Abstract
Preclinical studies suggest that dopamine D3 receptor (D3R) antagonists are promising for the treatment of drug abuse and addiction. However, few D3R antagonists have potential to be tested in humans due to short half-life, toxicity or limited preclinical research into pharmacotherapeutic efficacy. Here, we report on a novel D3R antagonist YQA14, which has improved half-life and pharmacokinetic profile and which displays potent pharmacotherapeutic efficacy in attenuating cocaine reward and relapse to drug-seeking behavior. Electrical brain-stimulation reward (BSR) in laboratory animals is a highly sensitive experimental approach to evaluate a drug’s rewarding effects. We found that cocaine (2 mg/kg) significantly enhanced electrical BSR in rats (i.e., decreased stimulation threshold for BSR), while YQA14 alone had no effect on BSR. Pretreatment with YQA14 significantly and dose-dependently attenuated cocaine-enhanced BSR. YQA14 also facilitated extinction from drug-seeking behavior in rats during early behavioral extinction, and attenuated cocaine- or contextual cue-induced relapse to drug-seeking behavior. YQA14 alone does not maintain self-administration in either naïve rats or in rats experienced at cocaine self-administration. YQA14 also inhibited expression of repeated cocaine-induced behavioral sensitization. These findings suggest that YQA14 may have pharmacotherapeutic potential in attenuating cocaine-taking and cocaine-seeking behavior. Thus, YQA14 deserves further investigation as a promising agent for treatment of cocaine addiction.
Keywords: Cocaine, Dopamine, D3 Receptors, YQA14, reward, reinstatement
1. Introduction
Cocaine addiction is a serious public health problem that is very difficult to treat due to high rates of relapse after abstinence. No effective pharmacotherapies are currently available to treat cocaine addiction in humans. The mesolimbic dopamine (DA) system originates in the midbrain ventral tegmental area (VTA) and projects to the nucleus accumbens (NAc) and prefrontal cortex (PFC); it is believed that this pathway is essential for both drug reward and relapse to drug-seeking behavior (Swanson, 1982, Wise, 1996, Luscher and Malenka, 2011). As cocaine significantly enhances extracellular DA by inhibiting the dopamine transporter, pharmacological blockade of DA transmission has been proposed to be effective in treating cocaine addiction (Di Chiara and Imperato, 1988, Torregrossa and Kalivas, 2008). Therefore, much attention has focused on various DA receptor antagonists as potential treatments for cocaine addiction (Heidbreder et al., 2005, Gorwood et al., 2012). In particular, interest has focused on the DA dopamine D3 receptor (D3R) since it is very locally and very highly expressed in the mesolimbic DA system (Bouthenet et al., 1991, Diaz et al., 1995, Heidbreder and Newman, 2010) and because a history of cocaine-taking behavior is associated with significant D3R alterations (Staley and Mash, 1996, Neisewander et al., 2004, Conrad et al., 2010). In fact, it has been proposed that highly selective D3R blockade may attenuate cocaine’s addictive effects with fewer, if any, unwanted effects (Sokoloff et al., 1992, Xi and Gardner, 2007, Song et al., 2012b).
To date, a number of D3R antagonists have been synthesized and tested in animal models of addiction. These compounds include SB-277011A, NGB2904, BP-897, S-33138 and PG-01037 (Yuan et al., 1998, Pilla et al., 1999, Reavill et al., 2000, Grundt et al., 2005, Peng et al., 2009, Higley et al., 2011, Song et al., 2012a). To date, SB277011A is the most well-studied D3R antagonist in multiple animal models of addiction, but further development of SB-277011A has been terminated due to problematic pharmacokinetics (particularly shorter half-life, ~30 min in primates) and toxicity problems (Macdonald et al., 2003). We have recently reported on a D3R antagonist – YQA14 –, which displays similar or higher affinity and selectivity than SB-277011A for D3Rs over D2Rs (Song et al., 2012a). Compared to SB-277011A, YQA14 has improved oral bioavailability (>40%) and longer half-life (>2 h) in the same human hepatic microsomal enzyme assays (unpublished data). Systemic administration of YQA14 significantly inhibits cocaine self-administration under both fixed-ratio (FR2) and progressive-ratio (PR) reinforcement conditions, but has no effect on oral sucrose self-administration and locomotor activity (Song et al., 2012a), suggesting that YQA14 may have translational potential for the treatment of drug addiction in humans.
