389/fnins.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Reel D, McCrady B, Hildebrandt T. Emphasizing interpersonal factors: an extension of the Witkiewitz and Marlatt relapse model. Addiction. 2009;104:1281–1290. doi: 10.1111/j.1360-0443.2009.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Duersteler-MacFarland KM, Weijers HG, Boening J, Wiesbeck GA. Social factors but not stress-coping styles predict relapse in detoxified alcoholics. Neuropsychobiology. 2006;54:100–106. doi: 10.1159/000096991. [DOI] [PubMed] [Google Scholar]


