Abstract
Plant species have long been regarded as possessing the principal ingredients used in widely disseminated ethnomedical practices. Different surveys showed that medicinal plant species used by the inhabitants of Jordan for the traditional treatment of diabetes are inadequately screened for their therapeutic/preventive potential and phytochemical findings. In this review, traditional herbal medicine pursued indigenously with its methods of preparation and its active constituents are listed. Studies of random screening for selective antidiabetic bioactivity and plausible mechanisms of action of local species, domesticated greens, or wild plants are briefly discussed. Recommended future directives incurring the design and conduct of comprehensive trials are pointed out to validate the usefulness of these active plants or bioactive secondary metabolites either alone or in combination with existing conventional therapies.
Keywords: Traditional medicine, Medicinal plants, Diabetes, Jordan, Ethnomedicine
Introduction
Diabetes mellitus (DM) is highly recognised as the most common metabolic and endocrine disorder worldwide. It is linked to disturbances in carbohydrate, fat, and protein metabolism [1]. It is especially important because the global prevalence of diabetes is projected to escalate relentlessly. At least 250 million individuals worldwide suffer from diabetes and this number will double by 2030. Increases in complications will undeniably follow increasing diabetes incidence rates [2]. More than 80% of diabetes deaths take place in low- and middle-income countries [3].
The regional prevalence of diabetes in MENA (Middle Eastern and North Africa) countries is 7.7%. Locally, endocrine, nutritional, and metabolic diseases represent 7.9% of deaths in Jordan [3–5]. With a prevalence rate at 10.1%, Jordan has the ninth highest incidence of diabetes among neighbouring countries. Several national surveys designated that the prevalence of type 2 diabetes and impaired fasting glycemia is unprecedentedly high, amounting to an epidemiological transition in Jordan [6–8].
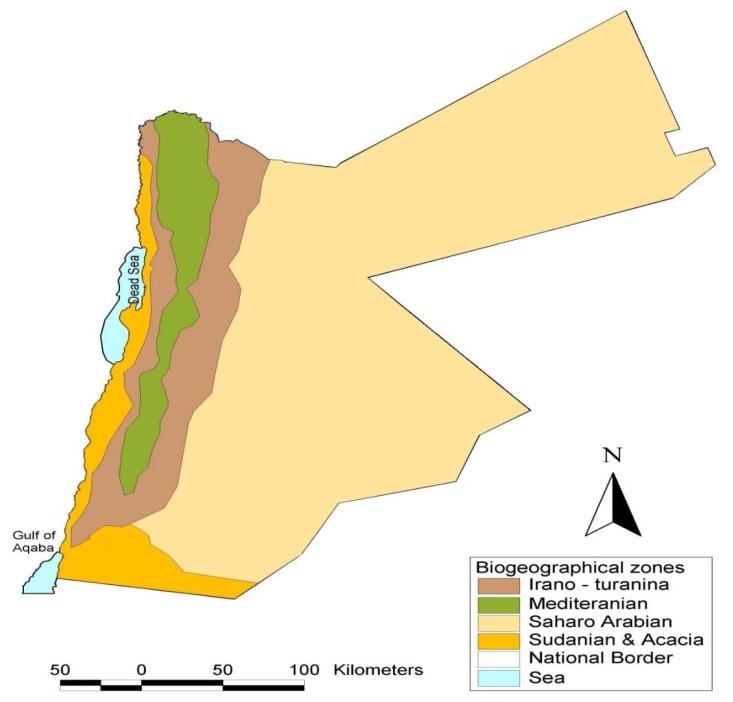
Undoubtedly, Jordan’s habitat is exceptional. It is at the intersection of arid desert, dense forest, and tropical geography, thus bestowing the country with a rich variety of plants and microorganisms that can be resourcefully studied (Fig. 1) [9]. The heterogeneous ecological conditions have favoured the proliferation of more than 2,500 wild plant species from 700 genera; of these, there are approximately 100 endemic species, 250 rare species, and 125 very rare species [9–11]. Unfortunately, this substantial biodiversity is principally understudied, or even worse, left unexplored [9–12].
Fig. 1.
