Abstract
Black jujube was made by aging dried jujube and its physiochemical characteristics, antioxidant activities and α-glucosidase inhibitory activities were evaluated. The moisture and sugar contents were increased depending on the period of aging times and the pH was reduced thereby increasing acidity. The color of black jujube extract was changed from red to black resulting in decreases of Hunter color values L, a and b. As the aging progressed, sucrose was decomposed by increasing glucose and fructose, indicating higher contents of the total reducing sugars. Among the six different types of organic acids extracted from dried jujube, the levels of oxalic acid and citric acid were increased as the aging progressed. The total polyphenol contents in ethanol and water extracts of dried jujube were 7.74 and 8.12 mg/g, respectively. The water extract of black jujube aged for 48 hr contained the highest polyphenol contents at 16.82 mg/g. The 5’-hydroxymethylfurfural (5’-HMF) contents of black jujube extract significantly increased by longer aging times, and contained higher contents in the ethanol extract than water extract. The ethanol extract of black jujube showed the highest 5’-HMF content with 338.89 mg% after aging for 3 days. Also, IC50 values of black jujube aged for 72 hr evaluated by DPPH and ABTS radical assays were 0.54 and 0.59 mg/mL, respectively. α-Glucosidase inhibitory activities of black jujube at the concentration of 3.33 mg/mL (ethanol extract) increased from 65 to 80 % after aging for 72 hr.
Keywords: black jujube, antioxidant, aging, α-glucosidase, organic acid
INTRODUCTION
Jujube (Ziziphus jujuba Miller) belongs to the family Rhamnaceae, which is widely distributed throughout tropical and subtropical climates around the world (1). Jujube has elliptical fruits with thin skin which are initially green but gradually change to reddish brown. Jujube fruits have been recognized as nutritious foods and have important uses in traditional medicine in oriental countries.
The jujube fruit has a light aroma complementing a sweet and slightly sour refreshing taste. In particular, jujube fruits are richer in sugar, vitamin C, bioflavonoids, edible fiber and minerals than other fruits (2). Medicinal ingredients such as sterols, alkaloids, saponins, serotonin, polyphenol, and flavonoids (3) along with triterpenoids (4) and c-GMP (5) are reportedly contained in jujube fruits. The methanol extracts, from the fruit, pharmacologically protect liver (6), suppress cancer cell proliferation (7) and generate antioxidant effects (8). Due to the increasing interest in healthy longevity in an aging society, researches on natural bioactive materials and functional and health foods are heightened.
Fresh jujube is difficult to keep for a long period of time and thus it is usually used after drying. The fruit is sun-dried where jujube is cultivated since its quality is largely affected by the drying method. However, the fruit often decomposes or softens during a rainy season, resulting in microbial contaminations as well as a poor hygienic status. Therefore, researches to improve its shelflife and functions are required to process and distribute jujube effectively.
The Maillard reaction, which generates non-enzymatic browning in food processes, greatly affects the flavors of foods including melanoidin generated from chemical reactions between reducing sugars and amino acid residues. Melanoidin has attracted interest due to a report on its beneficial effects on human health (9). In South Korea, black garlics have been produced in recent years by the chemical reaction of indigenous ingredients in garlic bulb through heat treatments in order to enhance the smell and taste of garlics (10). Black garlics are well known to have a superior organoleptic quality harmonizing the sweetness and the sourness taste when they are aged at a high temperature for a certain period, compared to unprocessed garlic. Recently, black jujubes were produced by aging for long periods and contained enhanced antioxidant activity by showing DPPH radical scavenging effect (11). However, research is lacking on the physiochemical and functional changes in composition and bioactive substances during the aging process. Production of black jujube by non-enzymatic browning, thought to be generated by an amino-carbonyl reaction, is possible because it contains sugar and amino acids. Previous studies have focused on the antioxidant activities of lipids (12) and the anti-mutagenesis activities by the products of the browning reaction, which are generated by the hydrogen-donating activity of reductone (13). The functions of melanoidin generated by the browning reaction includes strong antioxidant effects, radical scavenging activity and peroxidase activity (14). A previous study claims that the antioxidant effects of browning agents are caused by the substances created at the beginning of the browning process (14). However, a different study claims that the anti-oxidative activity increases as the browning progressed, leaving much debate about anti- oxidative activity (15). Furthermore, research needs to be conducted on the changes in palatability, including the taste, through the evaluation of changes in the components of black jujube during the non-enzymatic browning.
Therefore, in this study, dried jujube was effectively converted into black jujube with enhanced quality and functional properties for a short period. The organoleptic and physiochemical properties as well as the antioxidant activity and α-glucosidase inhibitory activity of water and ethanol extracts were evaluated. These results can be used as preliminary data to utilize black jujube as a novel ingredient for new functional and health foods.
MATERIALS AND METHODS
Materials
The dried jujube was obtained from Dae-Heung Nong San (Gyeongbuk, Korea). All the samples were washed under running water and dried at room temperature for 24 hr. Ethanol was purchased from Duksan Chemical Co. (Ansan, Korea) and the HPLC solvent used was of HPLC grade (J.T. Baker, Philipsburg, NJ, USA). DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS (2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), α-glucosidase and p-nitrophenyl-α-D-glucopyranoside were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Production of black jujube
Whole dried jujube with skin was placed in a heat-resistant plastic airtight container (30×20×20 cm) with a stainless steel net. To prevent drying, 100 mL of water was placed into the container and then the jujube was aged in a drying oven at 80°C for 72 hr. Black jujube was freeze-dried, packed in to sealing bags and kept at −20°C.
Measurement of moisture and sugar contents
The moisture contents of jujube and black jujube were measured by an infrared moisture determining balance (FD-720, KETT Electric Laboratory, Tokyo, Japan). The total sugar content was measured by a portable digital refractometer (Refractomer Pocket PAL-3, Atago, Tokyo, Japan).
