Abstract
This study was designed to investigate the anti-inflammatory effect of oyster shell extract on the production of pro-inflammatory factors [NO, reactive oxygen species (ROS), nuclear factor-kappa B (NF-κB), inducible nitric oxide synthase (iNOS) and cycloxygenase-2 (COX-2)] and pro-inflammatory cytokines [Interleukin-1β (IL-1β), Interleukin-6 (IL-6) and TNF-α] in the lipopolysaccharide (LPS)-stimulated Raw 264.7 cells. Cell viability, as measured by the MTT assay, showed that oyster shell extract had no significant cytotoxicity in Raw 264.7 cells. The treatment with oyster shell extract decreased the generation of intracellular reactive oxygen species dose dependently and increased antioxidant enzyme activities, such as SOD, catalase, GSH-px in LPS-stimulated macrophage cells. Oyster shell extract significantly suppressed the production of NO and also decreased the expressions of iNOS, COX-2 and NF-κB. Additionally, oyster shell extract significantly inhibited the production of IL-1β, IL-6, and TNF-α in LPS-stimulated Raw 264.7 cells. Thus, these results showed that the oyster shell extract had an anti-inflammatory effect on LPS-stimulated Raw 264.7 cells.
Keywords: oyster shells, anti-inflammation, Raw 264.7 cell
INTRODUCTION
Commonly acknowledged, inflammation plays important roles in the initiation and progress of many diseases including cancer in multiple organ sites (1). Inflammation, classified either as acute or chronic, has been described as the basis of many human diseases. Acute inflammation occurs from minutes to hours and days following tissue damage caused by physical force or an immune response. Chronic inflammation occurs over a longer period of time and is caused by pro-inflammatory mediators. Chronic inflammation in linked to rheumatoid arthritis, diabetes, atherosclerosis, and cancer (2–4). Therefore, inhibition of the production of pro-inflammatory mediators is an important goal in the treatment of various inflammatory diseases.
The lipopolysaccharides (LPS)-treated Raw 264.7 cells have been widely used to study inflammatory responses. Exposure of Raw 264.7 cells to external bacterial toxins like LPS has been extensively shown to stimulate the secretion of nitric oxide (NO), which is produced by the inducible isoforms of nitric oxide synthase (5). When the body is stimulated by pathologic injury, activated macro phages release numerous pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and inflammatory mediators, such as NO, inducible NOS (iNOS) and cycloocygenase-2 (COX-2) (6). COX-2 can convert arachidonic acid to prostaglandin and other eicosanoids. Aberrant functioning of COX-2 has been associated with carcinogenesis by promoting cell survival, angiogenesis, and metastasis (7–9). NO is generated enzymatically by synthases (NOS) and is formed by iNOS in macrophages and other cells that play a role in the inflammatory response. Enormous amounts of NO can stimulate many proteins and enzymes crucial to inflammatory reactions, such as nuclear factor-kappa B (NF-κB) (10). Therefore, various cytokines, NO, iNOS and COX-2 are important targets for anti-inflammation remedy.
Approximately 40,000 tons of oysters are annually harvested in Korea. Oyster shells, more than 90% of the oyster content, was wasted and has recently induced a serious environmental pollution (11). However, oyster shells contain a large amount of calcium carbonate (CaCO3), and relatively low amounts of calcium sulfate (CaSO4), calcium phosphate (CaPO4), and amino acids (12). Oyster shells are one of the most famous traditional antacid medicines in China, Japan and Korea. According to Bonchogangmok, oyster shells were used to medicine dermatitis. Recently, oyster shells are used as a substitute for calcium agents. However, no scientific proof or reports exist on the potential anti-inflammatory activity of oyster shells. Thus, this study examined the effect of oyster shell extract on antioxidant activities and anti-inflammatory response in LPS-stimulated Raw 264.7 cells.
