Abstract
We evaluated antioxidant activities of heated pear juice (HPJ) exposed to 120, 130, and 140°C for 2 hr. HPJ was partitioned using n-hexane, chloroform, ethyl acetate, n-butanol, and water. The ethyl acetate fraction treated at 130°C for 2 hr showed strong antioxidant activity; thus, this extract was isolated and purified using silica gel column chromatography and preparative high performance liquid chromatography. The structure of the purified compound was determined using ultraviolet and mass spectrometry, 1H-nucelar magnetic resonance (NMR), and 13C-NMR. Antioxidant activities of the isolated compound were evaluated and compared with α-tocopherol, ascorbic acid, and butylated hydroxytoluene (BHT) using DPPH and ABTS assays. The isolated compound was identified as 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP). The DPPH radical-scavenging activity (IC50) of DDMP occurred in the following order: ascorbic acid (45.3 μg/mL)> α-tocopherol (69.2 μg/mL)> DDMP (241.6 μg/mL)> BHT (268.0 μg/mL). Furthermore, DDMP showed strong ABTS radical-scavenging activity (569.0 mg AA eq/g).
Keywords: pear; antioxidant activity; 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one; heat treatment
INTRODUCTION
Antioxidants are chemical substances that reduce or prevent oxidation and have the ability to protect against the damaging effects of free radicals in cells and, thus, are believed to protect against cancer, arteriosclerosis, heart disease, and several other diseases. The major action of antioxidants is to prevent damage caused by the action of reactive oxygen species (ROS) such as superoxide radicals, hydroxyls, and peroxides (1,2). Several synthetic antioxidants such as butylated hydroxyanisole, butylated hydroxytoluene (BHT), and tertiary butyl hydroquinone, are commonly used antioxidants at present; however, their use is now restricted due to adverse side effects and possible toxicity (1,3,4). Thus, the search for anti-oxidants of natural origin has attracted increasing attention.
Pear (Pyrus pyrifolia Nakai) is one of the most important fruit crops in the world and is a good source of sugar, amino acids, and vitamin C, which are used as components in functional beverages (5). Pear is mostly consumed as fresh fruit and production is constantly being increased due to consumption demand. Moreover, pears have various physiological effects and contain various useful compounds such as phenolics and flavonoids. Therefore, pears have been receiving more attention recently as potential sources of natural antioxidants (5–7).
2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) was formed by the Maillard reaction (1-deoxyosone pathway) between carbonyl groups of reducing sugars and amino groups on proteins, peptides, amino acids, and organic amines (8). Numerous studies concerning DDMP have mainly focused on the detection, degradation pathways, and formation mechanisms using Maillard reaction model systems (8–11). Recently, Ban et al. (12) reported that DDMP from onion inhibits colon cancer cell growth by inducing apoptotic cell death through NF-κB inhibition.
Recent studies have shown that thermally processed foods, particularly fruits and vegetables, compared with fresh foods have increased biological activity caused by chemical changes during heat treatment (13–17). We previously evaluated the antioxidant activities of heated pear juice (HPJ) and confirmed that they increased relative to those of raw pear (14). Thus, the objective of the present study was to isolate and identify the antioxidant substances from HPJ and to investigate the antioxidant activity of the isolated compounds.
MATERIALS AND METHODS
Sample preparation
Pear (Pyrus pyrifolia Nakai) was purchased in Naju, South Korea in August 2007 and stored at −20°C. A heat treatment was performed using a temperature- and pressure-controlling apparatus (Jisico, Seoul, Korea). The pear was heated at temperatures of 120, 130, or 140°C for 2 hr. The heated samples were juiced and then filtered (Whatman filter paper No. 2, Maidstone, England) using a Büchner funnel under a vacuum. The pear juice was kept at −20°C until analysis (14).
Selection of the solvent layer
HPJ was partitioned consecutively in a separation funnel using solvents of increasing polarity: n-hexane, chloroform, ethyl acetate (EtOAc), n-butanol, and water. Solvent was evaporated using a rotary evaporator (Eyela N-1000, Tokyo, Japan) at 40°C. The dried residues of the HPJ extracts were measured for 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma-Aldrich, St. Louis, MO, USA) radical scavenging activity and 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS, Sigma-Aldrich) radical cation scavenging activity.