Measurement of color values
The Hunter color values of drying jujube and black jujube were obtained in terms of L (lightness), a (redness), and b (yellowness) values using a Hunter Lab colorimeter (Color Reader, CR-10, MINOLTA, Osaka, Japan). The 20 mL of distilled water was added to 5 g of sample and extracted for 30 min at room temperature. The solution was centrifuged at 1,000×g for 15 min and 1 mL of supernatant was measured in a screw cap test tube (PYREX, diameter 13 mm). ΔE value was calculated for the L, a, b values in the difference between the standard white plate, with L=97.37, a=0.12, b=1.82, respectively, and the sample.
Measurement of pH and total acidity
The pH of black jujube was determined with a pH meter (model 420A+, Thermo Fisher Scientific, Hudson, NH, USA). The titratable acidity was measured by determining the 0.1 N NaOH content necessary for adjusting to pH 8.3 and then expressed with tartaric acid content (%, v/v).
Free sugars and non-volatile organic acid contents
Two grams of freeze-dried black jujube was placed in a 50 mL conical tube and extracted for 30 min with 18 mL of distilled water. The extract was centrifuge at 1,000×g for 15 min and filtered through a 0.45 μm membrane filter. Glucose, sucrose, and fructose were quantified on a Knauer HPLC system (Knauer Co., Berlin, Germany) consisting of a refractive index detector, a column heater set at 35°C, and a Shodex monosaccharide column (Shodex, Tokyo, Japan); the isocratic mobile phase was 75% acetonitrile delivered at 1 mL/min. Non-volatile organic acid contents were quantified using a method adapted from Nakagawa et al. (16). Five grams of freeze-dried black jujube was placed in a 50 mL falcon tube and extracted for 30 min with 25 mL of distilled water. The extract was centrifuge at 1,000×g for 15 min and filtered through a 0.45 μm membrane filter and then applied to a Sep-pak plus C18 cartridges (55~105 μm, Waters Co., Milford, MA, USA). The Sep-pak column had been previously prepared by washing with 4 mL of methanol followed by 8 mL of boiling deionized-distilled H2O. The non-volatile organic acids were quantified on a Knauer HPLC system and a UV detector (210 nm), a column heater set at 30°C, and a Shodex Hypersil Gold aQ C18 column (4.6×250 mm) (Thermo Co., Waltham, MA, USA); the isocratic mobile phase was 20 mM H3PO4 delivered at 1 mL/min. Non-volatile organic acids were expressed as mg/100 g.
Preparation of black jujube extracts (BJE)
Dried jujube and black jujube seeds were removed and then freeze-dried. The powdered jujube was extracted with 10 volumes of 70% ethanol or distilled water for 12 hr 3 times at 25°C using a shaking incubator (SI-900R, JEIO TECH Co., Daejeon, Korea). The filtered extracts were concentrated by evaporation (EYELA, Rikakikai Co., Tokyo, Japan) under reduced pressure. After the extracts were thoroughly dried for complete removal of solvent, the dried extract was then stored in a deep freezer (−80°C).
5’-Hydroxymethylfurfural content of BJE
BJE samples (1 g) were dissolved with deionized water in a 10 mL volumetric flask. After thorough mixing, 50 mL acetone was added to the faction funnel and extracted 3 times. Samples were removed from the solvent completely and added to 2 mL of methanol. Samples were filtered through a 0.45 μm membrane filter (Millipore, Billerica, MA, USA) and analyzed by HPLC. The chromatographic determination was carried out on a Shimadzu LC-20A prominence manufactured by Shimadzu Company, with a SPD-20A UV/VIS detector, using μBondapack C18, 4.6×300 mm, 10 μm columns (Waters Co.). The isocratic HPLC system used a water/acetonitrile mix (80:20) as the mobile phase for analysis. The mobile phase flow rate was 0.6 mL/min, with the sample injection volume of 20 μL and the column temperature at 30°C. The HMF was detected in the UV region at 280 nm.
Determination of total polyphenol contents
The total polyphenol content of the BJE was determined according to the Folin-Ciocalteu method (17) with some modifications. One mL of prepared BJE (10 mg/mL concentration) was transferred into a test tube and adjusted to a total volume of 25 mL with distilled water. Folin-Ciocalteu’s reagent (0.5 mL) and sodium carbonate (10%, 1.5 mL) were added 3 min later. After the samples were mixed and left at 30°C for 1 hr, the absorbance at 750 nm was measured. Distilled water was used as the blank and the control. A calibration curve of garlic acid was prepared, and polyphenol contents were determined from the linear regression equation of this curve. The results are reported as garlic acid equivalents per mg extract.
DPPH radical scavenging activity
The DPPH free radical scavenging activity of BJE was evaluated by the Blois method (18). Different concentrations (0.1∼1.5 mg/mL) of BJE were prepared and diluted to 3 mL with ethanol. Then, 1 mL of ethanolic DPPH solution (0.1 mM) was added to the samples. These samples were mixed and then incubated in the dark at 30°C for 30 min. The absorbance was measured at 517 nm against blank samples. A decrease in absorbance indicates DPPH free radical scavenging activity. Butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and vitamin C were used as the positive control group.
ABTS radical scavenging activity
The ABTS radical scavenging activity of BJE was evaluated according to the method of Pellegrini et al. (19) with minor modifications. ABTS is blue-green in color with a characteristic absorbance at 734 nm. ABTS radical cation was produced by reacting ABTS (2 mM) in H2O and potassium persulphate (2.45 mM) at room temperature for 12 hr. The ABTS radical solution was diluted with phosphate buffer (0.1 M, pH 7.4) to achieve an absorbance of 0.750±0.025 at 734 nm. Then, 1 mL of ABTS radical solution was added to 3 mL of BJE in methanol at different concentrations (10~30 μg/mL). These samples were mixed, incubated in the dark for 30 min, and the absorbance at 734 nm was measured for each concentration relative to a blank. Decreased absorbance of the samples indicates ABTS radical cation scavenging activity. Vitamin C and trolox were used as a positive control group.