MATERIALS AND METHODS
Preparation of oyster shell extract
Oyster shell was collected from the Duckyen company in Tongyeong, South Korea. Salt, sand and epiphytes were removed using tap water. The samples were rinsed carefully with fresh water and air-dried. Dried oyster shell was ground and sifted through a 800 mesh. Ground oyster shell was extracted with 0.5 M citrate in water at 30°C for 12 h and centrifuged at 8,000 rpm at 4°C. After centrifuging for 20 min, the supernatant liquid was collected and filtered through an ultrafiltration system (QuixStand Benchtop, GE Healthcare, Piscataway, NJ, USA) at 15 psi and 100 rpm by hollow fiber membranes with molecular weight cut-off values of 10 kDa. Filtered liquid was vacuum-dried and the powder was stored at −80°C.
Cell culture and treatment
Mouse macrophage Raw 264.7 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37°C in 5% CO2/95% air. Cells in 96 well plates (2×104 cells/well) were treated with oyster shell extract (10, 25, 50, 100 μg/mL) for 2 h, and then incubated with LPS (1 μg/mL) for 20 h.
Cell viability
Cell viability was assessed using a modified 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (13). Briefly, cells (2×104 cells/well) were seeded in a 96 well plate and treated oyster shell extract. Following treatment, 100 μL of MTT solution (5 mg/mL in phosphate buffered saline) was added to each well and further incubated for 4 h at 37°C. Subsequently, 100 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve any deposited formazan. The optical density (OD) of each well was measured at 540 nm with a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Measurements of intracellular ROS level
Intracellular ROS levels were measured by the 2’,7’-dichlorofluorescein diacetate (DCF-DA) assay (14). DCF-DA can be deacetylated in cells, where it can react quantitatively with intracellular radicals to convert into its fluorescent product, DCF, which is retained within the cells. Therefore, DCF-DA was used to evaluate the generation of ROS in oxidative stress. Thereafter, the medium was removed and the cells were washed twice with phosphate buffered saline (PBS, pH 7.4) and incubated with 5 μL DCF-DA for 30 min at room temperature. Fluorescence was measured using a fluorescence plate reader (BMG LABTECH GmbH, Offenberg, Germany).
Measurement of antioxidant enzyme activities
Cells (2×104 cells/well) in a 96 well plate were pre-incubated with various concentrations (10, 25, 50, and 100 μg/mL) of oyster shell extract for 2 h, then further incubated with LPS for 20 h. The medium was removed and the cells were washed twice with PBS. 1 mL of 50 mmol/L potassium phosphate buffer with 1 mmol/L EDTA (pH 7.0) was added and the cells were scraped. Cell suspensions were sonicated three times for 5 sec on ice each time then centrifuged at 10,000×g for 20 min at 4°C. Cell supernatants were used for measuring anti-oxidant enzyme activities. The protein concentration was measured by using the Bradford method (15) with bovine serum albumin as the standard. Superoxide dismutase (SOD) activity was determined by monitoring the auto-oxidation of pyrogallol. A unit of SOD activity was defined as the amount of enzyme that inhibited the rate of oxidation of pyrogallol. Catalase activity was measured according to method of Aebi (16) by following the decreased absorbance of H2O2. The decrease of absorbance at 240 nm was measured for 2 min. Standards containing 0, 0.2, 0.5, 1 and 2 mmol/L of H2O2 were used for the standard curve. Glutathione peroxidase (GSH-px) activity was measured by using the method of Lawrence and Burk (17). One unit of GSH-px was defined as the amount of enzyme that oxidized 1 nmol of NADPH consumed per minute.
Measurement of NO level
Each 50 μL of culture supernatant was mixed with an equal volume of Griess reagent [0.1% N-(1-naphthyl)-ethylenediamine, 1% sulfanilamide in 5% phosphoric acid] and incubated at room temperature for 10 min, The absorbance at 550 nm was measured in a microplate absorbance reader (Bio-Rad Laboratories Inc.) and a series of known concentrations of sodium nitrite were used as the standards (18).