However, it remains unclear whether YQA14 has similar anti-cocaine effects as SB277011A in other animal models of addiction such as intracranial brain-stimulation reward, cocaine- or cue-induced reinstatement of drug-seeking behavior, and cocaine-induced behavioral sensitization. Therefore, in the present study, we investigated: 1) whether YQA14-induced D3R blockade attenuates cocaine-enhanced BSR; 2) whether YQA14 facilitates extinction from previous cocaine self-administration; 3) whether YQA14 inhibits cocaine- or cue-triggered reinstatement (relapse) to drug-seeking behavior; 4) whether YQA14 inhibits repeated cocaine-induced behavioral sensitization; and 5) whether YQA14 itself supports intravenous self-administration in rats experienced at cocaine self-administration.
2. Materials and methods
2.1 Animals
Male Long–Evans rats (Charles River Laboratories, Raleigh, NC) weighing 250–300 g were used. They were housed individually in a climate-controlled animal colony room on a reversed light/dark cycle with access to food and water ad libitum. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the United States National Academy of Sciences and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse.
2.2 Electrical brain-stimulation reward (BSR)
Surgery
Rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.) and placed in a stereotaxic frame, and a monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) was placed unilaterally into the lateral hypothalamus using standard aseptic surgical and stereotaxic techniques. The implant coordinates for the tips of the electrodes were AP −2.56, ML±1.9, and DV −8.6, according to the rat brain stereotaxic atlas of Paxinos and Watson (1998). The electrode was attached to the skull with jeweler’s screws and dental acrylic. A wire leading from the electrode was wrapped around a skull screw to serve as a current return.
Apparatus
The experiments were conducted in standard Med Associates operant chambers (32×25×33 cm). Each operant chamber had a lever located 6.5 cm above the floor, connected to an electrical stimulator.
General procedure
The general procedures for electrical BSR were the same as we have reported previously (Vorel et al., 2002, Hayes et al., 2003, Xi et al., 2006). Briefly, after 7 days of recovery from surgery, rats were allowed to self-train (auto shape) to lever-press for rewarding BSR. Each press on the lever resulted in a 500-ms train of 0.1-ms rectangular cathodal pulses through the electrode in the rat’s lateral hypothalamus, followed by a 500 ms ‘timeout’ in which further presses did not produce brain stimulation. The initial stimulation parameters were 72 Hz and 200 mA. If the animal did not learn to lever-press, the stimulation intensity was increased daily by 50 mA until the animal learned to press (45–60 responses/30 s) or a maximum of 800 mA was reached. Animals that did not lever-press at 800 mA or in which the stimulation produced unwanted effects (e.g., gross head or body movements, spinning, vocalization, or jumping) were removed from the experiment.
Rate-frequency BSR procedure
Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, animals responded for two 30-s time periods (‘bins’), following which the pulse frequency was decreased by 0.05 log units. Following each 30-s bin, the lever retracted for 5 s. Throughout the experiments, animals were run for three sessions a day. Response rate for each frequency was defined as the mean number of lever responses during two 30-s bins. Since lever-pressing behavior was variable during the first session (the ‘warm-up’ session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively. The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation.
Testing the effects of cocaine and/or YQA14 on BSR
Once a baseline θ0 value was achieved (<15% variation over 5 continuous days), the effects of cocaine and/or YQA14 on BSR were assessed. On test days, animals randomly received one of three different doses of YQA14 (12.5, 25 mg/kg i.p.) or vehicle (1 ml 25% 2-hydroxypropyl-β-cyclodextrin) 30 min prior to a cocaine injection (2 mg/kg i.p.). After each test, animals received an additional 5–7 days of BSR re-stabilization until a new baseline θ0 was established. The order of testing for various doses of YQA14 was counterbalanced. The effect of YQA14 on cocaine enhanced BSR was evaluated by comparing cocaine-induced alterations in θ0 value in the presence or absence of each dose of YQA14 pretreatment.
2.3 Cocaine or YQA14 Self-Administration
Surgery
Intravenous (i.v.) catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA). Rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.), and an i.v. catheter was inserted into a jugular vein using standard aseptic surgical procedures. During experimental sessions, the catheter was connected to an infusion pump via tubing encased in a protective metal spring from the head-mounted connector to the top of the experimental chamber. To prevent clogging, catheters were flushed daily with a gentamicin-heparin-saline solution (30 IU/ml heparin; ICN Biochemicals, Cleveland, OH).
Apparatus
Intravenous (i.v.) self-administration experiments were conducted in operant response test chambers (32 × 25 × 33 cm) from Med Associates Inc. (Georgia, VT, USA). Each test chamber had 2 levers: 1 active and 1 inactive, located 6.5 cm above the floor. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. A cue light and a speaker were located 12 cm above the active lever. The house light was turned on at the start of each 3 hr test session. Scheduling of experimental events and data collection was accomplished using Med-Associates software.
