Abstract
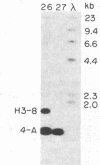
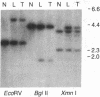
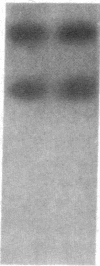
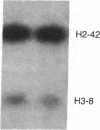
DNA fragments from a locus spanning 29 kilobases within chromosome band 13q14 detected deletions in 3 retinoblastomas out of 37 such tumors examined. Somatically occurring, homozygous deletions spanning at least 25 kilobases were detected in retinoblastomas from two unrelated patients. These deletions are bounded by the esterase D locus proximally. In a third patient, both tumor cells and leukocytes have a deletion of one chromosome 13 homolog, with one end of the deletion localized to a 1.55-kilobase fragment within the cloned region. It is likely that the cloned locus is within a few hundred kilobases of the retinoblastoma gene (i.e., the locus governing predisposition to such tumors) and that the deletions detected also involve the retinoblastoma gene. Further, it may be possible to base a successful approach to the isolation of the retinoblastoma gene on this assumed physical proximity of the two loci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Connolly M. J., Payne R. H., Johnson G., Gallie B. L., Allderdice P. W., Marshall W. H., Lawton R. D. Familial, EsD-linked, retinoblastoma with reduced penetrance and variable expressivity. Hum Genet. 1983;65(2):122–124. doi: 10.1007/BF00286647. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Morton C. C. Mapping of seven polymorphic loci on human chromosome 13 by in situ hybridization. Hum Genet. 1985;71(3):192–195. doi: 10.1007/BF00284571. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Epstein J., Goorin A. M., Weichselbaum R., Koufos A., Cavenee W. K. Chromosome 13 homozygosity in osteosarcoma without retinoblastoma. Am J Hum Genet. 1986 Jan;38(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Weichselbaum R., Bruns G. A. Chromosome 13 restriction fragment length polymorphisms. Hum Genet. 1984;65(4):320–324. doi: 10.1007/BF00291555. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Halloran S. L., Boughman J. A., Dryja T. P., Mukai S., Long D., Roberts D. F., Craft A. W. Accuracy of detection of the retinoblastoma gene by esterase D linkage. Arch Ophthalmol. 1985 Sep;103(9):1329–1331. doi: 10.1001/archopht.1985.01050090081036. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Mestriner M. A., Cortner J., Harris H. Esterase D: a new human polymorphism. Ann Hum Genet. 1973 Oct;37(2):119–137. doi: 10.1111/j.1469-1809.1973.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Kan Y. W. The William Allan Memorial Award address: Thalassemia: molecular mechanism and detection. Am J Hum Genet. 1986 Jan;38(1):4–12. [PMC free article] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lalande M., Dryja T. P., Schreck R. R., Shipley J., Flint A., Latt S. A. Isolation of human chromosome 13-specific DNA sequences cloned from flow sorted chromosomes and potentially linked to the retinoblastoma locus. Cancer Genet Cytogenet. 1984 Dec;13(4):283–295. doi: 10.1016/0165-4608(84)90073-6. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Middlesworth W., Colletti C. A., Aldridge J., Fischbeck K. H., Bartlett R., Pericak-Vance M. A., Roses A. D., Kunkel L. M. Detection of deletions spanning the Duchenne muscular dystrophy locus using a tightly linked DNA segment. 1985 Aug 29-Sep 4Nature. 316(6031):842–845. doi: 10.1038/316842a0. [DOI] [PubMed] [Google Scholar]
- Mukai S., Rapaport J. M., Shields J. A., Augsburger J. J., Dryja T. P. Linkage of genes for human esterase D and hereditary retinoblastoma. Am J Ophthalmol. 1984 Jun;97(6):681–685. doi: 10.1016/0002-9394(84)90497-5. [DOI] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Parker R. C., Davidson N. Representation of DNA sequences in recombinant DNA libraries prepared by restriction enzyme partial digestion. Gene. 1982 Sep;19(2):201–209. doi: 10.1016/0378-1119(82)90007-5. [DOI] [PubMed] [Google Scholar]
- Seed B. Theoretical study of the fraction of a long-chain DNA that can be incorporated in a recombinant DNA partial-digest library. Biopolymers. 1982 Sep;21(9):1793–1810. doi: 10.1002/bip.360210909. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Kalina R. E., Pagon R. A., Salk D. J., Disteche C. M. Separation of retinoblastoma and esterase D loci in a patient with sporadic retinoblastoma and del(13)(q14.1q22.3). Hum Genet. 1984;68(3):258–259. doi: 10.1007/BF00418397. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]