Biogeographic zones of Jordan
Apparently, there is a repository of ethnobotanical studies in the Mediterranean basin, providing a new and key tool for a quest after invaluable phytopharmaceuticals or the development of functional foods or nutraceuticals [13–20]. Traditional medicine practices, being part of the Jordanian culture, are considered responsible for an impartial role in primary health care despite modern medicine accessibility [21] where vegetables, culinary herbs, and medicinal plants are among the main choices in the management of diabetes [13, 21–30]. Essentially important, traditional medicine has not only survived, but thrived in the transcultural environment and intermixture of many ethnic traditions and beliefs despite the ‘aging’ or ‘vanishing’ of folk phytotherapy in the sense that the wealth of knowledge of medicinal plants resides mostly in elderly rural people with modest tuition [31]. Also, it is officially neither integrated in the health care system nor recognized in the national policies of the country. Furthermore, as the use of medicinal plant remedies constitutes the common legacy of Jordanians, reliability fractions on herbal medicine vary from rural and desert areas to heavily populated urban ones [21–24]. In the last decades, more plants have been added to the list of endangered species. This results in the urgent inevitability for local communities to implement nationwide conservation and sustainability programs [32].
The objective of this review is to emphasize the ethnopharmacological practices related to 20 selected ethnobotanicals with claimed antidiabetic properties in light of their comprehensive scientific evaluation and bioactive plant secondary metabolites. Considering the hugely diverse plant species in diabetes traditional medicine, the present manuscript can be complementary to our previous report of 30 indigenous plants [33]. In fact, all our attempts in this direction serve to bring together the Jordanian inventory of diabetes ethnomedicine. Still, further studies might also be integrated into this line of work.
Results and Discussion
Based on centuries of beliefs and observations, plants are primarily used in preparatory forms of infusions or decoctions in ethnomedicinal practices. Worldwide, more than 1,200 species of plants have been reported to be used empirically for their claimed antidiabetic activity [34] while in the Jordanian traditional medicine, almost 70 plant species are used by diabetic patients. Although indigenously grown plants are consumed in the countryside, in the vast cities, including the capital Amman, however, the herbalists’ shops display a wide variety of imported plant species, like Zingiber officinalis, Terminalia chebula, or Emblica officinalis, alongside the likely obtainable native ones [11, 23, 35, 36].
On the other hand, reports on the concomitant use of plants in orthodox therapy are evidently understated. In this aspect, interviews with diabetes patients in specialized health centres in Jordan further signified a more diversified list of selected plants [21, 26]. The reported plants were: Camellia sinensis, Pimpinella anisum, Zingiber officinale, Matricaria recutita, Salvia fruticosa, Trigonella foenum-graecum, Nigella sativa, Lupinus albus, Teucrium polium, Allium sativum, Cinnamomum zeylanicum, and Olea europea. It is tempting to speculate that the high frequency of use is related to the high efficacy and safety of the plant material, such as green tea, aniseed, or chamomile, although there are no clinical studies to indicate monitoring of glucose and haemoglobin A1c levels in diabetic patients using these plants [31]. Also, there is no information available on the protection from target organ damage by the long-term use of plant products. Interestingly, white lupin (Lupinus albus), fenugreek (Trigonella foenum-graecum), garlic (Allium sativum), olive leaves (Olea europea), ginger (Zingiber officinale), felty germander (Teucrium polium), or black fennel (Nigella sativa) were not the top/main preference herbs of choice by the Jordanian diabetic patients [21, 26], despite being scientifically appraised for their antidiabetic activities and frequent use in communities abroad. This has lent further weight to our major interests and concerns stemming from the unjustified claims and selection pressure of certain herbal ethnomedicines in the treatment of diabetes.
