Abstract
Purpose: An alternative autologous tissue for ocular surface reconstruction is a potential treatment for the patients with bilateral limbal stem cell deficiency. For the purpose of regenerative procedures in patients, it is desirable to eliminate the involvement of xenogeneic components, such as nonhuman sera and feeder cells. In the present study, we examined the behavior and phenotypic features of cultured conjunctival epithelial sheets generated in serum- and 3T3-free culture conditions when transplanted into the de-epithelialized limbal corneal surface.
Methods: Epithelial cells from normal conjunctiva obtained by neutral protease digestion were expanded by culture in a serum-free low-calcium medium and set in an air-liquid interface culture for 14 days. The resulting multilayered epithelial sheets were grafted onto rabbit ocular surfaces made epithelial-free by alkali treatment. Pre-grafted and post-grafted epithelia were analyzed by electron microscopy and immunohistochemistry.
Results: At graft time the cultured epithelial sheet consisted of 6–8 layers of properly stratified epithelium that displayed a CK19+/MUC5AC+/ CK3 -/CK12- phenotype, consistent with the conjunctival epithelial lineage. Two weeks after xeno-grafting the in vivo epithelium consisted of 5-6 well compacted layers expressing the precursor cell-related protein p63, the proliferation marker Ki67, desmosomes, hemidesmosomes and its integrin (β4), and the corneal specific cytokeratins CK3, and CK12. Conjunctival goblet cell mucin (MUC5AC) was not visible. The engrafted epithelium stained positively for the anti-human nuclei antibody, confirming that the epithelial cells on the rabbit corneas were of human origin.
Conclusions: Our results suggest that conjunctival epithelial sheets generated in serum- and 3T3-free culture conditions can acquire the corneal epithelial phenotype when transferred to the in vivo corneal stromal environment.
Introduction
Cultivated autologous epithelial sheets are used to treat limbal stem cell deficiency (LSCD) in unilateral cases. Autologous transplantation of limbal tissue or cultured limbal epithelial cells from the contralateral eye can result in the reestablishment of a long-term repopulating cell population in the recipient eye [1-5]. However, in bilateral cases only allogeneic limbal epithelial cells are available for homotypic transplantation, bringing about the attendant systemic adverse effects associated with postoperative immunosuppression. Furthermore, the success rate of allogenic transplantation shows great variability [6, 7] suggesting that either the intrinsic incompatibilities of the allogeneic transplantation and/ or the effects of the postoperative immunosuppressive drugs interfere with long-term engraftment. Accordingly, in preceding years, there has been strong interest in the use of alternative autologous ectodermal lineages to replace the limbal epithelium as a source tissue for ocular surface reconstruction in blind patients having bilateral LSCD. Pioneer studies were performed with oral mucosa [8-11].
The conjunctival epithelial (CjE) lineage, which shares with the limbal-corneal epithelium a common embryological origin and a somatic expression of the PAX6 morphogen [12], is particularly attractive as a heterotypic autologous source, provided that two phenotypic characteristics of conjunctival epithelium, vascular compatibility and mucinous cell generation, are spontaneously lost during the processes of in vivo cell expansion and reengraftement in the avascular cornea or are purposely eliminated by genetic manipulation. Ang et al. previously demonstrated that conjunctival epithelial cells grown on human amniotic membrane in a fetal bovine serum (FBS)-complemented medium with 3T3 mouse feeder cell support can modify their phenotype toward the corneal phenotype when grown in vivo on the corneal surface in a short-term human-to-rabbit xenograft transplantation model [13]. Recently, explant cultures from conjunctival biopsies made over human amniotic membrane in the presence of a FBS-complemented medium were used to attain corneal surface regeneration in patients having LSCD [14]. While the results of the cellular phenotype obtained under these conditions are highly encouraging for the purpose of regenerative procedures in patients, it will be desirable to eliminate the involvement of xenogeneic components, in particular the co-culture with animal cells and sera. We have previously demonstrated that the same culture approach used to generate conjunctival epithelial equivalents following a large in vivo expansion of the epithelial precursor populations [15] can be used to establish a CjE equivalent that recapitulates critical features of the source human CjE, including in selected cases, generation of mucinous, Goblet-like cells. The culture approach, derived from the original Boyce and Ham low-calcium media methodology for the expansion of epidermal precursors [16], avoids reliance on mouse feeder cells and fetal calf serum. Our research shows that when these human CjE equivalents are transplanted into the de-epithelialized ocular surface of rabbits with a carrier-free approach, after two weeks in the in vivo corneal environment, the human cells establish a five- to six-layer stratified epithelium that a) presents a defect-free outer surface; b) establishes a profusion of intercellular desmosomes and well developed substratum-anchoring hemidesmosomes; c) includes a layer of basal cells that is rich in nuclear p63, a marker of extended epithelial cells proliferative potential; and d) expresses the corneal-specific cytokeratins K12 and K3 rather than the cytokeratin K19 and MUC5AC proteins seen before transplantation. The studies demonstrate the feasibility of developing corneal epithelial regenerative approaches that use solely synthetic and human-derived culture materials.