Cocaine self-administration
After recovery from surgery, each rat was placed into a test chamber and allowed to lever-press for i.v. cocaine (1 mg/kg/infusion) delivered in 0.08 ml over 4.6 sec, on a fixed ratio 1 (FR1) reinforcement schedule. Each cocaine infusion was associated with presentation of a stimulus light and tone. During the 4.6 sec infusion time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 hr. FR1 reinforcement was used for 3–5 days until stable cocaine self-administration was established: a minimum of 20 presses on the active lever per test session and stability criterion of less than 10% variability in inter-response interval, less than 10% variability in number of infusions taken, and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. Then subjects were allowed to continue cocaine (0.5 mg/kg/infusion) self-administration under FR2 reinforcement. The dose of cocaine was chosen based on previous studies showing that 0.5 mg/kg/infusion of cocaine lies within the middle range of the descending limb of the cocaine dose-response self-administration curve, where reliable dose-dependent effects can be observed (Xi et al., 2005). In addition, we chose 0.5 mg/kg, rather than 1 mg/kg, of cocaine in order to increase the work demand (i.e., lever presses) of the animals for the same amount of drug intake. In our experience, this approach increases the sensitivity of measuring changes in drug-seeking behavior. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine injections per 3hr session.
YQA14 self-administration
Two additional groups of animals were used for YQA14 (0.5 mg/kg/infusion) self-administration or cocaine self-administration followed by YQA14 substitution for cocaine. The experimental procedures for YQA14 self-administration were the same as for cocaine self-administration except that YQA14 was substituted for cocaine. In the latter group of rats, after stable cocaine self-administration was established for at least 3 consecutive days, the animals were divided into 2 subgroups (n=6 each): 1) cocaine was replaced by YQA14 (0.5 mg/kg/infusion); and 2) cocaine was replaced by YQA14 (1.0mg/kg/infusion). Since animals might take several days to support self-administration for a novel reinforcer, each replacement test was continued for 7 days.
2.4 Relapse to Drug-Seeking Behavior
Testing the effects of YQA14 on extinction responding
After stable cocaine self-administration was established as described above, animals were divided into three groups for behavioral extinction of the cocaine-taking behavior. Each group of rats received either the vehicle (25% 2-hydroxypropyl-β-cyclodextrin) or one dose of YQA14 (12.5 and 25 mg/kg i.p.) 30 min before each daily extinction session for 8 days. Extinction conditions consisted of cocaine being replaced by saline, and the cocaine-associated cue-light and tone being turned off. Active lever-pressing led only to saline infusion. Daily 3 h extinction sessions for each rat continued until lever presses ≤ 10 times per 3 h session for at least 3 consecutive days. The effects of YQA 14 on extinction responding were evaluated.
Testing the effects of YQA14 on cocaine- triggered reinstatement of drug-seeking behavior
After stable cocaine self-administration was established, additional groups of animals were exposed to the extinction conditions, in which cocaine was replaced by saline, and the cocaine-associated cue-light and tone were turned off. Active lever-pressing led only to saline infusion. Daily 3 h extinction sessions for each rat continued until lever presses ≤ 10 times per 3 h session for at least 3 consecutive days. On the reinstatement test day, each group of rats received either the vehicle (25% 2-hydroxypropyl-β-cyclodextrin) or one dose of YQA14 (12.5 and 25 mg/kg i.p.). At 30 min after vehicle or YQA14 administration, all rats were given a priming injection of cocaine (10 mg/kg i.p.) immediately before reinstatement testing began in the same self-administration chambers. During the reinstatement test, lever-pressing responses did not lead to either cocaine infusions or presentation of the conditioned cues. Reinstatement test sessions lasted 3h. Cocaine-induced lever-pressing responses were recorded and compared between different dose groups of rats
Testing the effects of YQA14 on contextual cue-induced cocaine-seeking behavior
After stable cocaine self-administration was achieved, additional groups of rats were used to assess contextual cue-induced cocaine-seeking behavior. After 14 days of withdrawal from cocaine self-administration, animals were divided into 3 dose groups (0, 12.5, 25 mg/kg YQA14). At 30 min after injection on the test day, the rats were re-placed into the same self-administration chambers, and contextual cue-induced cocaine-seeking behavior was assessed under extinction conditions during which cocaine and the cocaine-associated cue light and tone were unavailable, and therefore, lever pressing did not result in any consequence. Each reinstatement test lasted for 3h.