DPPH radical scavenging activity
The DPPH radical scavenging activity of the extracts, fractions, and isolated compounds based on the scavenging activity of the stable DPPH free radical was measured according to the method of Hwang et al. (18). Aliquots of 0.8 mL of a 0.2 mM DPPH methanol solution were mixed with 0.2 mL of the samples. The mixture was shaken vigorously and then left to stand for 30 min under low light. The absorbance was measured at 520 nm using a spectrophotometer (DU 650; Beckman, Fullerton, CA, USA). The percent inhibition of activity was calculated as [(A0−A1)/A0]×100, where A0 is the absorbance without the sample and A1 is the absorbance with the sample. Sample concentrations providing a 50% inhibition concentration (IC50) were calculated from a graph of inhibition percentage versus sample concentration. All samples were analysed in triplicate.
ABTS radical scavenging activity
The ABTS radical cation scavenging activity of the extracts and fractions was measured according to the method of Hwang et al. (18). The ABTS radical cation was generated by adding 7 mM ABTS to 2.45 mM potassium persulfate (Sigma-Aldrich) solution and leaving the mixture to stand overnight in the dark at room temperature. The ABTS radical cation solution was diluted with distilled water to obtain an absorbance of 1.4∼1.5 at 735 nm. A 1 mL aliquot of diluted ABTS radical cation solution was added to 50 μL of the samples, ascorbic acid (Sigma-Aldrich) standard solution, or distilled water. The absorbance at 735 nm was determined using a spectrophotometer (DU 650; Beckman) after 60 min. The ascorbic acid equivalent antioxidant activity (AEAC) was calculated as (ΔA/ΔAAA)×CAA, where ΔA is the change in absorbance after addition of the sample, ΔAAA is the change in absorbance after adding the ascorbic acid standard solution, and CAA is the concentration of the ascorbic acid standard solution. The ABTS radical cation scavenging activity was expressed as AEAC in milligrams of ascorbic acid equivalents. All samples were analysed in triplicate.
Purification and identification of the antioxidant substance
Isolation of the active compound from the EtOAc layer of the HPJ treated at 130°C for 2 hr was subjected to column chromatography on a silica gel. HPJ (2.5 kg) was partitioned consecutively using various solvents. The EtOAc layer (2.14 g) was subjected to open-column (500×35 mm, i.d.) chromatography using silica gel (Kiesel gel 60, 70∼230 mesh; Merck, Darmstadt, Germany); elution was performed using a mixture of dichloromethane (DCM) : methanol (MeOH) with an increasing amount of MeOH (20:1, 10:1, 5:1, 1:1, 0:1, v/v, 400 mL). Five fractions were collected and assayed for antioxidant activity and then loaded onto silica-gel 60 F254 glass plates (0.25 mm thick, 20×20 cm; Merck), which were then developed with DCM : MeOH mixtures at different ratios. The plate was then sprayed with 20% sulfuric acid solution in 10% vanillin/ethanol solution to analyze the spot pattern. The active fraction A1 (1.04 g) was further purified by silica gel column (300×10 mm, i.d.) chromatography; elution was carried out using a mixture of DCM : MeOH with increasing amounts of MeOH (20:1, 10:1, 5:1, 1:1, 0:1, v/v, 200 mL). Thirty fractions were collected and assayed for antioxidant activity and then loaded onto a silica gel TLC plate in the same order as mentioned above. The active fractions B6–B13 (0.38 g) were purified by semi-preparative reverse-phase high performance liquid chromatography (RP-HPLC) (Discovery® C18 column; 250×10 mm, i.d., 5 μm, Supelco, Bellefonte, PA, USA; mobile phase: 3% acetonitrile; flow rate: 3.5 mL/min; detector: 298 nm) on a Younglin SP930D instrument (Anyang, Korea). The structure of the purified compound was determined using several spectroscopic methods. The UV spectrum in methanol was recorded on a spectrophotometer (UV-1650; Shimadzu, Kyoto, Japan). Gas chromatography-mass spectrometry (GC-MS) was performed (Agilent 6890 gas chromatograph/5973N; Agilent Technologies, Palo Alto, CA, USA). The 1H nuclear magnetic resonance (NMR, 500 MHz), 13C NMR (125 MHz), and distortionless enhancement by polarization transfer (DEPT) spectra were recorded on a spectrometer (Avance 500, Bruker, Karlsruhe, Germany) using CD3OD as a solvent.