Methods
Tissue specimens
Six 2×2 mm conjunctival biopsy specimens were obtained from the superior temporal bulbar conjunctiva of three patients (1 male, 2 females) undergoing routine pterygium surgery. Donors ranged in age from 43 to 65 years. Specimens used in these studies were from areas appearing normal by preoperative slit-lamp microscopic examination. Written informed consent was obtained from each patient, and the study protocol was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine of the Catholic University of Korea. All work was conducted in accordance with the Declaration of Helsinki.
Preparation of human conjunctival epithelial tissue equivalents
Conjunctival biopsies were incubated for 16–20 h at 4 °C in Dulbecco’s minimal essential medium (DMEM) containing 0.1% neutral protease (Sigma-Aldrich, St. Louis, MO). Epithelial cells were gently scraped off by a fluid stream from a pipette, dissociated into single-cell suspensions by repeated passage through a 200 µl pipette tip, and spun down. The pelleted cells were resuspended in Bronchial Epithelial Growth Medium (BEGM; Lonza, Wakerville, MD; contains 10 ng/ml epidermal growth factor or EGF) and cultured as previously described [15]. Briefly, 30,000 cells were seeded on 60 mm dishes. The medium was refreshed on days 1, 3 and 5, and on day 6 cells were harvested using Trypsin-EDTA and a trypsin-neutralizing solution (Lonza) and reseeded in BEGM at 2×105 cells on a 10 cm dish. When these cells reached 60%–70% confluence, they were harvested and seeded on Costar® Transwell-clear 3450 culture inserts (Corning, NY) using 105 cells per insert in a 1:1 (v/v) mixture of BEGM:Dulbecco’s minimal essential medium (DMEM) with the EGF concentration reduced to 0.5 ng/ml. At confluence (5–7 days of culture) the medium was removed to establish an air-liquid interface (ALI) culture to promote differentiation and stratification. These ALI conditions were continued for two weeks with underlying medium replacement daily.
Transplantation of cultivated human CjE sheets
At all times, the rabbits were housed and treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Our study was approved by the Catholic University of Korea Institutional Animal Care and Use Committee.
Adult New Zealand white rabbits (n=12) were obtained from Samtako (Osan, Korea). Four rabbits were selected as the control group. A paper disk embedded in 0.5N sodium hydroxide was applied over the cornea and limbal zones of each right eye for 30 s, and the eyes were thoroughly washed with PBS. The upper compartments of the transwell culture inserts were complemented with 1 ml PBS, and the stratified cell sheets were gently scraped at the edges, lifted by forceps, apposed onto the denuded scleral-limbal-corneal surfaces using TISSEEL fibrin sealant (Baxter Healthcare Corporation, Westlake Village, CA) to enhance the adhesiveness between the human CjE sheets and the de-epithelialized rabbit cornea. The graft was then covered with a therapeutic soft contact lens and secured with four peripheral anchoring sutures. Finally, total tarsorrhaphy was performed with 6–0 nylon sutures. Except the omission of transplantation of cultured epithelial sheet, the same procedures were performed on the control eyes. After surgery, the rabbits were treated with topical antibiotics (0.5% levofloxacin; Samil. Co., Ltd, Seoul, Korea), triamcinolone acetonide (0.2 ml injected subconjunctivally; Bristol-Myers Squibb Co., Tokyo, Japan), and systemic antibiotics (10 mg gentamicin/rabbit, delivered intramuscularly; Nacalai Tesque Inc., Kyoto, Japan). An intramuscular injection of FK506 (0.2 mg/kg; Astellas Co., Ltd., Tokyo, Japan) were administered daily to suppress xenogeneic reactions or nonspecific inflammation. Two weeks after transplantation the rabbits were sacrificed, and the engrafted corneas were divided into portions for histology, immunohistochemistry, and transmission electron microscopy (TEM).