Western blotting
TNF-α, IL-1β, IL-6, iNOS and COX-2 expressions and NF-κB DNA binding activity were determined by western blot analysis. Total protein for TNF-α, IL-1β, IL-6, iNOS, COX-2 and nuclear protein for NF-κB were electrophoresed through 10% sodium dodecyl sulfate-polyacrylamide gel. Separated proteins were transferred electrophoretically to a pure nitrocellulose membrane, blocked with 5% skim milk solution for 1 h, and then incubated with primary antibodies overnight at 4°C (19). After washing of the blots, they were incubated with goat anti-rabbit or goat anti-mouse IgG HRP conjugated secondary antibody for 1 h at room temperature. Each antigen-antibody (Abcam, Cambridge, UK) complex was visualized using ECL western blotting detection reagents and detected by chemiluminescence with LAS-1000 plus. Band densities were determined by an image analyzer ATTO densitograph and normalized to β-actin for total protein and nuclear protein.
Statistical analysis
The data were represented as mean±SD. The statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA). The values were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple range tests.
RESULTS AND DISCUSSION
Cell viability
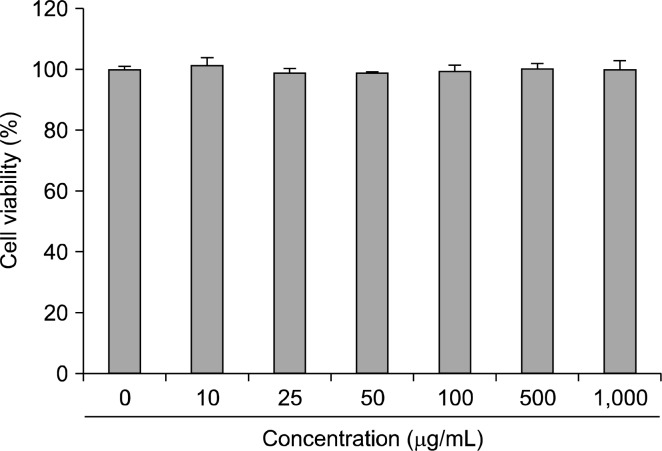
Raw 264.7 cells viability in the presence of oyster shell extract is shown in Fig. 1. These data indicate that oyster shell extract does not effect the viability of Raw 264.7 cells at the concentrations of 0~1,000 μg/mL.
Fig. 1.
Effect of oyster shell extract on cytotoxicity in Raw 264.7 cells. Cells in 96-well plates (2×104 cells/well) were incubated with and without indicated concentrations of oyster shell extract for 20 h. Each value is expressed as mean±SD in triplicate experiment.
Intracellular ROS generation
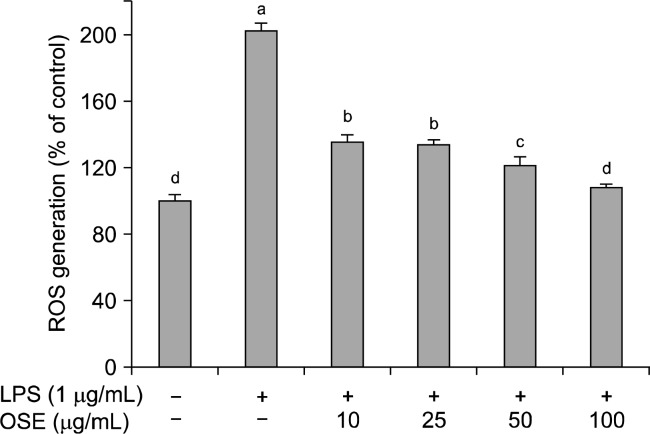
ROS are known to be crucial inflammatory mediators. During an inflammatory response, the excessive production of ROS can cause major damage to cells, which can lead to DNA damage and mutations (20,21). High ROS levels induce oxidative stress and inflammatory reactions, which can result in a variety of biochemical and physiological lesions (22). The effects of oyster shell extract on ROS production in LPS-stimulated Raw 264.7 cells is shown in Fig. 2. LPS significantly increase (p< 0.05) ROS generation in Raw 264.7 cells. However, oyster shell extract significantly reduced (p<0.05) LPS-generated ROS in a dose-dependent manner. Oyster shell extract at concentrations of 10, 25, 50 and 100 μg/mL reduced the intracellular ROS levels to 135.40%, 133.58%, 121.06%, and 107.75%, respectively. Oyster shell extract might effectively prevent inflammation by decreasing ROS production.