RESULTS AND DISCUSSION
Purification of the antioxidant substance from heated pear
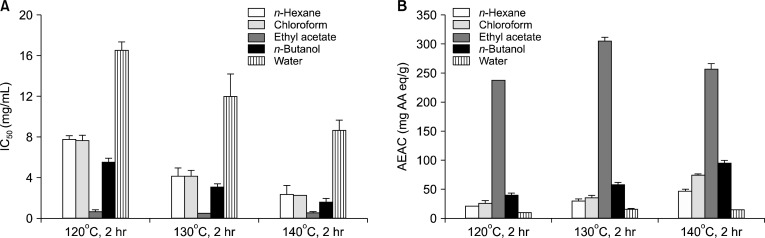
The optimum heating condition for pear was previously determined as 130°C for 2 hr (14). We attempted to isolate the antioxidant substance from HPJ exposed to 120, 130, or 140°C for 2 hr. Each HPJ sample was purified using a series of solvent fractionations with n-hexane, chloroform, EtOAc, n-butanol, and water. The antioxidant activity of the five fractions from HPJ is shown in Fig. 1. The antioxidant activity of the EtOAc fraction was higher than that of the n-hexane, chloroform, n-butanol, and water fractions. The EtOAc fraction of HPJ treated at 130°C for 2 hr showed strong antioxidant activity compared with those from the other heating conditions. Therefore, we isolated and purified the active compound from the EtOAc fraction of HPJ treated at 130°C for 2 hr. The EtOAc fraction (2.14 g) was subjected to silica gel column chromatography and eluted with an increasing concentration of MeOH in DCM. The greatest inhibitory activity of the first silica gel chromatography was observed in the A1 fraction (1.04 g; data not shown). Each fraction was separated on a TLC plate and then developed with DCM:MeOH (20:1, v/v). The TLC plate was sprayed with a vanillin/H2SO4 solution to analyze the spot pattern (data not shown). The A1 fraction was further purified using silica gel chromatography to obtain the B6 and B13 active fractions (0.38 g; data not shown), using the method described above. We then isolated the active compound from the B16 and B13 fractions using semi-preparative HPLC with a C18 column. The yield of the purified active compound was about 6.4 mg. The chemical structure of the isolated compound was determined using spectroscopic methods.
Fig. 1.
IC50 values of electron donating ability (A; %) and total antioxidant activities (B; AEAC) on the solvent fraction of heated pear juice at 120, 130, and 140°C for 2 hr. DPPH radical scavenging activity (%) on solvent fraction of raw pear juice (10 mg/mL): n-hexane, 44.37±6.33; chloroform, 10.11±1.34%; ethyl acetate, 27.70±5.57%; n-butanol, 13.40±4.22%; and water, 2.62±1.40%. AEAC (mg AA eq/g) on solvent fraction of raw pear juice: n-hexane, 9.90±0.54 mg AA eq/g; chloroform, 5.95±3.53 mg AA eq/g; ethyl acetate, 7.17±0.48 mg AA eq/g; n-butanol, 11.39±2.28 mg AA eq/g; and water, 3.91±0.98 mg AA eq/g.