Obviously, the significant efficacy of hypoglycaemic herbs, obtainable, via functioning as pancreatic insulin secretagogues and extrapancreatic insulin mimetics, enhancing glucose uptake by adipose and muscle tissues, or via inhibiting hepatic gluconeogenesis and intestinal carbohydrate digestibility and absorption, is comparable to conventional diabetes pharmacotherapeutics [37–39]. Literature surveys of botanicals with traditional uses, critically withstanding pharmacological appraisal, indicated that local target-based and mechanistic reports on diabetes interventional phytotherapies are primarily limited and inadequate. Gharaibeh et al. [40] investigated the hypoglycaemic effects of the aqueous extract of Teucrium polium in normal and streptozocin (STZ)-diabetic rats. Additionally, the hypoglycaemic effects of Ballota nigra[41] and Artemisia sieberi[42] were evidenced in alloxan-diabetic rats. Also, the antioxidative properties of an extensive list of Jordanian plants with diabetes ethnotherapeutic claims were closely discussed [43]. In other studies from Jordan, the pancreatic effects of the antidiabetic plants Eriobotrya japonica[44] and Ferula asafoetida were reported [45]. Further comprehensive in vitro and in vivo examinations of indigenous herbs valued as antidiabetic phytomedicines, including Achillea santolina, Eryngium creticum, Geranium graveolens, Paronychia argentea, Pistacia atlantica, Rheum ribes, Sarcopoterium spinosum, Teucrium polium, and Varthemia iphionoides, have been recognised with elaboration [46–49]. These research findings could collectively resonate with the prevention/modulation of postprandial hyperglycaemia, budding from the natural therapeutic inhibitors of α-amylase and α-glucosidase, with ethnopharmacological claims in the local communities.
Table 1, demonstrating the antidiabetic and/or other pharmacological activities of the compiled 20 plants, provides an updated overview of their reported phytoconstituents as well. In the present review, flavonoids are among the major classes of secondary metabolites detected in most of the tabulated plants. The antidiabetic activity is well-documented for numerous flavonoids [50]. Achillea santolina and A. fragrantissima are widely distributed in Jordan and used for their claimed antidiabetic activities. In STZ diabetic rats, hypoglycaemic activity was only evaluated for the former species though both species are rich in flavonoids among other similar volatile oil constituents. Hence, an antidiabetic activity can be likely assumed and verified for flavonoid-rich A. fragrantissima[51]. Also, the promoted antidiabetic activity of Anthemis pseudocotula might be due largely to its flavonoid content. On equal footing, similar postulations can be deduced for plant species with reported antioxidative capacities. Basically, natural antioxidants are well-linked with antidiabetic therapeutic/preventive pharmacology [34, 43, 52–55]. Consequently, despite the lack of scientific scrutiny, it can be speculated that the antioxidative propensities of Alhagi marourum, Alchemilla vulgaris, Cucurbita maxima, Juniperus phoenicea, Quercus coccifera, and Ambrosia maritima can in principle justify their reported phytotherapeutic claims and ethnomedicinal uses.
Tab. 1.
Antidiabetic plants indigenous to Jordan used for the treatment of diabetes in folk medicine in Jordan.
| No | Species | Reported antidiabetic efficacy and/or mechanism of action | Other reported pharmacological effects |
|---|---|---|---|
| Reported phytoconstituents | |||
| 1 | Asteraceae Achillea fragrantissima (Forsk.) Sch. Bip (Infusion of leaves and shoots [23]) Flavonoids [67–69]. Essential oil (santolina alcohol, artemisia alcohol, artemisia ketone, cis-thujone and trans-thujone, 1,8-cineole, fragranol, fragranyl acetate and terpin-4-ol) [70]. |
NONE | Antioxidative effects [43]. Lacked any antirheumatic or anti-inflammatory effects in carrageenan-induced acute inflammation in rats [71], but exerted antimicrobial and antiviral activities [70, 72–75]. Modulatory effects on rat ileum muscle contraction [74]. Beneficial in preventing/treating neuro-degenerative diseases [76]. Aqueous extract exhibited strong cytotoxicity and larvicidal activities [77, 78]. |
