Abstract
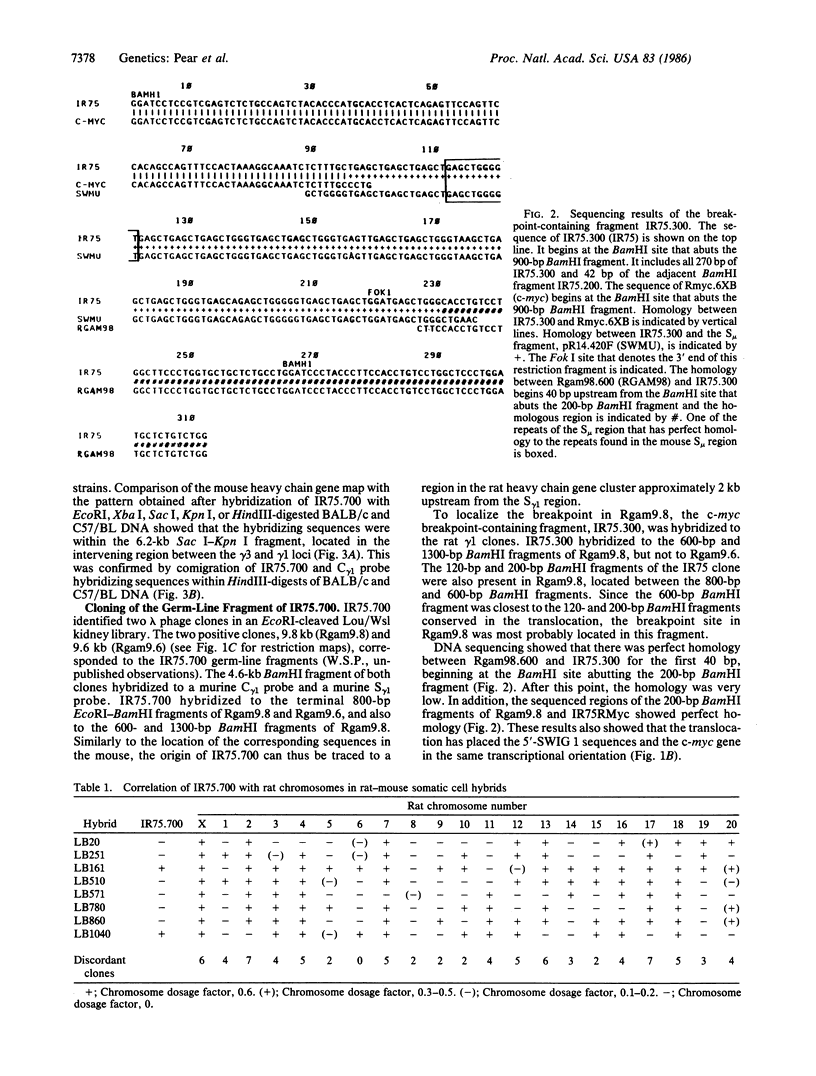
Spontaneously arising immunocytomas in Lou/Wsl rats contain a consistent translocation between chromosomes 6 and 7. The c-myc gene has been localized to chromosome 7 and has been shown to be rearranged in the majority of the rat immunocytomas. We now report the cloning of the rearranged 11-kilobase EcoRI c-myc fragment from the IgE-secreting IR75 tumor. Sequence analysis revealed that the cytogenetically visible t(6;7) translocation must have involved several events in this tumor. One event has led to the juxtaposition of c-myc and the switch mu region, in a head-to-head orientation. The breakpoint is approximately 850 base pairs upstream from the proximal c-myc promoter on chromosome 7. This area is distinct from the more common mouse plasmacytoma- and Burkitt lymphoma-associated translocation breakpoints and also differs from the known murine retroviral insertion sites. A second rearrangement has led to the transposition of sequences upstream from the switch gamma 1 region to the c-myc-distant end of the switch mu region, tail-to-tail. This requires at least two events, including one inversion. In addition to showing that identical loci (c-myc, immunoglobulin) are juxtaposed via chromosomal translocations in three different tumors (Burkitt lymphoma, mouse plasmacytoma, and rat immunocytoma) in different species (human, mouse, and rat), the multiple rearrangements in IR75 and some other tumors emphasize the selective value of c-myc activation by an immunoglobulin locus in the tumorigenic process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou-Pachnis A., Lohse M. A., Furano A. V., Tsichlis P. N. Insertion of long interspersed repeated elements at the Igh (immunoglobulin heavy chain) and Mlvi-2 (Moloney leukemia virus integration 2) loci of rats. Proc Natl Acad Sci U S A. 1985 May;82(9):2857–2861. doi: 10.1073/pnas.82.9.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Piechaczyk M., Henglein B., Blanchard J. M., Traub B., Kofler E., Wiest S., Lenoir G. M., Bornkamm G. W. Aberrant c-myc RNAs of Burkitt's lymphoma cells have longer half-lives. EMBO J. 1985 Dec 30;4(13B):3717–3725. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Erikson J., Miller D. A., Miller O. J., Abcarian P. W., Skurla R. M., Mushinski J. F., Croce C. M. The c-myc oncogene is translocated to the involved chromosome 12 in mouse plasmacytoma. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4212–4216. doi: 10.1073/pnas.82.12.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrlander P. D., Sümegi J., Yang J. Q., Wiener F., Marcu K. B., Klein G. Activation of the c-myc oncogene by the immunoglobulin heavy-chain gene enhancer after multiple switch region-mediated chromosome rearrangements in a murine plasmacytoma. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3746–3750. doi: 10.1073/pnas.82.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Lalley P. A., Moss W., Ivy J., Minna J. D. Gene mapping in Mus musculus by interspecific cell hybridization: assignment of the genes for tripeptidase-1 to chromosome 10, dipeptidase-2 to chromosome 18, acid phosphatase-1 to chromosome 12, and adenylate kinase-1 to chromosome 2. Cytogenet Cell Genet. 1977;19(2-3):57–84. doi: 10.1159/000130799. [DOI] [PubMed] [Google Scholar]