In vitro α-glucosidase inhibitory assay
Two grams of freeze-dried black jujube was placed in a 50 mL falcon tube and extracted for 30 min with 18 mL of distilled water or 70% ethanol. The α-glucosidase inhibitory assay of the BJE was determined using a modified procedure reported by Choe et al. (20). α-Glucosidase (EC 3.2.1.20) isolated from Saccharimyces cerevisiae and the substrate 4-nitrophenyl-α-D-glucopyranoside were purchased from Sigma-Aldrich. The initial concentrations of the enzyme and substrate solutions were 0.75 unit/mL and 2 mM in 50 mM potassium phosphate buffer (pH 6.5), respectively. The enzyme solution (20 μL/microtritre well plate) was mixed with the samples in a clear 96-well microplate (flat bottom) and the reaction was started by addition of the substrate solution (50 μL/well). The plates were incubated at 37°C for 30 min after shaking and the reaction was stopped by adding 0.1 M Na2CO3 (100 μL/well). Enzyme inhibition was determined by the absorbance of 4-nitrophenyl (product) at 405 nm, as measured with a microplate reader (Spectramax 340 pc 384, Molecular Device, LLC, CA, USA). Background absorbance was determined using a non-enzyme control microplate containing the potassium phosphate buffer (20 μL/well) and was subtracted from the absorbance of sample and controls. Acarbose was used as a positive control group.
Statistical analysis
All experiments were performed in triplicate. The results were expressed as the mean±standard error values (SE). One-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison test was performed. Statistical Package for the Social Science (SPSS, Version 20.0, SPSS Inc., Chicago, IL, USA) was used.
RESULTS AND DISCUSSION
Quality characteristics of black jujube produced from different aging periods
The jujube as a valuable fruit crop can be grown under a variety of environmental conditions, including those in dry areas. The soluble solid content of jujube fruit is about 20~40% (21). The carbohydrate content in Chinese jujubes can reach as high as 80~85%, including higher sugars and even protein contents (22). Therefore, as expected, the physicochemical properties of jujube fruit can be changed by the Maillard reaction. Conclusively, black jujube with higher palatability could be produced by aging at 80°C for 2 days. The moisture in foods greatly affects the appearance, texture, and sensual quality and plays an important role in spoilage of foods microbiologically (23). In the Maillard reaction, the brown pigment formation is also dependent on the moisture content of fruits and maximum browning occurs at 30% moisture, which corresponds to a water activity of 0.6~0.8 (24). Table 1 shows the changes in moisture content of jujube according to the aging period. The moisture content of jujube was about 36.56% and that of black jujube increased to 47.92% with longer aging periods. Thus, for the first stage of the Maillard reaction, dried jujube contained suitable moisture content for the browning reaction. The changes in moisture content during the aging process was also confirmed by a sensual evaluation performed by visual inspections. In conclusion, the flesh of jujube fruit absorbed moisture while dried jujube was aged in the condition of saturated humidity at a high temperature of 80°C.
Table 1.
The quality characteristics of black jujube extract according to aging time
| Sample | Aging time (hr) | Non drying | Freeze drying powder | ||
|---|---|---|---|---|---|
|
|
|
||||
| Water content (%) | Total soluble solids (°Bx) | pH | Titratable acidity (%) | ||
| Dry jujube | 0 | 36.56±0.66a | 77.90±0.99a | 4.96±0.40b | 1.08±0.03a |
| Black jujube | 24 | 45.73±1.51b | 79.80±1.10ab | 4.30±0.02a | 1.86±0.11b |
| 48 | 47.01±0.69b | 80.75±0.59b | 4.20±0.05a | 2.82±0.14c | |
| 72 | 47.92±1.27b | 81.71±0.97b | 3.86±0.01a | 3.33±0.10d | |
Data are expressed as mean±standard error values (n=3). Mean with different letters in each column are significantly different (p<0.05) by Duncan’s multiple range test.
The total soluble solid content of dried jujube was about 10.37°Bx and significantly increased by 77.90°Bx after freezing drying. During ripening, total soluble solid content of jujube increased and more than 75% of the pectin in the jujube was water-soluble pectin, supporting that the total soluble solid content of ripen jujube is relatively high. Interestingly, the total soluble solid content of black jujube slightly increased to 81.71°Bx after aging for 72 hr. The estimated increase of soluble solid content was stemmed from the transformation of insoluble carbohydrate into dextrin or monosaccharide during thermal processing (25).
The pH was reduced and the acidity increased when prolonging the aging process. The initial acidity of 1.08% increased to 3.33% after aging for 72 hr (Table 1). The pH of dried jujube was mildly acid with pH 4.96 and decreased to pH 3.86 as the aging period became longer (72 hr). In the Maillard reaction, nonvolatile components generated by sugars and peptides were largely affected by the reaction temperature. The pH was rapidly reduced as the reaction temperature increased, which was reported caused by organic acids created during the browning process (9). Black jujube has a strong sourness and some bitterness after aging for 72 hr, giving it a low palatability. During the aging at a high temperature, the sourness of jujube is largely increased and the texture becomes softer. However, the soluble solid content of aged jujube is partially increased. These results imply that aged jujube may have significantly different acids and sugar compositions. Therefore, the balance of sweetness and sourness should be considered to produce the good organoleptic properties of aged jujube. The ratio of Brix to acid is an important factor in considering the palatability of fruits. The Brix-acid ratio of aged jujube decreased to 42.9, 28.6 and 24.5 by aging for 1, 2, and 3 days, respectively. The aged jujube still had a higher soluble solid content compared with the Brix-acid ratio of other ripen fruits; however, the main fraction of soluble solid in jujube is considered to consist of soluble pectin instead of sweet sugar. Furthermore, Frank et al. (26) reported that the Maillard mixture contained the key compounds contributing the most to the intense bitter taste. Conclusively, the organoleptic properties of aged jujube could be manipulated by the aging time at a high temperature. These results confirmed that the palatable aged jujube, providing good taste and black color, was obtained by aging for 2 days under a saturated hot condition.