Histology and immunohistochemistry
Eight grafted corneas were evaluated for histology and immunohistochemistry. Frozen rabbit tissues, paraffin embedded rabbit tissues, and cultivated human CjE sheets were used. Seven-µm cryostat sections of specimens embedded in OTC compound (Tissue-Tek, Sakura Fine Technical Co., Ltd., Tokyo, Japan) were collected on silane-coated microscope slides (Matsunami, Osaka, Japan) and after air drying were fixed for 15 min in cold acetone. Fixed sections were blocked with PBS containing 10% fetal bovine serum for 1 h at room temperature, reacted with primary antibodies (Table 1) for 90 min at room temperature, and adequately washed with PBS three times, and incubated with FITC- or PE-conjugated secondary antibodies. The slides were washed with PBS three times, mounted in DAPI-supplemented Vectashield (Vector Laboratories, Burlingame, CA), and examined with a confocal laser scanning microscope (model LSM510 MET, Carl Zeiss Meditec, Jena, Germany). Four-µm sections of paraffin-embedded tissues and cultivated human CjE sheets were deposited on silane-coated microscope slides (Matsunami, Osaka, Japan) and stained with hematoxylin and eosin or microwaved for 20 min in Target Retrieval Solution (DAKO, Carpinteria, CA). The latter were washed with PBS (twice for 10 min each time), were incubated for 1 h in blocking buffer (10% BSA in PBS) and then overnight with primary antibodies in blocking buffer at 4 °C, and were subsequently treated with secondary antibodies as described above. The primary antibodies used for p63, Ki67, CK3, CK12, and MUC5AC are described in Table 1.
Table 1. Antibodies used in the study.
| Antigen | Dilution | Type of antibody | Company | Annotation |
|---|---|---|---|---|
| p63 |
X100 |
Mo |
Santa Cruz |
p53 homologous protein |
| Integrin β4 |
X100 |
Mo |
Chemicon |
Hemidesmosome component protein |
| CK3 |
X50 |
Mo |
Progen |
Major cytokeratin in corneal epithelium |
| CK12 |
X100 |
Po |
Santa Cruz |
Major cytokeratin in corneal epithelium |
| MUC1 |
X100 |
Mo |
Thermo Fisher |
A membrane bound mucin |
| MUC5AC |
X100 |
Mo |
Neomarkers |
Goblet cell mucin |
| Human nuclei |
X30 |
Mo |
Chemicon |
Distinguish human cells from other animal cells |
| Ki67(clone MIB-1) | X50 | Mo | Dako | Actively proliferating cells |
Mo, monoclonal; Po, polyclonal *Santa Cruz: Santa Cruz Biotechnology Inc., Santa Cruz, CA; Chemicon: CHEMICON International Inc., Temecula, CA; Progen: Progen Biotechnik GmbH, Heidelberg, Germany; Thermo Fisher: Thermo Fisher Scientific Lab Vision Corporation, Fremont, CA; Neomarkers, Freemont, CA; Dako: Dako North America, Inc., Carpinteria, CA.
