Abstract
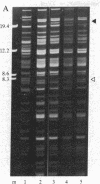
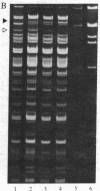
The genetic analysis of higher plant mitochondria has been limited by a scarcity of identified mutations with known progenitors. Correspondingly, few molecular studies have been directed at types of plant mitochondrial variation other than cytoplasmic male sterility. The maternally inherited nonchromosomal stripe (NCS) mutants of maize have profound deleterious effects on plant growth and yield. We report specific alterations in mitochondrial DNA (mtDNA) for two independent, phenotypically distinct NCS mutants. NCS2 plants have a distinctive 21-kilobase Xho I mtDNA band and very reduced amounts of DNA in an 8-kilobase band that is present in the progenitor. NCS3 plants have a distinctive 20-kilobase Xho I band and a reduction in a 16-kilobase band. Our studies confirm that the affected organelle in NCS plants is the mitochondrion. Because NCS-type plants appear with a certain frequency in a particular line (WF9), this line is a potential source of additional mutations for functional and molecular analyses of maize mitochondrial genes.
Keywords: mitochondrial mutants
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand H., Chan B. S., Griffiths A. J. Insertion of a foreign nucleotide sequence into mitochondrial DNA causes senescence in Neurospora intermedia. Cell. 1985 Jul;41(3):877–884. doi: 10.1016/s0092-8674(85)80068-4. [DOI] [PubMed] [Google Scholar]
- Gross S. R., Hsieh T. S., Levine P. H. Intramolecular recombination as a source of mitochondrial chromosome heteromorphism in Neurospora. Cell. 1984 Aug;38(1):233–239. doi: 10.1016/0092-8674(84)90545-2. [DOI] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus C. M., Earl A. J., Turner G., Küntzel H. Amplification of a mitochondrial DNA sequence in the cytoplasmically inherited 'ragged' mutant of Aspergillus amstelodami. Eur J Biochem. 1980 May;106(2):633–641. doi: 10.1111/j.1432-1033.1980.tb04611.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Pring D. R., Lonsdale D. M. Mitochondrial DNA rearrangements associated with fertile revertants of S-type male-sterile maize. Cell. 1985 Nov;43(1):361–368. doi: 10.1016/0092-8674(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Shumway L. K., Bauman L. F. Nonchromosomal stripe of maize. Genetics. 1967 Jan;55(1):33–38. doi: 10.1093/genetics/55.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. M., Horrum M. A., Cummings D. J. Are mitochondrial structural genes selectively amplified during senescence in Podospora anserina? Cell. 1982 Jun;29(2):505–515. doi: 10.1016/0092-8674(82)90167-2. [DOI] [PubMed] [Google Scholar]