Fig. 2.
Effect of oyster shell extract on intracellular ROS levels in LPS-stimulated Raw 264.7 cells. Cells (2×104 cells/well) in 96-well plates were first incubated with and without indicated concentrations of oyster shell extract for 2 h, and then incubated with LPS (1 μg/mL) for 20 h. Untreated is negative control without LPS treatment. Each value is expressed as mean± SD in triplicate experiments. Values with different alphabets are significantly different at p<0.05 as analyzed by Duncan‘s multiple range test. OSE: Oyster shell extract.
Antioxidant enzyme activities
Several antioxidant enzymes such as SOD, GSH-px, and catalase provide eukaryotic cells with a primary defense against ROS. This antioxidant defense becomes particularly important to phagocytes as macrophages are able to generate very high concentrations of ROS (23). Table 1 shows antioxidant enzyme activities of oyster shell extract in LPS-stimulated Raw 264.7 cells. The activity of SOD was decreased by LPS and significantly recovered (p<0.05) by oyster shell extract, with values from 45.83±2.56, 51.14±0.94, 63.89±2.63 and 65.74±1.14 μM/mg protein (10, 25, 50 and 100 μg/mL oyster shell extract, respectively). SOD is an enzyme that has anti-inflammatory capacity because of its ability to scavenge the superoxide free-radical (24). SOD catalyzes the dismutation of superoxide radicals to oxygen and hydrogen peroxide (H2O2). H2O2 is further reduced to H2O by the activity of catalase or glutathione peroxidase. SOD may increase hydrogen peroxide that sometimes plays a role as a second messenger in the production of inflammatory cytokines including TNF-α, IL-1β, and IL-6 (25). Catalase activities were significantly increased (p< 0.05) by oyster shell extract treatment from 1.24±0.01 to 1.56±0.01 μM/mg protein, with the addition of 10 μg/mL oyster shell extract. Recent studies have shown that in vivo or in vitro treatments of antioxidant enzymes, such as catalase or superoxide dismutase, are effective in reducing inflammation and cancer (26,27). Glutathione acts as an oxy radical scavenger by scavenging NO and other oxidants, thereby protecting cells against oxidative damage by reducing oxidants (28). GSH-px is a family of intracellular antioxidant enzymes that reduce H2O2 and organic hydroperoxides by oxidizing glutathione; these enzymes play critical protective roles in the detoxification of ROS produced during inflammation (29). In this experiment, the GSH-px activity in the Raw 264.7 cells showed a significant increase (p<0.05) upon treatment with oyster shell extract at 10, 25, 50 and 100 μg/mL, resulting in 3.41±0.04, 4.25±0.03, 4.57±0.02, 5.21±0.03 unit/mg protein, respectively. Thus, treatment of LPS-stimulated cells with oyster shell extract enhanced anti-oxidant enzyme activities that may be helpful in attenuating inflammation.
Table 1.