Transmission electron microscopy
Two control and four grafted corneas were evaluated. Specimens were fixed in 2.5% glutaraldehyde in PBS, washed three times in PBS, and post fixed for 1 h in 2% aqueous osmium tetroxide. They were then passed through a graded ethanol series, transferred to propylene oxide, and embedded in Epon-812 (TAAB, Berkshire, England). Ultrathin sections were cut with a diamond knife using a Leica ultracut microtome, stained with aqueous uranyl acetate and Reynolds lead citrate, and examined under a TEM (H-7000; Hitachi, Tokyo, Japan).
Results
Histology of air-liquid interface human CjE cultures
The histologic examination showed that at the end of the two-week ALI culture the epithelium contained six to eight cell layers, with spotted expression of Goblet cell mucin MUC5AC (Figure 1A). In our previous work we demonstrated that in selected cases these cultures can generate well developed Goblet cell profiles and profusely express the conjunctival epithelial marker CK19 [15] (Figure 1B). The corneal epithelial specific cytokeratin CK3 and CK12 were not observed (Figure 1C,D). Together these results suggest that the basic characteristics of the human CjE phenotype have been preserved in these cultures. In addition, the CjE sheets expressed p63, a strong marker of extended proliferative potential for the epidermal keratinocytes and other epithelial ectodermal lineages [17, 18] in a fraction of the basal cell (Figure 1E).
Figure 1.
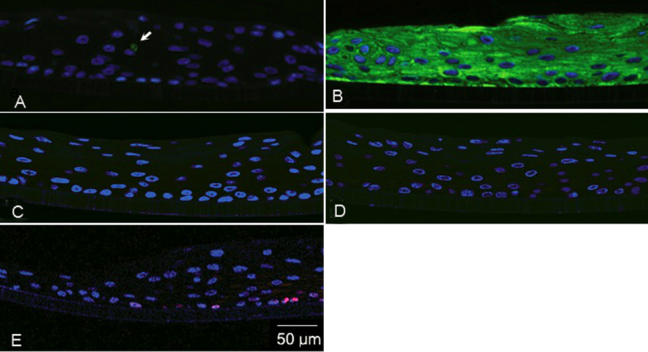
Immunohistochemical examinations of the cultivated human conjunctival epithelia (CjE) equivalent. The cultivated human CjE equivalent was stained green for MUC5AC (A), CK19 (B), CK3 (C), CK12 (D), red for p63 (E) by indirect immunofluorescence as described in Methods. Nuclei have been counterstained with DAPI. A: The arrow points to positive cell for MUC5AC. Representative images of three independent cultures are shown.
Transplantation of cultivated human CjE sheets
Cultivated CjE sheets were transplanted onto the corneas of rabbits. Engraftment was patent in all eight tests; sheets were well maintained on the recipient’s corneal surface; and there was no retraction or dislodgement of the graft during follow-up. The covered ocular surfaces remained transparent, smooth, and devoid of epithelial defects for two weeks (Figure 2A,C). In contrast, eyes treated in an identical manner but without the CjE graft showed incipient neovascularization and an almost complete absence of epithelial barrier function (Figure 2B,D).
Figure 2.
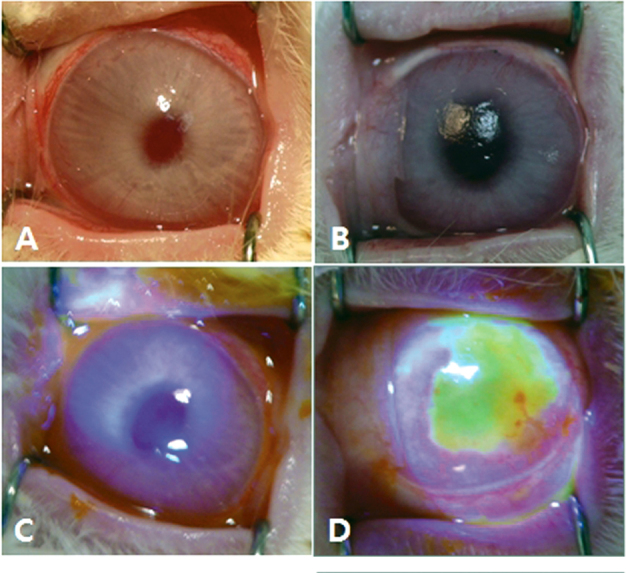
Micrographs of the rabbit ocular surface two weeks after transplantation of human conjunctival epithelia (CjE) sheets onto alkali-treated ocular surfaces. A, C: In these eyes with transplants, the ocular surfaces are smooth and show no fluorescein penetration. B, D: The eyes were treated exactly as A and C but without human CjE sheet application. Note the incipient corneal neovascularization in B and the extensive corneal epithelial defect in D. Representative images of eight grafted corneas are shown.