2.5 Cocaine-induced behavioral sensitization
Before receiving cocaine or YQA14, rats were placed in a locomotor detection chamber (Accuscan, Columbus, OH, USA) for habituation for 1 hour per day for 3 days. Rats were then divided into three groups. One group of rats were used to study the effects of daily administration of YAQ14 (0, 12.5, 25 mg/kg/day, 20 min prior to cocaine, n=8 per group) on acquisition of cocaine (15 mg/kg/day × 7 days)-induced locomotor sensitization. The second group of rats were used to study the effects of a single injection of YQA14 (0, 12.5, 25 mg/kg, n=9–12 per dose group) on expression of cocaine-induced locomotor sensitization in rats 7 days after the last cocaine injection. The third group of rats were used to study the effects of repeated administration of YQA14 (25 mg/kg × 7 days) alone on basal locomotor activity. On the test day, rats were placed into the locomotion detection chambers to measure locomotion immediately after received 10 mg/kg cocaine or YQA14. Total distance counts were used to evaluate the effect of YQA14 on basal and cocaine-induced behavioral sensitization.
2.6 Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. YQA-14 was synthesized at the Beijing Institute of Pharmacology and Toxicology and dissolved in vehicle, i.e., 25% 2-hydroxypropyl-β-cyclodextrin (Sigma, St Louis, MO).
2.7 Data analysis
All data are presented as means (±SEM). One- or two-way analysis of variance (ANOVA) was used to analyze the data reflecting the effects of YQA14 on brain stimulation reward or on cocaine-self-administration in rats. Individual group comparisons were performed with the Student-Newman-Keuls method.
3. RESULTS
3.1 YQA14 inhibits cocaine-enhanced BSR
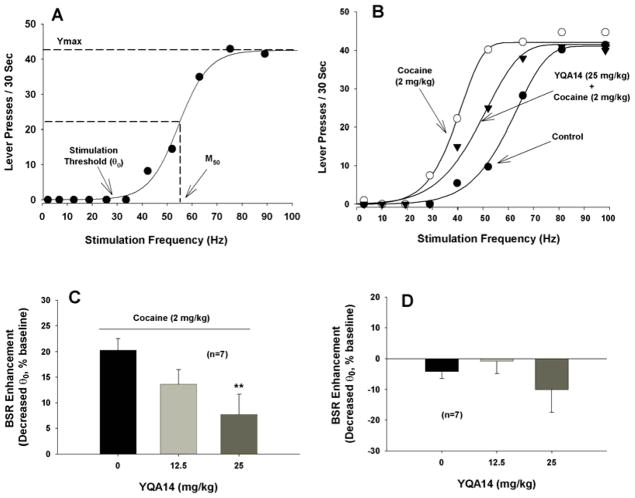
As we reported before (Xi et al., 2006), systemic administration of cocaine (1, 2, and 10 mg/kg, i.p.) produced significant and dose-dependent enhancement of BSR indicated by a decrease in BSR threshold (θ0 value) (Fig. 1A, 1B). In the present research, we chose the median dose of 2 mg/kg cocaine, which produced a significant decrease in BSR threshold in rats (Fig. 1B). Pretreatment with YQA14 (12.5, 25 mg/kg, i.p.) significantly inhibited the enhanced BSR produced by 2 mg/kg of cocaine (Fig. 1C). YQA14 alone (12.5, 25 mg/kg, i.p.) had no effect on BSR (Fig. 1D, F2, 22=1.12, p=0.33). However, one way-ANOVA for the data shown in Fig. 1C revealed a significant YQA14 treatment main effect on cocaine-enhanced BSR (F2, 30=6.21, p<0.01). Individual group comparisons revealed a statistically significant reduction in cocaine-enhanced BSR after 25 mg/kg YQA14 (q=4.98, p<0.01) when compared to vehicle group.
Figure 1.
Effects of YQA14 on cocaine-enhanced BSR in rats. A: A representative rate-frequency function curve for BSR, illustrating BSR stimulation threshold (θ0), M50 and Ymax; B: Representative rate-frequency function curves for BSR, illustrating that cocaine (2 mg/kg, i.p.) shifted the rate-frequency function curve to the left, lowering the BSR threshold θ 0 value (i.e., enhancing BSR), without a change in the Ymax level. C: Systemic administration of YQA14 dose-dependently decreased cocaine-enhanced BSR. D: Systemic administration of YQA14 alone had no effect on basal BSR. **p<0.01 compared to vehicle control group.
3.2 YQA14 does not maintain self-administration
Figure 2 shows the results of the YQA14 self-administration tests. Figure 2A shows that cocaine (0.5 mg/kg, i.v.) quickly produced robust self-administration behavior. YQA14, at the same or higher dose (0.5 or 1.0 mg/kg/injection), failed to produce self-administration behavior under the same experimental conditions in drug naïve rats. Figure 2B shows that the same doses of YQA14 were also unable to replace cocaine in maintaining self-administration behavior in rats (n=8) already displaying behaviorally stable cocaine self-administration under FR1 reinforcement conditions. Compared to the stable pattern of self-administration maintained by cocaine, YQA14 substitution failed to maintain stable self-administration. Instead, gradual extinction behavior was observed. This pattern of extinction was essentially identical to saline substitution for cocaine, as we reported previously (Xi et al., 2006). Two-way ANOVA for repeated measures over time revealed a significant time main effect (F2, 21=58.91, p<0.001), showing that YQA14 does not maintain self-administration in cocaine self-administration rats.