| 2 | Asteraceae Achillea santolina L. (Infusion of leaves, flowering branches [24]) Flavonoids such as luteolin, quercetin, cosmosiin, hyperoside and cynaroside [79–81], terpenoids [82]. Essential oil (1,8-cineole, fragranol, fragranyl acetate and terpin-4-ol) [70]. |
Hypoglycemic activity in STZ rats due to antioxidative potential [51, 83–84]. Lack of significant inhibition of α-amylase and α-glucosidase in vitro despite acute antihyperglycemic trend in starch fed rats [48]. | Enhancement of antimicrobial efficacy against antibiotic resistant E. coli and other microorganisms [85, 86]. Potent anti-inflammatory and immunomodulatory activities [87]. |
| 3 | Asteraceae Ambrosia maritima L. (Infusion of herb [24]) Sesquiterpenes and sesquiterpene lactones [88–90]. Thiophene A and thiophene A diol as major polyacetylenes [91]. |
NONE | Cytotoxicity [88]. Effective molluscicidal activity [92–96] but little or no effect on the larvae of Anopheles stephensi and Aedes aegypti[97, 98] as well as hepatoprotective and antioxidant properties [99]. Antifungal activity of its sesquiterpenes [89]. |
| 4 | Asteraceae Anthemis pseudocotula Boiss (Infusion of flowering heads, leaves [24]) Flavonoids (apigenin, apigenin-7-glucoside) and coumarins (scopoletin and herniarin) [100]. Essential oil [101], sesquiterpenes and sesquiterpene lactones [102, 103]. |
NONE | NONE |
| 5 | Asteraceae Varthemia iphionoides Boiss and Blanche (Decoction of shoots, leaves [23, 25]) Eudesmane sesquiterpene [104]. Flavonoids: jaceidine, kumatakenine, xanthomicrol, seven 3-methoxyflavones [105–107]. Essential oil [108, 109]. |
Inhibitory activity against porcine pancreas α-amylase [110]. Highly significant dose dependent dual anti-α-amylase and anti-α-glucosidase efficacies in vitro[49]. Significant decreases in the blood glucose levels of the STZ hyperglycaemic rats and hypoglycaemic activity in the diabetic sand rats [111, 112]. | Antiplatelets benefits [113] as well as antioxidative effects [43, 105, 110, 114]. Cytotoxic effect on human leukemia (HL-60) and antitumor properties [105, 115]. Pronounced antibacterial and antifungal propensities [86, 105, 106, 116]. |
| 6 | Capparaceae Cleoma droserifolia (Forskal) Delil (Decoction of leaves [24]) Terpenes, flavonoids (quercetin, kaempferol, and isorhamnetin) and phenolic acids [117–122]. |
Hypoglycaemic efficacy via potentiation of peripheral and hepatic insulin sensitivity, thus decreasing hepatic glucose output. Also decreasing intestinal glucose absorption, which was evident by blunting plasma glucose levels throughout the oral glucose challenge in tetracycline-induced fatty liver rats [123]. Insulin induction activity [124]; restored the blood glucose level, plasma malondialdehyde, and urine sugar to near the physiological values [121]. In alloxan-induced diabetic mice reduced oxidative stress in addition to antihyperglcemic activity [125]. | Suppressive effect on NO production in activated macrophages in vitro[117]. Hepato-protective effect [119]. Hypocholesterolemic and protective anti-atherogenic benefits in tetracycline induced fatty liver in rats [123]. Hypolipidemic, antioxidative and anti-Schistosomiasis mansoni properties [124–126]. Hepatotoxicity in co-culture systems [127]. Significant cytotoxic activity against breast (MCF7) and colon (HCT116) cancer cell lines [122]. |
| 7 | Cucurbitaceae Cucurbita maxima Duchesne (Dry seeds [23]) Spinasterol [128]. Carotenoids (violaxanthin, beta-carotene) and lutein [129]. Tocopherols, fatty acids (oleic, linoleic, and palmitic acids), beta sitosterol and phenolic acids [130–133]. Water soluble polysaccharide fraction [134]. Volatile compounds, such as lipid aldehydes, ethyl acetate, 2,3-butanedione, and dimethylsulfide [135]. |