Identification of the isolated antioxidant substance
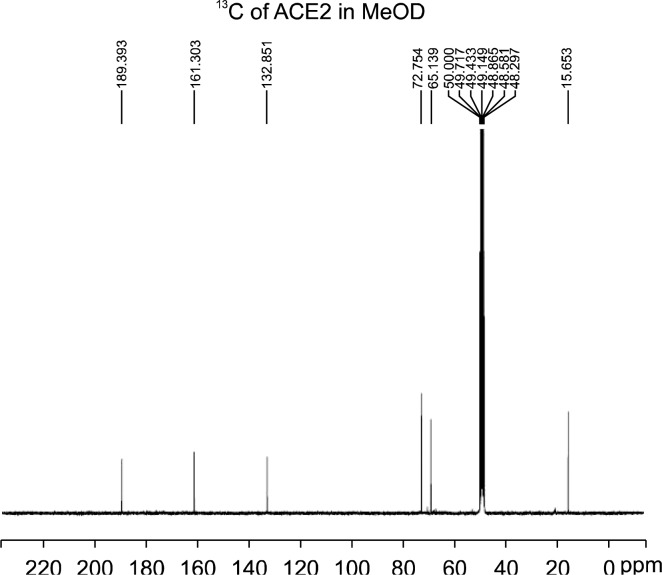
The purified compound was analysed by UV, GC/MS, 1H NMR, 13C NMR, and DEPT. The ultraviolet absorption spectrum was 298 nm (MeOH). The GC-MS spectrum showed a molecular ion peak at m/z 144. 1H NMR δ (ppm): 2.038 (3H, δC 15.653), 4.087 (2H, δC 72.764), and 4.189 (1H, δC 69.138). 13C NMR δ (ppm): 15 (CH3), 69 (CH), 72 (CH2), 132 (quaternary carbon), 161 (carbonyl carbon, C=O), and 189 (ketone carbon, CO) (Fig. 2). The chemical structure of the compound is shown in Fig. 3. The isolated compound was identified as 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP). The MS and NMR data were consistent with those of DDMP reported by Kim and Baltes (8) and Davidek et al. (9).
Fig. 2.
13C-NMR spectrum for the isolated compound.
Fig. 3.
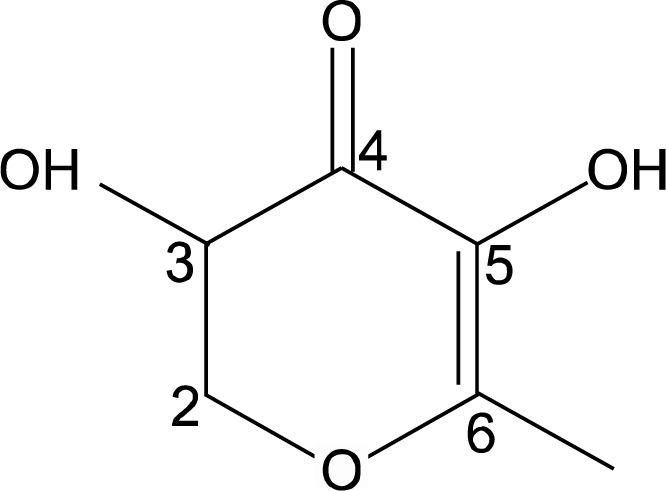
Chemical structure of the isolated compound of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) from heated pear.
Antioxidant activity of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one
DPPH and ABTS radical-scavenging activities of DDMP were 1.96-fold and 1.87-fold higher, respectively, than those of EtOAc fraction of HPJ treated at 130°C for 2 hr. The antioxidant activity of DDMP was evaluated and compared to that of α-tocopherol, ascorbic acid, and BHT using the DPPH and ABTS assays (Table 1). The DPPH radical-scavenging activity (IC50) of DDMP occurred in the following order: ascorbic acid (45.3 μg/mL)> α-tocopherol (69.2 μg/mL)> DDMP (241.6 μg/mL)> BHT (268.0 μg/mL). Furthermore, DDMP showed strong ABTS radical-scavenging activity (AEAC: 569.0 mg AA eq/g).
Table 1.
Antioxidant activities of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) isolated from heated pear
| IC501) (μg/mL) | AEAC2) (mg AA eq/g) | |
|---|---|---|
| DDMP | 241.6±29.23) | 569.0±13.4 |
| Vitamin E | 69.2±0.5 | NT4) |
| Vitamin C | 45.3±4.2 | NT |
| BHT | 268.0±12.8 | NT |
IC50 value is the half maximal (50%) inhibitory concentration of DPPH radical.
The ascorbic acid equivalent antioxidant activity.
Values are mean±SD of three experiments, each performed in triplicate.