The browning of dried jujube to black jujube is generated by the amino-carbonyl reaction between sugars and amino acids present in the jujube fruit. Therefore, to turn dried jujube into black jujube occurs by maturation process rather than fermentation because the microorganisms will not be able to survive (27). The color of black jujube becomes dark brown or black as the aging period becomes longer (Fig. 1). Table 2 shows the changes in colors of dried jujube and aged jujube. The brightness of dried jujube (L) was 19.68 before aging and then became less and less as the maturation progressed, leading to 14.23 after aging for 72 hr. The red chromaticity (a) and the yellow chromaticity (b) were recorded as 2.45 and 3.87, respectively, at the initial stage and were also significantly reduced to 1.18 and 0.77, respectively, after aging for 24 hr while still decreasing afterwards.
Fig. 1.
Comparison of dry jujube and black jujube aged for different aging periods. (A) dry jujube, (B) aged jujube (24 hr), (C) aged jujube (48 hr), (D) aged jujube (72 hr).
Table 2.
The color values of black jujube extract according to aging time
| Aging time (hr) | L (lightness) | a (redness) | b (yellowness) | ΔE |
|---|---|---|---|---|
| 0 | 19.68±1.33c | 2.45±0.15c | 3.87±0.07c | 20.21±1.30d |
| 24 | 17.15±0.33b | 1.18±0.04b | 0.77±0.08b | 17.21±0.33b |
| 48 | 16.04±0.58b | 1.44±0.12b | 0.88±0.01b | 16.13±0.59b |
| 72 | 14.23±0.73a | 0.70±0.27a | 0.30±0.09a | 14.25±0.72a |
Data are expressed as mean±standard error values (n=3). Mean with different letters in each column are significantly different (p<0.05) by Duncan’s multiple range test.
Free sugar and nonvolatile organic acid contents of black jujube
Table 3 shows the free sugar contents of dried and black jujube according to the aging period. The sugars, including fructose, glucose and sucrose, were extracted from dried jujube, but sucrose was disappeared after maturation for 24 hr; however, fructose and glucose gradually increased. The higher fructose and glucose contents of black jujube were thought to be caused by the decomposition of sucrose during the aging at a high temperature. According to a previous report, acid hydrolysis of sucrose occurred in continuous thermal processing at pasteurization temperature (28). Therefore, the sucrose in jujube could have converted into monosaccharides during aging at a high temperature. In conclusion, the fructose and glucose determined the sweetness of black jujube. The total sugar content of dried jujube was 370.10 mg/g, but this increased to 439.20 mg/g after aging for 72 hr. Table 4 shows the changes in nonvolatile organic acid contents according to the aging of dried jujube. Six different types of organic acids were extracted from dried jujube. The oxalic acid content was the highest with 436.08 mg%, followed by citric acid (198.92 mg%), and lactic acid (40.27 mg%). These organic acid contents, except lactic acid, appeared to increase as the aging time become longer. Although the organic acid contents were similar to those in a study by Lee (29), the citric acid content (330 mg%) was reportedly the highest in that study. Conclusively, the overall taste of aged jujube could be dependent upon the acid content which increased during the aging process. Thus, further experiments are needed to determine the optimum aging period for producing wholesome aged jujube.
Table 3.
Change in free sugar contents of black jujube extract from different aging times
| Free sugar contents (mg/g) | Aging time (hr) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 24 | 48 | 72 | |
| Fructose | 153.10±7.38a | 181.76±2.70b | 211.84±10.20c | 222.50±3.54c |
| Glucose | 160.60±3.22a | 174.90±4.59a | 209.84±5.85b | 216.70±8.56b |
| Sucrose | 56.40±1.50 | ND | ND | ND |
| Total contents | 370.10 | 356.66 | 421.68 | 439.20 |
Data are expressed as mean±standard error values (n=3). Mean with different letters in each row are significantly different (p<0.05) by Duncan’s multiple range test.
Table 4.
Change in nonvolatile organic acid contents of black jujube extract according to aging times
| Organic acid contents (mg/100 g) | Aging time (hr) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 24 | 48 | 72 | |
| Oxalic acid | 436.08±10.50a | 697.97±8.01b | 720.49±5.58b | 755.67±12.93c |
| Pyruvic acid | 22.03±3.36a | 44.60±2.90b | 82.34±3.80c | 45.44±1.58b |
| Lactic acid | 40.27±2.91a | 45.56±5.83a | 41.92±10.40a | 42.89±3.92a |
| Acetic acid | 5.24±1.95a | 8.79±1.56ab | 8.55±0.97ab | 12.96±2.39b |
| Malic acid | 1.25±0.78a | 3.86±0.91b | 5.47±0.83b | 5.86±1.05b |
| Citric acid | 198.92±13.11a | 384.52±8.29b | 426.23±13.63c | 498.54±17.13d |
Data are expressed as mean±standard error values (n=3). Mean with different letters in each row are significantly different (p<0.05) by Duncan’s multiple range test.