Wistar rats treated for 70 days with pumpkin seed flour exhibited significant decrease in glucose and triacylglycerides [136]. | Antigenotoxic principle [128] and antioxidative benefits [134]. Trypsin inhibition [137, 138]. Larvicidal, ovicidal and repellent properties against mosquito bites [139]. |
| 8 | Cupressaceae Juniperus phoenicea L. (Decoction of fruits, leaves [13]) Lignans [140]. Phenylpropane glycosides [141], essential oil (α-pinene, α-and β-phellandrenes, α-terpinyl acetate, Δ3 carene and myrcene) [142–156]. Oxygenated diterpenes [157]. Terpenic hydrocarbon fraction dominance [158–160]. Polyphenols, flavonoids and essential oil from the fleshy cones [161–164] |
NONE | Anticancer constituents [140] and cytotoxicity against 5 cell lines [156, 157]. Antimicrobial properties and helpful in the prevention of aflatoxin contamination for many foods [144, 150, 153, 154, 156, 159, 160, 163–166]. Potent activity against Candida albicans[143]. Antiparasitic, nematicidal and antifouling constituents [155, 167] with tick repellent properties [168]. Antioxidative [152, 159, 160, 162, 164, 166] propensities. Remarkable effect in enhancing liver and kidney functions in CCl4 treated rats, and may thus be of therapeutic potential in treatment of hepatotoxicity and nephrotoxicity [169, 170]. Wound-healing effect [171]. Anticholinesterase activity [148, 166]. |
| 9 | Fagaceae Quercus coccifera L. (Decoction of galls [13]) Polyphenols and tannins (pedunculagin, castalagin, phillyraeoidin A, and acutissimin B) [164, 172, 173]. Sesquiterpenes [174]. |
NONE | Antioxidant and antibacterial properties [164]. Anti-lipoperoxidant properties-related gastroprotective and anti-ulcerogenic effects [173, 175]. Anthelmintic activity against parasitic nematodes [176]. |
| 10 | Geraniaceae Geranium graveolens L. (Decoction of leaves [13, 24]) Essential oils [177–182]. |
Dual inhibition of α-amylase and α-glucosidase in vitro, confirmed by highly significant and potent acute antihyperglycemic trends in starch-fed rats [49]. | Fumigant antitermitic activity [179]. Antioxidant activity [182]. Repellent effect against host-seeking nymphs of Ixodes ricinus[183] with antimicrobial qualities [180, 184, 185]. Mosquito repellent property [186]. Improves the immune cell count of cancer patients receiving chemotherapy and/or radiotherapy to prevent leucopenia and immune impairment that usually occurs during cancer therapy [187]. |
| 11 | Labiatae Ajuga iva L. (Schreber) (Decoction of herb [24]) 14,15-dihydroajugapitin [188] Ecdysones [189] and phytoecdysteroids [190, 191]. Iridoids, such as 8-O-acetylharpagide [192, 193]. |
Its phytoecdysteroids are beneficial for correcting the hyperglycaemia and preventing diabetic complications in liver, pancreas and kidneys in alloxan diabetic rats [191]. Acute and subchronic antihyperglycemic effects in normoglycemic and STZ-diabetic rats [194, 195]. | Hypolipidemic and hypocholesterolemic activities that may reduce intestinal cholesterol absorption [195–200] as well as antiatherogenic efficacy [199]. Vasorelaxant effect in rat aorta [196]. Reducing the oxidative stress in hyper-cholesterolemic rats by increasing the antioxidant enzymes activity [200]. Antioxidative benefits [201]. Inhibits crystallization of calcium oxalate in the urine [202]. Insecticidal properties [203, 204]. |
| 12 | Leguminoseae Alhagi maurorum Medicus (Decoction of roots [24]) Flavonoids (isorhamnetin-3-O-[-α-1-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside; 3′-O-methylorobol and quercetin 3-O-β-D-glucopyranoside) [205, 206]; cinnamic acids, phenolic acids, β-sitosterol and its glucoside [205, 207]. Three flavones (2-phenyl-1,4-benzopyrone derivatives) [208]. Polymethoxy substituted flavanenol [209] and triterpenoid lupeol [210]. Tannins and anthraquinones [211]. |
NONE | Antioxidative [206, 207, 212], anti-inflammatory [208, 210, 213, 214], antifungal [211] and anti-gastric ulcer [208, 214–216] activities. Antinociceptive [217] and antidiarrhoeal effects [218]. Spasmolytic and urether relaxing benefits [209, 219, 220]. ACE- and NADH oxidase-inhibitory activity [221]. Antibacterial activity [222]. Potent allelopathic activity [223]. |