Effects of oyster shell extract on antioxidant enzyme activities in LPS-stimulated Raw 264.7 cells
| Untreated | Oyster shell extract (μg/mL)+LPS (1 μg/mL) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 10 | 25 | 50 | 100 | ||
| SOD (μM/mg protein/min) | 69.37±3.61a | 26.09±2.55e | 45.83±2.56d | 51.14±0.94c | 63.89±2.63b | 65.74±1.14ab |
| Catalase (μM/mg protein/min) | 1.66±0.04a | 1.24±0.01d | 1.56±0.01c | 1.59±0.01bc | 1.62±0.02ab | 1.63±0.03ab |
| GSH-px (unit/mg protein) | 4.26±0.01c | 2.71±0.04e | 3.41±0.04d | 4.25±0.03c | 4.57±0.02b | 5.21±0.03a |
Cells (2×104 cells/well) in 96-well plates were first incubated with or without indicated concentrations of oyster shell extract for 2 h and then incubated with LPS (1 μg/mL) for 20 h. Untreated is negative control without LPS treatment. Values with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. SOD: Superoxide dismutase, GSH-px: Glutathione peroxidase.
NO production
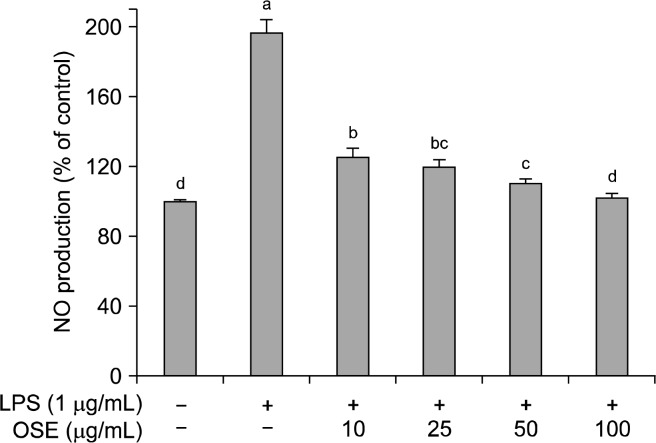
NO is a pluripotent signaling molecule synthesized by a family of nitric oxide synthase isoforms (NOS) found in most tissues (30,31). NO has beneficial roles in host defense system against tumor cells, viral replication and other factors. However, over production of NO causes various inflammatory diseases (32). Namely, NO is an important mediator and regulator of inflammatory responses. In an inflammatory response, the overproduction of NO reacts with superoxide creating cytotoxicity and tissue damage in an organism (33). The effect of oyster shell extract on NO inhibition was determined by treating the Raw 264.7 cells of LPS stimulation (Fig. 3). LPS significantly increased NO production in Raw 264.7 cells. The level of NO production induced by LPS decreased significantly (p<0.05) in a dose-dependent manner when treated with different concentrations of oyster shell extract; with 10, 25, 50 and 100 μg/mL of oyster shell extract, NO production was 125.12%, 120.03%, 110.21%, 101.67%, respectively, compared with LPS treatment alone to 196.67%. In this study, oyster shell extract effectively decreased NO production, indicating oyster shell extract might be useful to suppress the inflammatory process.
Fig. 3.
Effects of oyster shell extract on NO levels in LPS-stimulated Raw 264.7 cells. Cells (2×104 cells/well) in 96-well plates were first incubated with and without indicated concentrations of oyster shell extract for 2 h, and then incubated with LPS (1 μg/mL) for 20 h. Untreated is negative control without LPS treatment. Each value is expressed as mean±SD in triplicate experiments. Values with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. OSE: Oyster shell extract.