Histology, ultrastructure, and immunohistochemistry of engrafted human CjE
Histological examination demonstrated that at two weeks after transplantation, the epithelium covering the rabbit central corneal zone displayed a typical in vivo corneal appearance with cuboidal or columnar basal cells, wing-shaped supra basal cells, and flattened superficial cells (Figure 3A). There were no visible epithelial cells on the permeable corneas where transplantation was omitted (Figure 3B). TEM examination of a representative transplanted cornea revealed fully developed hemidesmosomes at the basal cell layer (Figure 3C), desmosomes at the inter-epithelial junctions (Figure 3D), and apical microvilli (not shown). Goblet cell profiles were not visible at either the histological or TEM levels.
Figure 3.
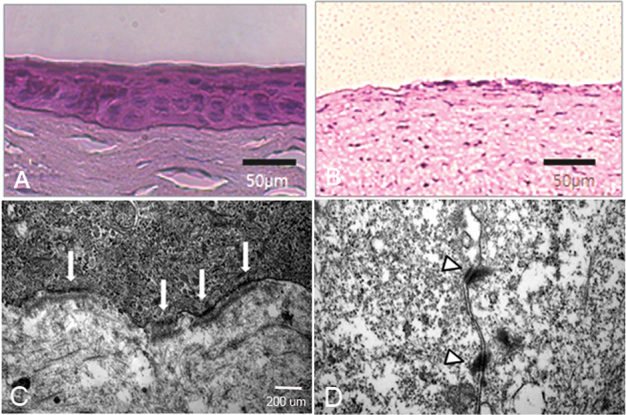
Hematoxylin and Eosin (H and E), and transmission electron microscopy (TEM) of the engrafted human conjunctival epithelia (CjE). A, B: H and E stain of corneas with or without transplanted epithelial sheets two weeks after transplantation. C, D: TEM of the grafted tissue showing hemidesmosomal profiles at the basal cell-central corneal stromal interface (C, arrows) and desmosomes between wing shaped cells (D, arrowheads). Representative images shown of four grafted corneas.
The engrafted sheets uniformly stained positively for the anti-human nuclei antibody(Figure 4A), confirming that the epithelial cells in the central rabbit corneas originated from the transplanted human CjE cells rather than from zonal repopulation from host epithelial cells (limbal and or conjunctival) surviving the alkali treatment at the periphery. The basal cell layer intensively expressed p63 (Figure 4B). Many of those cells also expressed Ki67 (Figure 4C), indicating an active proliferative state. Consistent with the abundance of anchoring hemidesmosomes observed by TEM, there was substantial integrin β4 expression at the basement-facing side of the basal cells (Figure 4D), and the ubiquitous MUC1 antigen was seen throughout the strata (Figure 4E).
Figure 4.