Figure 2.
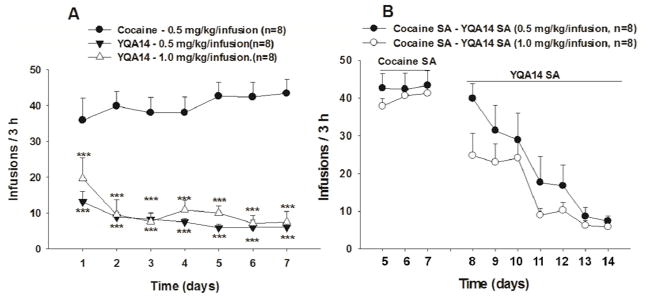
Cocaine and YQA14 self-administration. Intravenous administration of cocaine, but not YQA14, induced self-administration in drug naïve rats (A). YQA14 substitution for cocaine failed to maintain drug self-administration behavior in rats previously self-administering cocaine. ***p<0.001 compared to vehicle control group.
3.3 YQA14 facilitates extinction of cocaine-seeking behavior
Figure 3 shows responses on active and inactive levers in the presence of vehicle or YQA14 pretreatment under extinction conditions in which drug and drug-associated cues were unavailable. Daily YQA14 (12.5, 25 mg/kg, i.p.) treatment significantly facilitated extinction from cocaine-seeking behavior. Two-way ANOVA for repeated measures revealed a statistically significant YQA14 treatment main effect on active lever presses (Fig. 3A, F2, 184=33.3, p<0.001), but not on inactive lever presses (Fig. 3B, F2, 184=0.68, p>0.05) during extinction.
Figure 3.
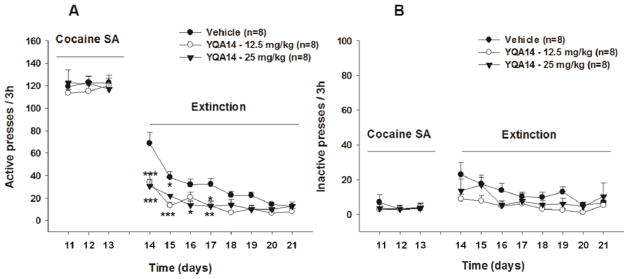
Effects of YQA14 on drug-seeking behavior during extinction. Systemic administration of YQA14 dose-dependently decreased active lever presses (A), but not inactive lever presses (B), in rats during extinction conditions in which cocaine or cocaine-associated cue-light and tone were not available. *p<0.05, **p<0.01, ***p<0.001 compared to vehicle control group.
3.4 YQA14 inhibits cocaine-triggered reinstatement of drug-seeking behavior
Figure 4 shows total numbers of active lever presses observed during the last session of cocaine self-administration, the last session of extinction, and the reinstatement test session in two different YQA14 dose groups. A single non-contingent cocaine prime (10 mg/kg) evoked robust reinstatement of cocaine-seeking behavior in rats extinguished from previous cocaine self-administration (Fig. 4A). Pretreatment with YQA14 (12.5, 25 mg/kg) significantly attenuated cocaine-triggered reinstatement of drug-seeking behavior by ~60% (Fig. 4A, F2, 46=8.98, p<0.001). Individual group comparisons revealed a statistically significant reduction in cocaine-seeking behavior after 12.5 mg/kg YQA14 (t=3.25, p<0.01) or 25 mg/kg YQA14 (t=3.55, p<0.01), when compared to vehicle control group. In contrast, YQA14 pretreatment did not alter inactive lever response under the same experimental conditions (Fig. 4B, F2, 46=0.24, p>0.05), suggesting a specific effect on cocaine-induced drug-seeking behavior.
Figure 4.
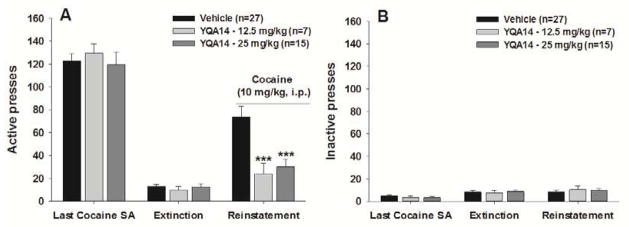
Effects of YQA14 on cocaine-triggered reinstatement of drug-seeking behavior. Systemic administration of YQA14 (12.5 or 25 mg/kg i.p., 20 min prior to test) significantly inhibited cocaine-triggered reinstatement of drug-seeking behavior in rats extinguished from daily cocaine self-administration. ***p<0.001, when compared with the vehicle pretreatment group.