Effects of oyster shell extract on TNF-α, IL-1β and IL-6 expressions
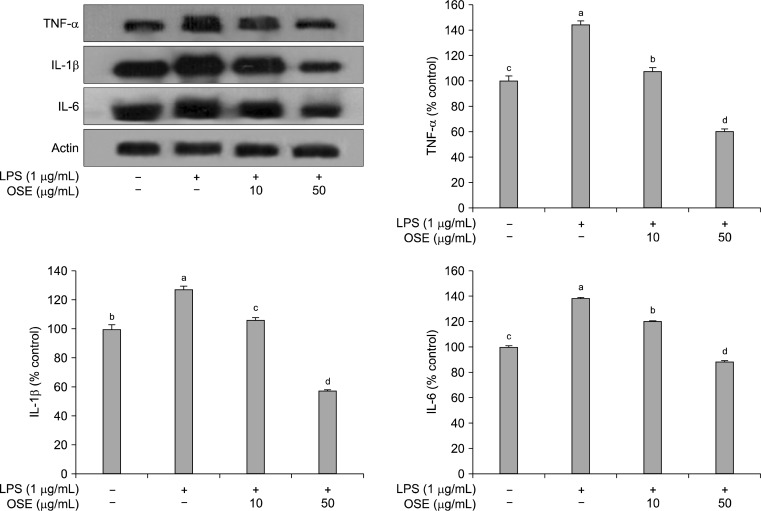
Macrophages, important components in the human immune defense system, respond actively to inflammation by releasing pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6; high levels of these cytokines can cause systemic complications (34). TNF-α is a pleiotropic inflammatory cytokine and can stimulate the production or expression of IL-1β and IL-6 (35). IL-6 is a pivotal pro-inflammatory cytokine synthesized mainly by macrophages and plays a role in the acute-phase inflammation response (36). IL-1β is also considered to be another pivotal pro-inflammatory cytokine (37). LPS stimulation of Raw 264.7 cells increased the production of TNF-α, IL-1β, and IL-6. As shown in Fig. 4, after treatment with oyster shell extract, these increases were significantly reduced (p<0.05) in a dose-dependent manner.This result indicates that oyster shell extract can attenuate the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6.
Fig. 4.
Inhibitory effects of oyster shell extract on TNF-α, IL-1β and IL-6 production in LPS-stimulated Raw 264.7 cells. Equal amounts of cell lysates (30 μg) were subjected to electrophoresis and analysed for TNF-α, IL-1β and IL-6 production by Western blot. Raw 264.7 cells were preincubated with LPS (1 μg/mL) for 20 h, and then incubated without or with indicated concentrations of oyster shell extract for 20 h. Each value is expressed as mean±SD (n=3) and values with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. OSE: Oyster shell extract.
Effects of oyster shell extract on NF-κB, iNOS and COX-2 expressions
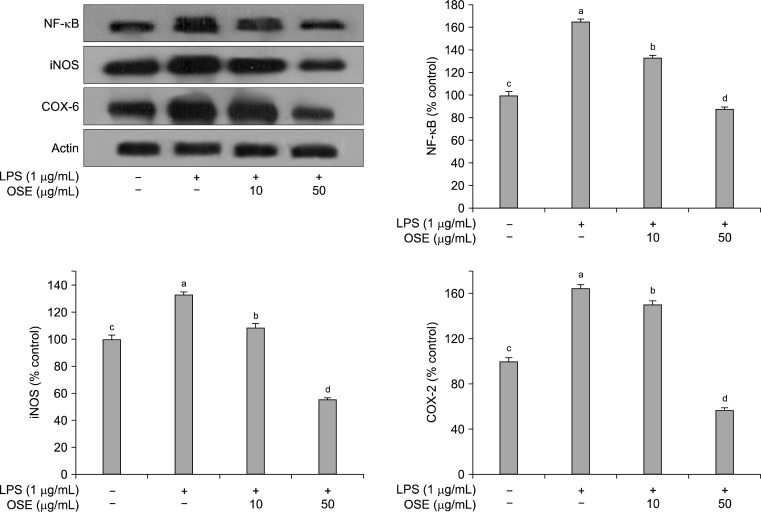
As shown in Fig. 5, NF-κB was increased in LPS-stimulated Raw 264.7 cells, and this increase was significantly reduced (p<0.05) by treatment with oyster shell extract in a concentration-dependent manner. Activation of the NF-κB family plays a central role in inflammation through its ability to induce transcription of pro-inflammatory genes (38). NF-κB is one of the principal factors for the expression of COX-2 and iNOS as mediated by the LPS or pro-inflammatory cytokines (39). LPS stimulation of the Raw 264.7 cells strongly upregulated the iNOS and COX-2 protein expression levels. However, when oyster shell extract was added to the Raw 264.7 cells, the iNOS and COX-2 activities were significantly suppressed (p< 0.05) than that treated with the LPS only (Fig. 5). Usually, activated inflammatory cells produce high quantities of NO, which in turn produce iNOS. NO is necessary for maintaining prolonged COX-2 gene expression, a central mediator in inflammation (40,41). Both iNOS and COX-2 are inducible forms of enzymes up-regulated in response to inflammation challenge. Expression of iNOS and COX-2 can be regulated by the activation of NF-κB (42). In this study, the production of NF-κB, iNOS and COX-2 were inhibited by oyster shell extract.