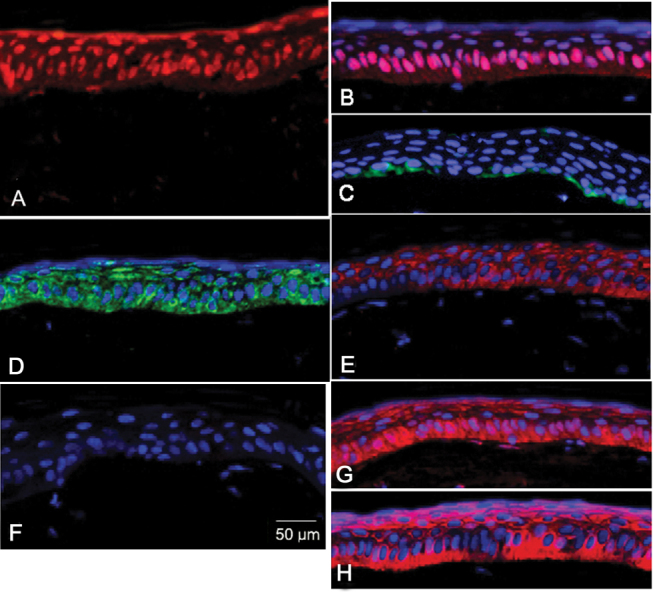
Immunohistochemistry of the engrafted human conjunctival epithelia (CjE). The engrafted human CjE over the rabbit cornea surfaces were stained red for human nuclei (A), p63 (B), MUC1 (E), CK3 (G), and CK12 (H) by indirect immunofluorescence as described in Methods. The engrafted human CjE was stained green for Ki67 (C) and Integrin β4 (D). MUC5AC expression was not detected (F). Nuclei have been counterstained with DAPI except sections stained with anti-human nuclei antibody. Represented images of eight engrafted human CjEs over rabbit cornea surfaces.
Finally, in contrast to the phenotype staining patterns observed in the epithelial sheets before grafting, MUC5AC expression could not be detected after the two-week in vivo culture (Figure 4F), but the engrafted sheets were found to abundantly express CK3 and CK12 (Figure 4G,H), providing a strong indication of a transdifferentiation event.
Discussion
Cultivated corneal limbal stem cell transplantation has critical limitations for patients with bilateral LSCD is fine. Even in the case of unilateral disease, harvesting limbal tissue from the contralateral eye risks inflicting iatrogenic LSCD on the donor eye. For this reason, cultivated oral epithelium has been tested as an alternative heterotypic source for autologous treatment of bilateral ocular surface disease. Short- to mid-term results have shown great promise [8-11]. However, the oral tissue is non-ocular in origin and may retain certain undesirable features of the tissue of origin, such as parakeratinization. In this context, the CjE appears to be an attractive alternative. First, a small amount of healthy conjunctiva can be obtained with minimal risk of iatrogenic injury. Second, the limbal corneal and conjunctival epithelia lineages are closely related, having developed via divergent paths from the same set of pre-ocular PAX6-positive head ectodermal cells [12]. Third, differentiation in the adult stage is under the control of PAX6 in either tissue [12]. On the downside, the CjE displays a bi-potent differentiation phenotype; under normal physiologic conditions a fraction of the cells become mucin secreting Goblet cells, an undesirable feature if manifested at the central corneal surface. Nevertheless, human CjE Goblet cell generation appears to be a fastidious process that requires exquisite conditions (e.g., their numbers dwindle rapidly when physiologic conditions are disturbed in dry eyes, and in vitro fully developed Goblet cells are rarely observed).
Our study shows that if human CjE cells have undergone a large expansion in the differentiation-delaying conditions of optimized Boyce and Ham type low-calcium, serum-free culture media [16] and have been used to generate a stratified CjE equivalent in an ALI culture, such cells undergo a phenotypic conversion toward the corneal phenotype when the stratified equivalent is directly grafted on de-epithelialized stroma of rabbits and allowed to grow in vivo for two weeks. This conjunctival epithelial tissue equivalent expressed biochemical features of the source epithelium, including CK19 and MUC5AC expression and in some cultures even morphologically distinguishable Goblet cells [15]. Two weeks after application of the conjunctival epithelial equivalent to the denuded surface of the rabbit cornea, histology and TEM revealed good human epithelial cell stratification, apical microvilli, manifest intercellular compaction, hemidesmosomes at the basal cell-stromal lamina interphase, and a profusion of intercellular desmosomes, features that are essential for the optical and physical attributes required for the epithelium overlying the corneal surface. The abundant expression of p63 and the presence of Ki67 positive cells indicate that at the end of these two weeks this basal layer consists of actively proliferating cells that have moderate to high repopulating capacity. Last but not least, as previously shown by Ang et al. [13], the human CjE cells have acquired the tonofilament pattern that characterizes the corneal epithelial cells, the expression of CK3 and CK12. With respect to CK12, gene expression comparisons by microarray [19] and histology have shown that certain areas of the human CjE the epithelium may spontaneously express a substantial level of the CK12 mRNA [20]. However, no such observation has been made in regard to CK3. Additionally, the engrafted epithelium appears to have ceased to express the CjE phenotype-associated protein MUC5AC, strengthening the notion that a cultivated human CjE sheet may lose phenotypic characteristics when growing over the corneal surface.