3.5 YQA14 inhibits contextual cue-induced cocaine-seeking behavior
Figure 5 shows lever responses on both active and inactive levers in rats after 14 days of withdrawal from cocaine self-administration. Pretreatment with YQA14 (12.5, 25 mg/kg) on the test day significantly and dose-dependently attenuated contextual cue-induced cocaine-seeking behavior. One-way ANOVA revealed a statistically significant YQA14 treatment main effect on active lever presses (Fig. 5A, F2, 15=5.60, p<0.05). Individual group comparisons revealed a significant reduction in active lever pressing after 25 mg/kg (q=4.52, p<0.05), when compared to vehicle control group. In contrast, systemic administration of YQA14 did not alter inactive lever responses (Fig. 5B, F2, 15 = 1.22, p>0.05).
Figure 5.
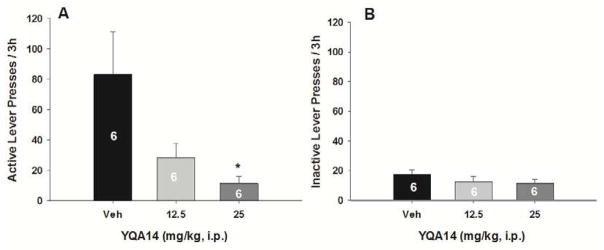
Effects of YQA14 on contextual cue-triggered cocaine-seeking behavior. Systemic administration of YQA14 (12.5 or 25 mg/kg, i.p., 20 min prior to test) significantly inhibited contextual cue-triggered drug-seeking behavior in rats after 14 days of withdrawal from previous cocaine self-administration. *p<0.05, when compared with the vehicle pretreatment group.
3.6 YQA14 inhibits cocaine-induced locomotor sensitization
Figure 6A shows that pretreatment with YQA14 (12.5, 25 mg/kg), administered 20 min prior to each cocaine injection for 7 days, did not produce a statistically significant inhibitory effect on acquisition of repeated cocaine-induced locomotor sensitization. Two-way ANOVA for repeated measures over time revealed a statistically significant time main effect (F6,126 = 5.16, p<0.001), but non-significant YQA14 treatment main effect (F2, 21 = 2.83, p>0.05) nor treatment × time interactions (F6,126 = 1.28, p>0.05). Repeated administration of YQA14 alone failed to alter spontaneous locomotion when compared to the basal levels of locomotion after saline injection (Fig. 6A, F9,36 = 0.39, p>0.05). Figure 6B shows that pretreatment with YQA14 significantly inhibited the expression of cocaine-induced locomotor sensitization measured 7 days after the last cocaine injection (F2,27 = 41.40, p<0.05).
Figure 6.
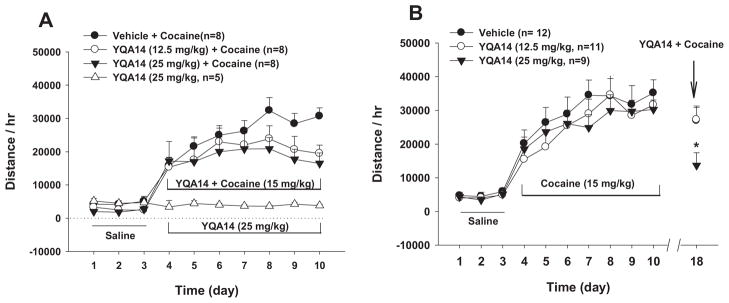
Effects of YQA14 on basal level of locomotion and cocaine-induced behavioral sensitization. A: Pretreatment of YQA14 (12.5, 25 mg/kg, i.p., 20 min prior to each cocaine injection) did not produce a statistically significant inhibition on acquisition of cocaine-induced locomotor sensitization. Repeated administration of YQA14 (25 mg/kg) alone also failed to alter basal levels of locomotion. B: A single injection of YQA 14 (12.5, 25 mg/kg, i.p.) significantly inhibited expression of cocaine-induced locomotor sensitization in rats 7 days after the last cocaine injection.