Fig. 5.
Inhibitory effects of oyster shell extract on NF-κB, iNOS and COX-2 production in LPS-stimulated Raw 264.7 cells. Equal amounts of cell lysates (30 μg) were subjected to electrophoresis and analysed for NF-κB, iNOS and COX-2 production by Western blot. Raw 264.7 cells were preincubated with LPS (1 μg/mL) for 20 h, and then incubated with or without indicated concentrations of oyster shell extract for 20 h. Each value is expressed as mean±SD (n=3) and values with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. OSE: Oyster shell extract.
In conclusion, oyster shell extract significantly decreased intracellular ROS levels and increased anti-oxidant enzyme activities. Also, oyster shell extract blocked NO production and the expressions of TNF-α, IL-1β, IL-6, NF-κB, iNOS and COX-2 in LPS stimulated Raw 264.7 cells. Together, these results suggest oyster shell extract could be useful as a natural anti-oxidant and anti-inflammatory resource.
Oyster shell extract contains an abundant amount of calcium carbonate. Therefore, we suggest that calcium carbonate in oyster shell could be effective for inhibition of the inflammation process. However, further studies are needed to identify which ingredients in oyster shell extract inhibit the inflammation.
Acknowledgments
This research was a part of the project titled “Osteoarthritis health functional food development that use oyster shell” funded by the Ministry of Land, Transport and Maritime Affairs, Korea (KIMT-2012-D11110411H3900 00111).
REFERENCES
- 1.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo LY, Hung TM, Bea KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of schisandra chinensis baill. J Pharmacology. 2008;597:293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 3.Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M, Lundberg JO, Hellstrom PM. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12:3386–3392. doi: 10.3748/wjg.v12.i21.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. Biofactor. 2002;12:129–133. doi: 10.1002/biof.5520120120. [DOI] [PubMed] [Google Scholar]
- 5.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Lmmunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Xu W. TLR4-mediated activation of macrophages by the polysaccharide fraction form polyporus umbellatus. Fries J Ethnopharmacology. 2010;135:1–6. doi: 10.1016/j.jep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 9.Liu XH, Kirschenbaum A, Yao S, Stearns ME, Holland JF, Claffey K, Levine AC. Uppregulation of vascular endothelial growth factor by cobalt chloride-simulated hypoxia is mediated by persistent induction of cyclooxygenase-2 in a metastatic human prostate cancer cell line. Clin Exp Metastasis. 1999;17:687–694. doi: 10.1023/a:1006728119549. [DOI] [PubMed] [Google Scholar]
- 10.DeFranco AL, Hambleton J, McMahon M, Weinstein SL. Examination of the role of MAP kinase in the response of macrophages to lipopolysaccharide. Prog Clin Biol Res. 1995;392:407–420. [PubMed] [Google Scholar]
- 11.Kim GH, Jeon YJ, Byun HG, Lee YS, Lee EH, Kim SK. Effect of calcium compounds from oyster shell bouind fish skin gelatin in calcium deficient rats. J Korean Fish Soc. 1998;31:149–159. [Google Scholar]
- 12.Fujita T, Fukase M, Nakada M, Koishi M. Intestinal absorption of oyster shell electrolysate. Bone Miner. 1998;11:85–91. [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colormetric assay for cellular growth and survival application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Periquet A, Fernandez Y, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 18.D’ Agostino P, Ferlazzo V, Milano S, La Rosa M, Di Bella G, Caruso R, Barbera C, Grimaudo S, Tolomeo M, Feo S, Cillari E. Anti-inflammatory effects of chemically modified tetracyclines by the inhibition of nitric oxide and inteleukin-12 synthesis in J774 cell line. Intern Immunopharmacol. 2001;1:1765–1776. doi: 10.1016/s1567-5769(01)00100-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim EK. Purification and characterization of antioxidative peptides from enzymatic hydrolysates of venison. 2008. PhD Dissertation. Pusan National University, Busan, Korea.