| 13 | Poaceae Zea mays L. (Decoction of kernel [26]) Feruloylated oligosaccharide [224]. Flavone C-glycosides and sesquiterpenes [225, 226]. Phenolics (proto-catechuic acid mainly) [227]. Hydroxycinnamic acids [228]. Anthocyanins (cyanidin 3-glucoside and cyanidin-3-(6″-Qmalonylglucoside) [229]. |
In vitro inhibition of glycation [225]. Suppressed the progression of diabetic glomerular sclerosis in STZ- diabetic rat [230]. Decreasing blood glucose and protective action on the kidney and pancreas injury of STZ diabetic rats [231]. Inhibition of hyperglycaemia-relevant α-glucosidase but not α-amylase [227, 232]. Antidiabetic activity might be due PPAR activation [233]. Possible renoprotective role in diabetic nephropathy [229]. | Antioxidative [227, 234] action. Inhibited significantly the hypertension-relevant angiotensin I-converting enzyme [227]. Litholytic effects of herbal extracts on cystine urinary calculi [235]. Attenuating high-glucose-induced mesangial fibrosis and inflammation [229]. |
| 14 | Polygonaceae Rheum ribes Linn. (Decoction of roots [23]) Tannins and hydroxyanthracene derivatives (rhein, physcion, aloe-emodin, chrysophanol, physcion-8-O-glucoside, aloe-emodin-8-O-glucoside, sennoside A, rhaponticin) [236, 237], minerals [238], phenolics (pyrocatechol) and flavonoids (quercetin equivalents) [239]. |
Insulin releasing effects in healthy mice [240] and hypoglycemic activity in alloxan-diabetic animals [241]. Significant dose dependent dual inhibition of α-amylase and α-glucosidase in vitro[48]. | Antiviral [242] and antibacterial activities [243] with nutritional value [238]. Antioxidative potential [239, 244, 245]. Cytotoxic effects [246, 247] and anti-ulcer activity [248] as well as treating mild to moderate major depression disorders [249]. |
| 15 | Rhamnaceae Zizyphus spina- christi (L.) Desf. (Infusion of fruits, leaves, bark [27]) Saponin glycosides [250–252]. Flavonoids [253, 254]. Essential oil [255, 256]. Amino acid, carbohydrate and lipid composition [257, 258]. |
Insulinotropic hypoglycaemic effects in diabetic rats [251, 259, 260]. Antidiabetic effect in alloxan-diabetic dogs [261]. | Cytoprotective against liver aflatoxicosis [262, 263] and CCl4-fibrosis [264], vasoconstrictive effect in rat aorta [265]. Antiviral, antifungal and antibacterial activities [253, 266]. Its lipid fraction showed antimicrobial activity against Bacillus subtilis, Escherichia coli and Streptococcus pyogenes[257]. Its fruit and seed are good source of protein, mineral and energy foods [258]. Antinociceptive effect in mice and rats [267, 268]. Antidiarrhoeal benefits [269]. Mild dose dependent CNS depressant effect [270]. Molluscicidal property [94]. |
| 16 | Rosaceae Alchemilla vulgaris L.(Decoction of leaves, roots [28]) Polyphenols [271–273], flavonoids [274–276], tannins [277], gallic acid [278]. |
Weight reduction in obese subjects [279] despite lack of antihyper-glycemic activity in STZ diabetes mice [280]. | Antioxidative properties [271, 273, 275]. Mouth ulcers and wound-healing properties associated with pro-mitotic activity in epithelial cells and myofibroblasts [281, 282]. Activation of thyroid hormone synthesis [272]; antimicrobial with antiradical [277, 283, 284] as well as anxiolytic properties [285]. |
| 17 | Rosaceae Sarcopoterium spinosum (L.) Spach. [Syn Poterium spinosum L.] (Infusion, decoction of roots [13, 24, 27–29]) Triterpenoids [286]. α-tocopherol [287], proanthocyanidines [288]. |
Traditionally used in the treatment of diabetes [289]. Hypoglycaemic effect, evidenced in rabbits, with fluctuations [290–292]. Antidiabetic properties viz. insulinotropic, and insulin sensitizing [293, 294]. Starch blocker due to duality of inhibition of α-amylase and α-glucosidase [48]. | Action potential changes induced by its polyflavane on normal or hypoxic guinea pig myocardial strips [295]. Tumour inhibitory effects [296] and antioxidative properties [43]. Inhibited isoproterenol-induced lipolysis in 3T3-L1 adipocytes [293]. |