4. DISCUSSION
In the present study, we found that systemic administration of YQA14, a novel potent and selective D3R antagonist significantly attenuates cocaine’s rewarding effects, as assessed by electrical BSR, and relapse to drug-seeking behavior triggered by cocaine priming or contextual environmental cues. In addition, YQA14 also facilitated extinction from drug-seeking behavior (e.g., decreased extinction responding in the absence of cocaine) and inhibited expression of cocaine-induced locomotor sensitization. YQA14 itself had no reinforcing effect in naïve rats nor in rats previously experienced at cocaine self-administration, and did not by itself produce any significant effect on BSR or locomotion. Together, these data suggest that D3Rs play a critical role in cocaine-induced reward and relapse, and that YQA14 or other D3R antagonists show exceptional promise of having therapeutic potential in the treatment of cocaine dependence.
We have previously reported that YQA14 significantly inhibits cocaine self-administration in rats and mice, an effect mediated by selective blockade of D3Rs based on findings in D3R gene-deleted (KO) mice (Song et al., 2012a, Song et al., 2012b). To further explore this finding, we here used the BSR paradigm to study the pharmacological effects of YQA14 on cocaine’s rewarding effects. BSR is a highly sensitive experimental procedure to assess whether a substance is rewarding/pleasurable or aversive/dysphoric (Stein and Ray, 1960, Gardner, 2005, Wise RA, 2004). In the present study, we found that systemic administration of cocaine (2 mg/kg) significantly and dose-dependently enhanced BSR, consistent with our previous reports (Xi et al., 2006, Xi et al., 2008, Xi et al., 2010). Pretreatment with YQA14 significantly inhibited this enhanced BSR, suggesting a reduction in cocaine’s rewarding effects after YQA14 pretreatment. This result is consistent with our previous finding that YQA14, at the same doses, inhibits cocaine self-administration (Song et al., 2012a). It is also consistent with our findings that blockade of brain D3Rs by SB-277011A, NGB2904, PG01037, or S33138 significantly attenuates cocaine- or methamphetamine-enhanced BSR in rats (Vorel et al., 2002, Xi et al., 2006, Peng et al., 2009, Higley et al., 2011). Moreover, YQA14 itself produced neither a rewarding or aversive effect as assesses by BSR. This is congruent with our previous findings with NGB2904 and SB-277011A (Vorel et al., 2002, Xi et al., 2004, Xi et al., 2006, Campos AC, 2004). Taken together, all these data strongly support the conclusion that selective D3R antagonists are effective in reducing cocaine’s rewarding efficacy, while themselves having no unwanted rewarding or aversive effects.
In addition, the present study, for the first time, demonstrates that repeated daily administration of YQA14 significantly accentuates behavioral extinction from daily cocaine self-administration. This reduction was not due to sedation or locomotor impairment since repeated administration of YQA14 alone for 7 days did not produce significant effects on basal and cocaine-enhanced locomotion. The neuronal mechanisms underlying this effect are unclear. Since extinction responding is measured in drug self-administration chambers that were previously associated with cocaine-taking behavior, it has been proposed that extinction responding is maintained predominantly by drug-associated environmental cues (Bouton, 2002, Shalev et al., 2002a, Quirk and Mueller, 2008, Nic Dhonnchadha and Kantak, 2011, Xue et al., 2012). It is well documented that drug-associated cues can increase DA (and glutamate) release in the mesolimbic DA system, especially in the NAc (Ito et al., 2000, Volkow et al., 2006, Day et al., 2007, Volkow et al., 2008, Aragona et al., 2009). This may well explain why blockade of D3Rs by YQA14 facilitates extinction of cocaine self-administration behavior in the present study.
Cocaine addiction is well characterized by a persistent susceptibility to drug relapse. Drug re-exposure has been identified as one of the most important determinants of relapse in humans and in experimental animals (Shaham and Stewart, 1994, Shalev et al., 2000, Stewart, 2000). Using a classical reinstatement model, we found that pretreatment with YQA14 significantly attenuated cocaine-triggered reinstatement of cocaine-seeking behavior. This finding is consistent with previous research using other D3R antagonists (Vorel et al., 2002, Gal and Gyertyan, 2006, Xi et al., 2006, Peng et al., 2009, Higley et al., 2011).
In addition, we also found that systemic administration of YQA14 significantly inhibited contextual cue-induced relapse to cocaine-seeking behavior. In this model, animals are withdrawn from cocaine self-administration without behavioral extinction of the previously-reinforced operant responding. After 2 weeks of withdrawal in their home cages, animals are re-exposed to the environmental context previously paired with drug self-administration, i.e., the self-administration chambers. Subsequent operant responding (e.g., drug-seeking) leads to no consequence (no drug, no conditioned cue light or tones) (Grimm et al., 2003). This procedure has been thought to model contextual cue-induced drug-seeking behavior in humans (Lu et al., 2004). As stated above, the mesolimbic DA system is critically involved in cocaine- or cue-induced drug-seeking behavior (Shalev et al., 2002b, Kalivas and McFarland, 2003). In addition, growing evidence suggests that brain D3Rs (particularly in the NAc) are up-regulated in rats after prolonged withdrawal from chronic cocaine self-administration in both experimental animals and humans (Staley and Mash, 1996, Neisewander et al., 2004, Conrad et al., 2010). Accordingly, blockade of D3Rs might well be explained to antagonize cocaine- or cue-induced cocaine-seeking behavior. This is consistent with the present findings. It is also consistent with our recent report that microinjections of SB-277011A into the NAc or amygdala significantly inhibited contextual cue-induced cocaine seeking under the same experimental conditions (Xi et al., 2012).