5′- Hydroxymethylfurfural contents of BJE
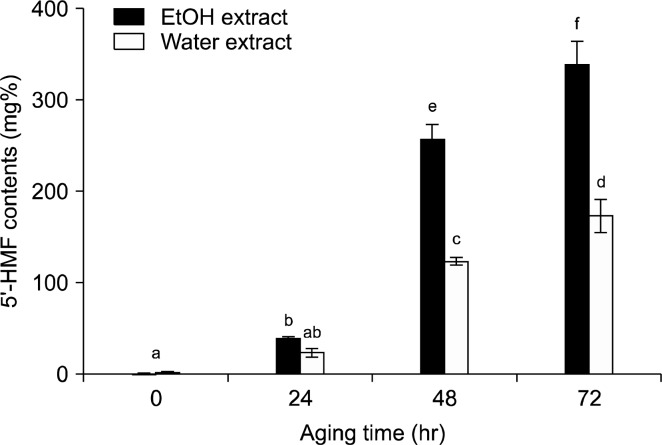
5’-Hydroxymethylfurfural (5’-HMF) is a common Maillard reaction product generated during heat-processing. HMF occurs as a product of decomposing sugars, including glucose and sucrose, in the presence of acid. Although 5’-HMF has been proposed to have harmful effects, its beneficial effects include antioxidant, cytoprotective and antitumor effects and have become increasingly apparent. A recent study found that the extract of aged black garlic shows anti-inflammatory properties when administered to human umbilical vein endothelial cells (30). The 5’-HMF contents of black jujube extract significantly increased with longer aging times, and contained even higher contents in the ethanol extract than the water extract (Fig. 2). The 5’-HMF content of ethanol extract showed the highest value with 338.89 mg% after aging for 3 days. Compared to the 5’-HMF content (1.34 mg%) in non-aging jujube, that in aged jujube increased by approximately 250 times. Interestingly, Park et al. (11) reported that after 50 days of aging, HMF content of jujube extract concentrate was 38.84 mg/g. The dehydrated fruit such as prune contains about 220 mg% of HMF. The important factors in the development of HMF in the Maillard reaction are temperature and time so that the formation of browning products and HMF increase with extended heat exposure and aging time.
Fig. 2.
5’-HMF contents of black jujube extract according to aging time. Data are expressed as mean±standard error values (n=3). Mean with different letters are significantly different (p<0.05) by Duncan’s multiple range test.
Total polyphenol contents of BJE
Table 5 shows the polyphenol contents from dried jujube and black jujube extract according to the aging period. The polyphenol contents in the ethanol extract of dried jujube was 7.74 mg/g compared to the water extract was 8.12 mg/g, which both increased as the aging progressed. The polyphenol content from ethanol extract after aging for 72 hr was increased to 13.79 mg/g and that of water extract increased to 16.82 mg/g after only 48 hr, indicating the highest levels. The reasons for the increased polyphenol contents by aging dried jujube at a higher temperature was thought to be caused by the transformation of some ingredients into soluble polyphenols or the efficient extraction of polyphenol compounds due to the softening of fruit pulp during the humid heat processing. Jumnongpon et al. (31) reported that the Maillard reaction of proteins does not only result in the generation of flavors and colors, but also changes the texture of foods. Shim (32) reported that the polyphenol contents of black jujube aged for a week at a certain humidity was 2.61%, which was higher than that of dried jujube. Kim et al. (33) reported that total phenol and flavonoid contents increased when black garlic was produced by a heat treatment at high pressure.
Table 5.
The total polyphenol contents of black jujube extract according to aging times
| Sample | Aging time (hr) | Total polyphenol contents (mg/g) |
|---|---|---|
| Ethanol extract | 0 | 7.74±0.42a |
| 24 | 12.44±0.71b | |
| 48 | 13.39±0.55b | |
| 72 | 13.79±0.57b | |
| Water extract | 0 | 8.12±0.60a |
| 24 | 13.11±0.52b | |
| 48 | 16.82±0.58d | |
| 72 | 15.17±0.64c |
Data are expressed as mean±standard error values (n=3). Mean with different letters in each column are significantly different (p<0.05) by Duncan’s multiple range test.
DPPH radical scavenging effects of BJE
DPPH (1,1-diphenyl-2-picryl-hydrazyl) can be deoxidized by ascorbic acid, tocopherol, polyhydroxy aromatic compounds and aromatic amines, decolorizing its violet color which is used to measure electron releasing levels of antioxidants (34). Electron releasing antioxidants are characterized by the ring structure containing more than one hydroxyl group and a non-polar group, such as methyl, or non-polar hydrocarbon chain. The polyphenol contents in foods are the representative means for measuring antioxidant effects.
Table 6 shows the results of anti-oxidative effects of dried jujube and black jujube using DPPH free radical scavenging activity. The value of IC50 of dried jujube extract was about 1 mg/mL but the ethanol extract and water extract of black jujube after aging for 72 hr were 0.54 mg/mL and 0.56 mg/mL as DPPH radical scavenging activity increased. Shim (32) reported that the level of DPPH radical scavenging activity of the hydrothermal extract of black jujube was superior to that of the 70% ethanol extract of black jujube, 97.94% and 93.05%, respectively, at the concentration of 1 mg/mL. Moreover, Song (35) reported that the levels of DPPH radical scavenging activity increased to more than 99% after 5 days when jujube was aged for 25 days. The levels of DPPH radical scavenging activity of black jujube matured for 20 days were 70.90% at the concentration of 50 mg/mL and 89.42% at the concentration of 100 mg/mL, supporting the claim that the activity level was increased as the maturation period became longer (25). Generally, the reason for the higher level of DPPH radical scavenging activity of black jujube than dried jujube is thought to be stemmed from the increased polyphenol and flavonoid contents and the melanoidin generated during the aging process. As previously reported and according to our results, the Maillard reaction products provided anti-oxidant properties (36).
Table 6.