- Francke U., Taggart R. T. Assignment of the gene for cytoplasmic superoxide dismutase (Sod-1) to a region of chromosome 16 and of Hprt to a region of the X chromosome in the mouse. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5230–5233. doi: 10.1073/pnas.76.10.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R., Lang R. B., Diamond M. S., Marcu K. B. A switch region inversion contributes to the aberrant rearrangement of a mu immunoglobulin heavy chain gene in MPC-11 cells. Nucleic Acids Res. 1982 Dec 11;10(23):7751–7761. doi: 10.1093/nar/10.23.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Obata M., Yamawaki-Katoaka Y., Kataoka T., Kawakami T., Takahashi N., Mano Y. Cloning and complete nucleotide sequence of mouse immunoglobulin gamma 1 chain gene. Cell. 1979 Oct;18(2):559–568. doi: 10.1016/0092-8674(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., McCoy C., Blanc D., Trucy J., Devaux C., Schmitt-Verhulst A. M., Fitch F., Hood L., Malissen B. Direct evidence for chromosomal inversion during T-cell receptor beta-gene rearrangements. Nature. 1986 Jan 2;319(6048):28–33. doi: 10.1038/319028a0. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Yang J. Q., Blanchard J. M., Jeanteur P., Marcu K. B. Posttranscriptional mechanisms are responsible for accumulation of truncated c-myc RNAs in murine plasma cell tumors. Cell. 1985 Sep;42(2):589–597. doi: 10.1016/0092-8674(85)90116-3. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton L. W., Yang J. Q., Eckhardt L. A., Harris L. J., Birshtein B. K., Marcu K. B. Products of a reciprocal chromosome translocation involving the c-myc gene in a murine plasmacytoma. Proc Natl Acad Sci U S A. 1984 Feb;81(3):829–833. doi: 10.1073/pnas.81.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki H., Kanazawa S. New restriction endonucleases from Flavobacterium okeanokoites (FokI) and Micrococcus luteus (MluI). Gene. 1981 Dec;16(1-3):73–78. doi: 10.1016/0378-1119(81)90062-7. [DOI] [PubMed] [Google Scholar]
- Szpirer J., Levan G., Thörn M., Szpirer C. Gene mapping in the rat by mouse-rat somatic cell hybridization: synteny of the albumin and alpha-fetoprotein genes and assignment to chromosome 14. Cytogenet Cell Genet. 1984;38(2):142–149. doi: 10.1159/000132047. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Spira J., Bazin H., Szpirer J., Levan G., Klein G. Rat c-myc oncogene is located on chromosome 7 and rearranges in immunocytomas with t(6:7) chromosomal translocation. Nature. 1983 Dec 1;306(5942):497–498. doi: 10.1038/306497a0. [DOI] [PubMed] [Google Scholar]
- Wiener F., Babonits M., Spira J., Klein G., Bazin H. Non-random chromosomal changes involving chromosomes 6 and 7 in spontaneous rat immunocytomas. Int J Cancer. 1982 Apr 15;29(4):431–437. doi: 10.1002/ijc.2910290412. [DOI] [PubMed] [Google Scholar]
- Wiener F., Ohno S., Babonits M., Sümegi J., Wirschubsky Z., Klein G., Mushinski J. F., Potter M. Hemizygous interstitial deletion of chromosome 15 (band D) in three translocation-negative murine plasmacytomas. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1159–1163. doi: 10.1073/pnas.81.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschubsky Z., Ingvarsson S., Carstenssen A., Wiener F., Klein G., Sümegi J. Gene localization on sorted chromosomes: definitive evidence on the relative positioning of genes participating in the mouse plasmacytoma-associated typical translocation. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6975–6979. doi: 10.1073/pnas.82.20.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]