- 20.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation. Int J Cancer. 2011;128:1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidi SF, Ahmed K, Yamamoto T, Kondo T, Usmanghani K, Kadowaki M, Sugiyama T. Effect of resveratrol on Helicobacter pylori-induced interleukin-8 secretion, reactive oxygen species generation and morphological changes in human gastric epithelial cells. Biol Pharm Bull. 2009;32:1931–1935. doi: 10.1248/bpb.32.1931. [DOI] [PubMed] [Google Scholar]
- 22.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, Tubaro A, Menichini F. The protective ability of Mediterranean dietary plants against the oxidative damage: the role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009;112:587–594. [Google Scholar]
- 23.Vivancos M, Moreno JJ. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic Biol Med. 2005;34:91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Bulkey GB. The role of oxygen free radicals in human disease processes. Surgery. 1983;94:407–411. [PubMed] [Google Scholar]
- 25.Yasui K, Baba A. Therapeutic potential of superoxide dismutase for resolution of inflammation. Inflamm Res. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 26.Jarvinen K, Pietarinen-Runtti P, Linnainmaa K, Raivio KO, Krejsa CM, Kavanagh T. Antioxidant defense mechanisms of human mesothelioma and lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:696–702. doi: 10.1152/ajplung.2000.278.4.L696. [DOI] [PubMed] [Google Scholar]
- 27.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 29.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 30.Clancy RM, Amin AR. The role of nitric oxide in inflammation and immunity. Atrhritis Rheun. 1998;14:1141–1151. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Salas M, Gironella M, Salasa M. Nitric oxide supplementation ameliorates dextran sulfate sodium-induced colitis in mice. Lab Invest. 2002;82:597–607. doi: 10.1038/labinvest.3780454. [DOI] [PubMed] [Google Scholar]
- 32.Kin SB, Seong YA, Jang HJ, Kim GD. The anti-inflammatory effects of Persicaria thunbergii extracts on lipopolysaccharide-stimulated RAW 264.7 cells. J Life Sci. 2011;21:1689–1697. [Google Scholar]
- 33.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitirite: the good, the bad, and ugly. Am J Physiol. 1996;271:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 34.Bosca L, Zeini M, Traves PG, Hortelano S. Nitiric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Fur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 36.Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179:294–304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 37.Jung WK, Choi I, Lee DY, Yea SS, Choi YH, Kim MM, Park SG, Seo SK, Lee SW, Lee CM, Park YM, Choi IW. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in Raw 264.7 macrophages via the p38/ERK and NF-κB pathways. Int J Biochem Cell Biol. 2008;40:2572–2584. doi: 10.1016/j.biocel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Paul PT, Cary SF. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM, Byun HG, Chio YH, Park SG, Choi IW. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J Agric Food Chem. 2009;57:4439–4446. doi: 10.1021/jf9003913. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Han W, Wang MH. Antioxidant activity of hawthom fruit in vitro. J Appl Biol Chem. 2010;53:8–12. [Google Scholar]
- 41.Perkins DJ, Kniss DA. Blockade of nitric oxide formation down-regulates cyclooxgenase-2 and decreases PGE2 biosynthesis in macrophages. J Leukoc Biol. 1999;65:792–799. doi: 10.1002/jlb.65.6.792. [DOI] [PubMed] [Google Scholar]
- 42.Vane JR, Bottinf RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 1998;47:78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]