DPPH radical scavenging effects of black jujube extract according to aging times
| Sample | Aging time (hr) | IC501) (mg/mL) |
|---|---|---|
| Ethanol extract | 0 | 1.08±0.10d2) |
| 24 | 0.68±0.03b | |
| 48 | 0.56±0.03a | |
| 72 | 0.54±0.02a | |
| Water extract | 0 | 0.94±0.02c |
| 24 | 0.69±0.06b | |
| 48 | 0.60±0.01a | |
| 72 | 0.56±0.06a | |
| BHT3) | 2.62±0.92 μg/mL | |
| BHA4) | 9.63±0.31 μg/mL | |
| Vitamin C | 6.38±0.50 μg/mL | |
Concentration required for 50% reduction of DPPH radical at 30 min after starting the reaction.
Data are expressed as mean±standard error values (n=3). Mean with different letters in each column are significantly different (p<0.05) by Duncan’s multiple range test.
Butylated hydroxy toluene.
Butylated hydroxy anisole.
ABTS radical scavenging effects of BJE
ABTS radical cation is eradicated by the anti-oxidative reagent resulting in the decolorization of the unique green-blue color of radicals, when ABTS and potassium persulfate are kept in the dark to generate ABTS radical cations, and can be expressed in optical density to measure the ABTS radical scavenging activity (37). Table 7 shows the results of ABTS radical scavenging activity of dried jujube and black jujube according to the aging periods. The results resembled the results of DPPH radical scavenging activity in that the activity was increased as the maturation period became longer. The IC50 value of water extract of dried jujube was about 2 mg/mL whereas the IC50 value of water extract of black jujube was 0.66 mg/mL after aging for 72 hr. In a previous report, the ABTS radical scavenging activity increased as dried jujube with red skin was becoming black jujube with black skin (38), which was measured in trolox equivalent antioxidant capacity after aging for 5 days with up to 3.186 mmol/L and little difference afterwards. Byun et al. (39) concluded that the anti-oxidative effects of the ethanol extract of garlic reduced to about 50% with treatment at 100°C compared to 120°C, implying that the anti-oxidative effects are related to the intermediated products of the Maillard reaction generated by the higher heat treatment. The Maillard reaction for browning can occur in most foods and this non-enzymatic browning takes place during thermal food or storage processes. Melanoidin, the brown colored polymer, is generated by the reaction between amino acids and sugars during the maturation of traditional Korean condiments and has a strong anti-oxidative effect known to prevent cancer (40). Thus, the Maillard reaction product in black jujube is a beneficial ingredient providing coloring and anti-oxidative effects.
Table 7.
ABTS radical scavenging effects of black jujube extract according to aging times
| Sample | Aging time (hr) | IC501) (mg/mL) |
|---|---|---|
| Ethanol extract | 0 | 2.09±0.04e2) |
| 24 | 0.73±0.02c | |
| 48 | 0.60±0.01a | |
| 72 | 0.59±0.01a | |
| Water extract | 0 | 1.99±0.02e |
| 24 | 0.82±0.02d | |
| 48 | 0.70±0.02b | |
| 72 | 0.66±0.01b | |
| Vitamin C | 67.29±5.94 μg/mL | |
| Trolox | 57.55±1.28 μg/mL | |
Concentration required for 50% reduction of ABTS radical.
Data are expressed as mean±standard error values (n=3). Mean with different letters in each column are significantly different (p<0.05) by Duncan’s multiple range test.
α-Glucosidase inhibitory activity of BJE
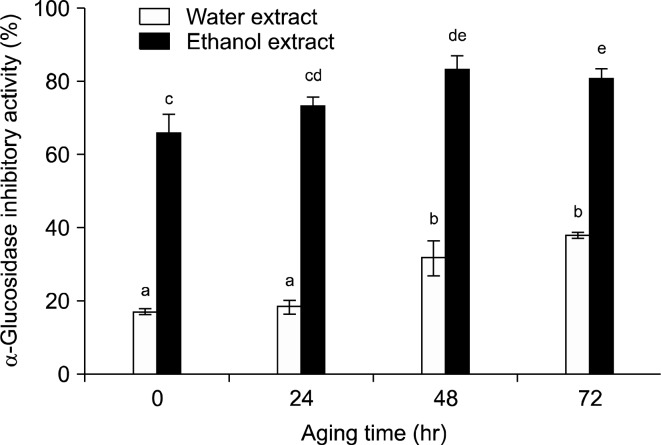
α-Glucosidase is the digestive enzyme in the brush-border membrane of small bowel and hydrolyzes disaccharides and polysaccharides into monosaccharides which is the absorbable form of carbohydrates (41). α-Glucosidase inhibitory substances may prevent the increase of blood glucose level after a carbohydrate intake diet. Fig. 3 shows the results of α-glucosidase inhibitory activity of black jujube at the concentration of 3.33 mg/mL according to the aging periods. Overall, the levels of α-glucosidase inhibitory activity were superior in the 70% ethanol extract compared to the water extract. Dried jujube in the water extract and 70% ethanol extract showed the inhibitory activity of 17.04% and 65.7%, respectively. As the aging progressed, the inhibitory activity level of 70% EtOH extract increased to 80.69% and after 72 hr. Hwang et al. (42) reported that the fraction isolated from the fructose-tyrosine Maillard reaction products showed strong α-glucosidase inhibitory activity. Thus, higher α-glucosidase inhibitory activity of black jujube may be related to the HMF compound which is highly extracted with 70% ethanol. Furthermore, tannins, condensed tannins and related polyphenols showed the inhibitory effects of enzymes such as xanthine oxidase. The inhibitory activity of several oligomeric hydrolyzable tanins seemed particularly low, and the degree of polymerization in proanthocyanidins was also shown to remarkably affect the strength of the inhibition (43). In addition, water soluble Maillard polymers were prepared from HMF, glucose and amino acids (44). Thus, polyphenols in jujube seem to be polymerized during the Maillard reaction and their compounds may affect enzyme activity.
Fig. 3.