We must mention that, since it is precisely the invasion of the adjacent conjunctiva that elicits the neovascularization of the corneal surface leading to blindness in LSCD, the distinct outcome of transplantation of a cultured conjunctival epithelium is an intriguing phenomenon from both clinical and scientific perspectives. In LSCD conjunctivalization implies the migration of conjunctival epithelial cells over the corneal surface with preservation of the original cell phenotype. In the expansion-transplantation cycle the extensive cell cycles outside the natural phenotypic-inducing or -preserving environment may destabilize the cellular phenotype to a degree that facilitates transition to the phenotype induced by the host environment (i.e., the cornea). While this notion is speculative, it is supported by recent advances in our understanding of direct cell reprograming as well as the information arising from studies on the generation of induced pluripotent cells [21, 22]. Early-passage cells of this type seem to retain an epigenetic memory of their origins [23–26]. As they undergo further cell division, this memory fades away, and the cells approach the pluripotent nature of embryonic stem cells. Thus, due to the shared developmental origins of the corneal and the conjunctival epithelia, the latter may be the most natural source for autologous heterotypic transplantation to treat bilateral LSCD; plasticity to achieve full transdifferentiation may require a minimal number of duplications in culture and may be favored by certain conditions of the culture. Interestingly, notwithstanding the remarkable clinical outcome of their studies, Ricardo et al. [14] found that the cells overlying the corneal surface of patients treated with conjunctival epithelial cells grown on an amniotic membrane displayed not only what appears to be a mixed phenotype that expresses the corneal specific keratin 3 but also Goblet cells that express the conjunctival specific MUC5AC mucin. Studies to define the culture conditions that facilitate conjunctival-to-corneal epithelial transdifferentiation may be of great value for future clinical application and of great scientific value as well.
Overall, from both technical and clinical perspectives, our protocol seems to provide several advantages over that used in the work of Ang et al. [13] and Ricardo et al. [14]. First, the culture system avoids the use of bovine serum and, more importantly, the co-culture with live mouse cells. Using an optimized low-calcium Boyce and Ham type culture medium allows for a very large expansion of the donor precursor population, reducing the amount of autologous tissue needed. Although to date we have used a BPE-complemented media formulation to follow our own established protocol for the generation of human CjE equivalents [15], with advances in synthetic formulations [21, 22], it should be possible to transition to a completely animal-free autologous culture process. Second, with the use of an extensive cell expansion protocol, it may be possible to cryopreserve a fraction of the cells for repeated cycles of engraftment. Third, grafting is achieved by direct apposition of the mechanically floated pure epithelial equivalent to the keratomized rabbit stroma covered with fibrin glue (i.e., without resorting to an intercalated carrier amniotic membrane, derived from unrelated human subjects).
The present results notwithstanding, it should be emphasized that from a purely scientific perspective it remains to be determined a) whether other non-CjE epithelia keratin also become expressed (i.e., the degree of selectivity for the keratin expression changes); b) whether the disappearance of MUC5A reflects an irreversible phenotypic change or a modulatory effect of the environment similar to the disappearance of Goblet cells under dry eye and/or inflammation in vivo [23, 24] or in most culture conditions [25, 26]; and c) whether the phenotype change ultimately transcends the keratin and mucin expressions to encompass the large differences between the two lineages at the gene level revealed in our prior microarray studies [19].
The chemically induced immunodeficiency state needed to complete these experiments in the rabbit preclude long-term follow-up to determine whether the cells retain problematic pro- vascular activity like that of the CjE. Longer term follow-up studies in an immunologically humanized animal model are needed to further assess the benefits and risks of the outlined approach for the treatment of intractable bilateral LSCD in patients.