Another important finding of the present study is that YQA14 cannot induce or maintain self-administration in drug naïve rats or substitute for cocaine in the self-administration paradigm. It also failed to alter electrical BSR, suggesting that it has neither rewarding nor aversive effects by itself. This is consistent with our previous study with another D3R antagonist NGB2904 (Xi et al., 2006).
In conclusion, the present study demonstrates that YQA14 attenuates cocaine’s rewarding effects, facilitates extinction from drug-seeking, inhibits cocaine- or cue-induced relapse to drug-seeking behavior, and attenuates cocaine-induced behavioral sensitization. YQA14 by itself has no apparent sedative, rewarding or aversive effects. These data suggest that YQA14 or other selective D3 receptor antagonists may be promising as pharmacotherapetic agents for treating cocaine addiction.
Highlights.
YQA14 attenuates cocaine-enhanced brain-stimulation reward
YQA14 facilitates extinction from cocaine-seeking behavior
YQA14 attenuates cocaine- or cue-induced relapse to drug-seeking behavior
YQA14 inhibits cocaine-induced behavioral sensitization
YQA14 itself has no addictive effects
Acknowledgments
This work was supported by the U.S. National Institute on Drug Abuse Intramural Research Program, the National Basic Research Program of China (No. 2009CB522008) and the Natural Science Foundation of China (Grant No. 81102425).
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflicting financial interests.
Author Contribution: R.S. designed and conducted experiments, analyzed data and wrote the first draft of the manuscript. R.-F.Y. synthesized YQA14. G.-H.B. and H.-Y. Z. carried out behavioral experiments. E.L.G., J.L. and Z.-X.X. supervised the experiments and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 48–97. [Google Scholar]
- Gorwood P, Le Strat Y, Ramoz N, Dubertret C, Moalic J-M, Simonneau M. Genetics of dopamine receptors and drug addiction. Hum Genet. 2012;131:803–822. doi: 10.1007/s00439-012-1145-7. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su T-P, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology. 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby Cr., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi Z-X, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR., Jr Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))- phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3 -benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J Med Chem. 2003;46:4952–4964. doi: 10.1021/jm030817d. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LTL, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99:229–244. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi Z-X, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-Q, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaër E, Muńoz C, Gardner EL, Xi Z-X. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Andrieux M, Besançon R, Pilon C, Bouthenet M-L, Souil E, Schwartz J-C. Localization and function of the D3 dopamine receptor. Arzneimittelforschung. 1992;42(2A):224–230. [PubMed] [Google Scholar]
- Song R, Yang R-F, Wu N, Su R-B, Li J, Peng X-Q, Li X, Gaál J, Xi Z-X, Gardner EL. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2012a;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang H-Y, Li X, Bi G-H, Li J, Gardner EL, Xi Z-X. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012b;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang H-Y, Peng X-Q, Su R-B, Yang R-F, Li J, Xi Z-X, Gardner EL. Dopamine D3 receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L, Ray OS. Brain stimulation reward “thresholds” self-determined in rat. Psychopharmacologia (Berl) 1960;1:251–256. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav. 2008;90:261–272. doi: 10.1016/j.pbb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Gardner EL. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2. Oxford, England: Oxford University Press; 2004. pp. 683–697. [Google Scholar]
- Xi Z-X, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost&variable-payoff cocaine self administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Kiyatkin M, Li X, Peng X-Q, Wiggins A, Spiller K, Li J, Gardner EL. N-acetyl-aspartatylglutamate (NAAG) attenuates cocaine-enhanced brain-stimulation reward and cocaine self-administration in rats. Neuropharmacology. 2010;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Li X, Li J, Peng X-Q, Song R, Gaál J, Gardner EL. Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol. 2013;18:665–677. doi: 10.1111/j.1369-1600.2012.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Newman AH, Gilbert JG, Pak AC, Peng X-Q, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng X-Q, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- Xue Y-X, Luo Y-X, Wu P, Shi H-S, Xue L-F, Chen C, Zhu W-L, Ding Z-B, Bao Y-P, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JWF, Thurkauf A. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]