α-Glucosidase inhibitory activity of black jujube extract according to aging time. All sample concentration was 3.33 mg/mL. Data are expressed as mean±standard error values (n=3). Mean with different letters are significantly different (p<0.05) by Duncan’s multiple range test.
Shin et al. (45) reported that α-glucosidase inhibitory activity levels of the water extract of browned garlic made by hot-air drying for 30 hr at 80°C were not much different at the concentration level less than 2.5 mg/mL; but, the inhibitory activity levels increased up to 22.22% depending on the concentration level above 2.5 mg/mL. In addition, they also reported that α-glucosidase inhibitory activities of fresh garlic, red garlic and black garlic extracts were measured at 21.23%, 21.54% and 37.84%, respectively, at the concentration of 2 mg/mL, indicating the inhibitory effect due to the increase of browning substances (46).
In diabetes, free radicals can be generated by auto-oxidation of glucose and various oxidative stresses and can result in tissue damage (47). Recently, an increasing effort exists to identify α-glucosidase inhibitors from natural materials (41). Therefore, managing diabetes milletus may be more effective when both α-glucosidase inhibitory activity and anti-oxidative activity levels in black jujube are increased. Conclusively, aged black jujube could be a valuable ingredient to help alleviate diabetes milletus indirectly. Furthermore, black jujube produced by aging for 2 days could be a natural food ingredient substituting for caramel pigment as well as providing palatable taste.
Acknowledgments
This research was financially supported by the Ministry of Education, Science Technology (MEST) and National Research Foundation of Korea (NRF) through the Human Resource Training Project for Regional Innovation and supported (in part) by High Value-added Food Technology Development Program, Ministry for Food, Agriculture Forestry and Fisheries, Republic of Korea.
REFERENCES
- 1.Mukhtar HM, Ansari SH, Ali M, Naved T. New compounds from Zizyphus vulgaris. Pharm Biol. 2004;42:508–511. [Google Scholar]
- 2.Woo KS, Son SI, Jeong HS, Lee JS, Lee HB. Effects of grape fruit stem extracts treatment on the storage property of fresh jujube (Zizyphus jujuba) J Korean Soc Food Sci Nutr. 2006;35:192–198. [Google Scholar]
- 3.Ziiaev R, Irgashev T, Israilov IA, Abdullaev ND, Yunusov MS, Yunusov S. Alkaloids of Ziziphus jujuba structure of yuziphine and yuzirine. Khim Prir Soedin. 1997;2:239–243. [Google Scholar]
- 4.Bae KH, Lee SM, Lee ES, Lee JS, Kang JS. Isolation and quantitative analysis of betulinic acid and alphitolic acid from Zizyphi fructus. Yakhak Hoeji. 1996;40:558–562. [Google Scholar]
- 5.Tomoda M, Takahashi M, Nakatsuka S. Water soluble carbohydrates of Zizyphi fructus. II. Isolation of two polysaccharide sand structure of an arabinan. Chem Pharm Bull. 1973;21:707–711. [Google Scholar]
- 6.Zhang Y, Lu P, Wang H, Zhang JX, Li H, Liu JZ. Simultaneous HPLC determination of cAMP and cGMP in commercial jujube juice concentrate. Food Sci. 2009;30:321–322. [Google Scholar]
- 7.Choi KS, Kwon KI, Lee JG, Lee RK. Studies on the chemical compositions and antitumor activities of jujube tea products. J Resour Develop. 2003;22:23–29. [Google Scholar]
- 8.Kim HK, Joo KJ. Antioxidative capacity and total phenolic compounds of methanol extract from Zizyphus jujuba. J Korean Soc Food Sci Nutr. 2005;34:750–754. [Google Scholar]
- 9.Lan X, Liu P, Xia S, Jia C, Mukunzi D, Zhang X, Xia W, Tian H, Xiao Z. Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose-soybean peptide system: further insights into thermal degradation and cross-linking. Food Chem. 2010;120:967–972. [Google Scholar]
- 10.Jang EK, Seo JH, Lee SP. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J Food Sci Technol. 2008;40:443–448. [Google Scholar]
- 11.Park HJ, Lee SH, Kim Y, Jang GY, Hwang IG, Woo KS, Kwon OS, Lee JS, Jeong HS. Changes in chemical components and antioxidant activity of dried jujube with different aging temperatures and durations. J Korean Soc Food Sci Nutr. 2012;41:591–597. [Google Scholar]
- 12.Yamaguchi N, Tokoo Y, Koyama Y. Studies on the browning reaction product yielded by reducing sugar and amino acid. Part 1. Effect of browning reaction products on the stability of fats contained in biscuits and cookies. J Food Sci Technol Japan. 1964;11:184–189. [Google Scholar]
- 13.Wattenberg LW. Inhibitors of chemicals carcinogenesis. J Environ Pathol Toxicol. 1980;3:35–52. [PubMed] [Google Scholar]
- 14.Lee JW, Park CK, Do JH. Antioxidative activity of the water soluble browning reaction products from Korean red ginseng. J Ginseng Res. 2005;29:44–48. [Google Scholar]
- 15.Kirigaya N, Kato H, Fujimaki M. Studies on antioxidant of nonenzymatic browning products. part 1, Relations of color intensity and reductones with antioxidant activity of browning reaction products. J Agric Chem Soc. 1968;32:289–290. [Google Scholar]
- 16.Nakagawa S, Kusuga S, Matsuura H. Prevention of liver damage by aged garlic extract. Phytotheraphy Res. 1989;3:50–53. [Google Scholar]
- 17.Singleton VL, Orthofer R, Lamuela-Raventŏs RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 18.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 19.Pellegrini N, Proteggente A, Pannlala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Choe M, Kim DJ, Lee HJ, You JK, Seo DJ, Lee JH, Chung MJ. A study on the glucose regulating enzymes and antioxidant activities of water extracts from medicinal herbs. J Korean Soc Food Sci Nutr. 2008;37:542–547. [Google Scholar]
- 21.Sun HY, Shao LW, Lui XW, Miao WF, Chen SY, Zhang XY. Determination of water consumption and the water-saving potential of three mulching methods in a jujube orchard. Europ J Agronomy. 2012;43:87–95. [Google Scholar]
- 22.Li JW, Fan LP, Ding SD, Ding XL. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007;103:454–460. [Google Scholar]
- 23.Park MH. Study on the quality and preparation processing of jujube fruits Jungkwa. 2009. MS Thesis. Daegu Haany University, Gyeongbuk, Korea.