NT, not tested.
REFERENCES
- 1.Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J Agric Food Chem. 2002;5:4989–4993. doi: 10.1021/jf020225o. [DOI] [PubMed] [Google Scholar]
- 2.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- 3.Williams GM, Iatropoulos MJ, Whysner J. Safety assessment to butylated hydroxyanisol and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol. 1999;37:1027–1038. doi: 10.1016/s0278-6915(99)00085-x. [DOI] [PubMed] [Google Scholar]
- 4.Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg. 2009;10:627–632. [Google Scholar]
- 5.Choi OJ, Park HR, Chough SH. Variation of free sugar and amino acid contents of pears during the ripening period. Korean J Food Sci Technol. 1998;14:250–254. [Google Scholar]
- 6.Zhang YB, Choi HS, Han HS, Park JH, Son JH, Bae JH, Seung TS, An BJ, Kim HG, Choi C. Chemical structure of polyphenol isolated from Korean pear (Pyrus pyrifolia Nakai) Korean J Food Sci Technol. 2003;35:959–967. [Google Scholar]
- 7.Choi HJ, Park JH, Han HS, Son JH, Son GM, Bae JH, Choi C. Effect of polyphenol compound from Korean pear (Pyrus pyrifolia Nakai) on lipid metabolism. J Korean Soc Food Sci Nutr. 2004;33:299–304. [Google Scholar]
- 8.Kim MO, Baltes W. On the role of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one in the maillard reaction. J Agric Food Chem. 1996;44:282–289. [Google Scholar]
- 9.Davidek T, Clety N, Devaud S, Robert F, Blank I. Simultaneous quantitative analysis of maillard reaction precursors and products by high-performance anion exchange chromatography. J Agric Food Chem. 2003;51:7259–7265. doi: 10.1021/jf034794n. [DOI] [PubMed] [Google Scholar]
- 10.Wirth DD, Baertschi SW, Johnson RA, Maple SR, Miller MS, Hallenbeck DK, Gregg SM. Maillard reaction of lactose and fluoxetine hydrochloride, a secondary amine. J Pharm Sci. 1998;87:31–39. doi: 10.1021/js9702067. [DOI] [PubMed] [Google Scholar]
- 11.de Lerma NL, Peinado J, Moreno J, Peinado RA. Antioxidant activity, browning and volatile Maillard compounds in Pedro Ximénez sweet wines under accelerated oxidative aging. LWT. 2010;43:1557–1563. [Google Scholar]
- 12.Ban JO, Hwang IG, Kim TM, Hwang BY, Lee US, Jeong HS, Yoon YW, Kim DJ, Hong JT. Anti-proliferate and pro-apoptotic effects of 2,3-dihydro-3,5-dihydroxy-6-methyl- 4H-pyranone through inactivation of NF-κB in human colon cancer cells. Arch Pharm Res. 2007;30:1455–1463. doi: 10.1007/BF02977371. [DOI] [PubMed] [Google Scholar]
- 13.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional values of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 14.Hwang IG, Woo KS, Kim TM, Kim DJ, Yang MH, Jeong HS. Change of physicochemical characteristics of Korean pear (Pyrus pyrifolia Nakai) juice with heat treatment conditions. Korean J Food Sci Technol. 2006;38:342–347. [Google Scholar]
- 15.Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. [Google Scholar]
- 16.Woo KS, Hwang IG, Kim TM, Kim DJ, Hong JT, Jeong HS. Changes in the antioxidant activity of onion (Allium cepa) extracts with heat treatment. Food Sci Biotechnol. 2007;16:828–831. [Google Scholar]
- 17.Kim HY, Woo KS, Hwang IG, Lee YR, Jeong HS. Effects of heat treatments on the antioxidant activities of fruits and vegetables. Korean J Food Sci Technol. 2008;40:166–170. [Google Scholar]
- 18.Hwang IG, Woo KS, Kim DJ, Hong JT, Hwang BY, Lee YR, Jeong HS. Isolation and identification of an antioxidant substance from heated garlic (Allium sativum L.) Food Sci Biotechnol. 2007;16:963–966. [Google Scholar]