Acknowledgments
This work was supported by a grant from the Korea Health technology R and D Project, Ministry of Health and Welfare, Republic of Korea (A120225, SHC) and a grant RO1EY014878 from the USPHS and NYSTEM (JMW).
References
- 1.Tsai RJ, Sun TT, Tseng SC. Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology. 1990;97:446–55. doi: 10.1016/s0161-6420(90)32575-7. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 3.Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- 5.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Sotozono C, Bentley AJ, Mano S, Inatomi T, Koizumi N, Koizumi N, Fullwood NJ, Kinoshita S. Long-term phenotypic study after allogeneic cultivated corneal limbal epithelial transplantation for severe ocular surface diseases. Ophthalmology. 2010;117:2247–54. doi: 10.1016/j.ophtha.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–9. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–4. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–96. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 10.Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118:1524–30. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Sotozono C, Inatomi T, Nakamura T, Koizumi N, Yokoi N, Ueta M, Matsuyama K, Miyakoda K, Kaneda H, Fukushima M, Kinoshita S. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120:193–200. doi: 10.1016/j.ophtha.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Koroma BM, Yang JM, Sundin OH. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38:108–20. [PubMed] [Google Scholar]
- 13.Ang LP, Tanioka H, Kawasaki S, Yamasaki K, Do TP, Thein ZM, Koizumi N, Nakamura T, Yokoi N, Komuro A, Inatomi T, Nakatsukasa M, Kinoshita S. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2010;51:758–64. doi: 10.1167/iovs.09-3379. [DOI] [PubMed] [Google Scholar]
- 14.Ricardo JR, Cristovam PC, Filho PA, Farias CC, de Araujo AL, Loureiro RR, Covre JL, de Barros JN, Barreiro TP, dos Santos MS, Gomes JA. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221–8. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 15.Chung SH, Lee JH, Yoon JH, Lee HK, Seo KY. Multi-layered culture of primary human conjunctival epithelial cells producing MUC5AC. Exp Eye Res. 2007;85:226–33. doi: 10.1016/j.exer.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 17.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 18.Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol. 2010;5:349–71. doi: 10.1146/annurev-pathol-121808-102117. [DOI] [PubMed] [Google Scholar]
- 19.Turner HC, Budak MT, Akinci MA, Wolosin JM. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest Ophthalmol Vis Sci. 2007;48:2050–61. doi: 10.1167/iovs.06-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki S, Tanioka H, Yamasaki K, Yokoi N, Komuro A, Kinoshita S. Clusters of corneal epithelial cells reside ectopically in human conjunctival epithelium. Invest Ophthalmol Vis Sci. 2006;47:1359–67. doi: 10.1167/iovs.05-1084. [DOI] [PubMed] [Google Scholar]
- 21.Lu R, Bian F, Zhang X, Qi H, Chuang EY, Pflugfelder SC, Li DQ. The beta-catenin/Tcf4/survivin signaling maintains a less differentiated phenotype and high proliferative capacity of human corneal epithelial progenitor cells. Int J Biochem Cell Biol. 2011;43:751–9. doi: 10.1016/j.biocel.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Wan P, Duan H, Zhou J, Tan B, Liu Y, Zhou Q, Zhou C, Huang Z, Tian B, Li C, Wang Z. ES micro-environment enhances stemness and inhibits apoptosis in human limbal stem cells via the maintenance of telomerase activity. PLoS ONE. 2013;8:e53576. doi: 10.1371/journal.pone.0053576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984;102:1049–51. doi: 10.1001/archopht.1984.01040030851031. [DOI] [PubMed] [Google Scholar]
- 24.Murube J, Rivas L. Biopsy of the conjunctiva in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:246–56. doi: 10.1177/112067210301300302. [DOI] [PubMed] [Google Scholar]
- 25.Ang LP, Tan DT, Seah CJ, Beuerman RW. The use of human serum in supporting the in vitro and in vivo proliferation of human conjunctival epithelial cells. Br J Ophthalmol. 2005;89:748–52. doi: 10.1136/bjo.2004.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, Siemasko KF, Stern ME. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–74. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]