- 24.Eichner K, Karel M. The influence of water content and water activity on the sugar-amino browning reaction in model systems under various conditions. J Agric Food Chem. 1971;20:218–223. [Google Scholar]
- 25.Shin SR, Lee SH, Yoon KY, Kim KS. Changes in the physical characteristics and components of the jujube fruits by drying methods. Korean J Postharvest Sci Technol. 1998;5:346–349. [Google Scholar]
- 26.Frank O, Jezussek M, Hofmann T. Sensory activity, chemical structure and synthesis of Maillard generated bitter-tasting 1-oxo-2,3-dihydro-1H-indolizinium-6-olates. J Agric Food Chem. 2003;51:2693–2699. doi: 10.1021/jf026098d. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama N, Morigychi T, Morihara N, Saito H. Ameliorative effect of S-allylcysteine, a major thioallyl constituent in aged garlic extraction learning deficits in senescence accelerated mice. J Nutr. 2001;131:1093–1095. doi: 10.1093/jn/131.3.1093S. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro TA, Oliverira F. Application of the acid hydrolysis of sucrose as a temperature indicator in continuous thermal process. J Food Eng. 1999;40:181–188. [Google Scholar]
- 29.Lee HS. Effects on antioxidative capacity and lipid improvement of black garlic according to different aging periods. 2010. PhD Dissertation. Kyungsung University, Busan, Korea.
- 30.Kim HK, Choi YW, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim YT, Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother Res. 2011;25:965–974. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- 31.Jumnongpon R, Chaiseri S, Hongsprabhas P, Healy JP, Meade SJ, Gerrard JA. Cocoa protein crosslinking using Maillard chemistry. Food Chem. 2012;134:375–380. [Google Scholar]
- 32.Shim DW. Studies on the process method and quality of black jujube. 2011. MS Thesis. Daegu Haany University, Gyeongbuk, Korea.
- 33.Kim KJ, Do JR, Kim HK. Antimicrobial, antihypertensive and anticancer activities of garlic extracts. Korean J Food Sci Nutr. 2008;37:1174–1181. [Google Scholar]
- 34.Nieva MM, Sampietro AR, Vattuone MA. Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71:109–114. doi: 10.1016/s0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 35.Song XJ. Physical and biochemical changes of jujube as affected by different drying conditions. 2010. MS Thesis. Kyungpook University, Daegu, Korea.
- 36.Rao MS, Chawla SP, Chander R, Sharma A. Antioxidant potential of Maillard reaction products formed by irradiation of chitosan-glucose solution. Carbohyd Polym. 2011;83:714–719. [Google Scholar]
- 37.Kim JE, Joo SI, Seo JH, Lee SP. Antioxidant and α-glucosidase inhibitory effect of Tartary buckwheat extract obtained by the treatment of different solvents and enzyme. J Korean Soc Food Sci Nutr. 2009;38:989–995. [Google Scholar]
- 38.Kwon YI, Jung IC, Kim SH, Kim SY, Lee JS. Changes in properties of pitted jujube during drying and extraction. Agric Chem Biotechnol. 1997;40:43–47. [Google Scholar]
- 39.Byun PH, Kim WJ, Yoon SK. Changes of functional properties of garlic extracts during storage. Korean J Food Sci Technol. 2001;33:301–306. [Google Scholar]
- 40.Morales FJ, Jimeanez S. Free radical scavenging capacity of maillard reaction products as related to color and fluorescence. Food Chem. 2001;72:119–125. [Google Scholar]
- 41.Lee DS, Kim JG, Lee SH. Inhibition of α-glucosidase activity by quercetin. Korean J Microbiol Biotechnol. 2006;34:368–372. [Google Scholar]
- 42.Hwang IG, Kim HY, Woo KS, Hong JT, Hwang BY, Jung JK, Lee J, Jeong HS. Isolation and characterization of an α-glucosidase inhibitory substance from fructose-tyrosine Maillard reaction products. Food Chem. 2011;127:122–126. [Google Scholar]
- 43.Hatano T, Yasuhara T, Yoshihara R, Agata I, Noro T, Okuda T. Effects of interaction of tannins with co-existing substances. VII. Inhibitory effects of tannins and related polyphenols on xanthine oxidase. Chem Pharm Bull. 1990;38:124–129. doi: 10.1248/cpb.38.1224. [DOI] [PubMed] [Google Scholar]
- 44.Feather MS, Nelson D. Maillard polymers from D-glucose, D-fructose, 5-(hydroxymethyl)-2-furaldehyde, glycine and methionine. J Agric Food Chem. 1984;32:1428–1432. [Google Scholar]
- 45.Shin JH, Kang MJ, Lee SJ, Yang SM, Ryu JH, Sung NJ. Biological activity of dried garlic, red ginseng and their mixture. J Korean Soc Food Sci Nutr. 2009;38:1633–1639. [Google Scholar]
- 46.Shin JH, Kang MJ, Kim RJ, Ryu JH, Kim MJ, Lee SJ, Sung NJ. Biological activity of browning compounds from processed garlics separated by dialysis membrane. J Korean Soc Food Sci Nutr. 2011;40:357–365. [Google Scholar]
- 47.Baynes JW, Thorpe SR. Role of oxidative stress in diabetes complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]