| 18 | Umbelliferae Ferula persica Wild. (Decoction of roots and resin [23, 30]) Sesquiterpenes, persicasulphides A, B and C and umbelliprenin [297–303]. Several coumarins (farnesiferol A, B, badrakemone, gummosin) and a new coumarin, farnesiferone A) [303, 304]. Sesquiterpene coumarin glycosides [305, 306]. Essential oil [307–310]. |
Did not demonstrate any α-amylase inhibitory activity, thus lacking on significant hypoglycaemic effects in normoglycemic and STZ-hyperglycaemic rats [46]. | Matrix metalloproteinases inhibition [297]. Umbelliprenin from F. persica roots inhibits the red pigment production in Serratia marcescens[299]. Antifungal activity [300]. Antioxidant, anti-inflammatory and lipoxygenase inhibitory properties and cancer preventive activity of umbelliferin [302, 303]. Farnesiferol A significantly inhibited the P-glycoprotein activity [305]. Antimicrobial effects [309]. Antigenotoxic activity via prevention of oxidative damage to DNA of rat lymphocytes [311] as well as cytotoxicity [312]. Umbelliprenin induced apoptosis in CLL cell lines [313]. |
| 19 | Urticaceae Urtica dioica L. (Decoction of herb [26]) Polyphenolics [314–316]. Flavonoids [317–319]. Essential oil [320, 321]. Lignan glucosides [322]. Carotenoids [323]. |
Antidiabetic effect on high fructose fed rats [324]. Alpha-amylase inhibitory activity [325]. Antihyperglycemia in animal models via reduction of intestinal glucose absorption [326] and enhancement of insulin secretion by Langerhans Isletes [327] or inhibition of α-glucosidase [328]. Hypoglycemic and protective activities of β-cells of Langerhans in hyperglycemic rats [329]. Proliferation of the beta cells of the diabetic rats [330]. Chronic exposure (24 h) to U. dioica significantly enhanced glucose uptake in L6-GLUT4myc myoblast cells [331]. Anti-hyperglycemic effect in STZ-rats via potentiating insulin activity, thus enhancing glucose utilization [332] and plausible activation of the human peroxisome proliferator-activated receptor in glucose homeostasis [333]. Protective effect on hepatocytes of STZ rats [334], neuro-protective effect in diabetes-induced loss of pyramidal cells [335]. | Antioxidant, antiradical, antimicrobial and antiulcerogenic effects [314–316, 336]. Antimicrobial activity [337]. Promotes learning performance in the brain of rats [338]. Immunostimulatory activity of the flavonoid fraction and intracellular killing activity of the isolated flavonoid glycosides suggesting that they could possibly be useful for treating patients suffering from neutrophil function deficiency and chronic granulomatous diseases [317]. Immunostimulatory activity [317, 318, 339]. Cardiovascular effects like hypotensive responses, through a vasorelaxing effect mediated by the release of endothelial NO and the opening of potassium channels, and through a negative inotropic action [340]. Beneficial for treatment of benign prostatic hyperplasia [341]. Platelet inhibitory activity [342]. Hepatoprotective in CCl4 treated rats [343] and protective effect on the liver in hepatic ischemia-reperfusion-injured rats [344]. Antifungal role [266]. Regulation of inflammatory gene expression [345]. Aromatase inhibitory activity [346]. |
| 20 | Zygophyllaceae Peganum harmala Linn. (Decoction of seeds [30]) Flavonoid glycosides [347] and major β-carboline alkaloids (Harmaline, harmine, harmalol, harmol and tetrahydroharmine) [348–350]. |
Antidiabetic activity in C57BL/KsJ-db/db mice [351]. | Antiplasmodial and vasorelaxant benefits [352]. Antileishmanial [353, 354], analgesic [355], anti-inflammatory [356], and antiplatelet activities [357]. Insecticidal activity [358–360], antibacterial, antifungal and antiviral propensities [361–366]. ACE-inhibitory activity [367, 368] and inhibition of human monoamine oxidase (MAO) [369]. In vitro cell-toxicity on cancerous cell-lines [370–372] as well as herbicidal activity [373]. |
Six of the enlisted plants, namely Ajuga iva, Cleoma droserifolia, Urtica dioica, Sarco-poterium spinosum, Rheum ribes, Zea mays, and Geranium graveolens exhibited hypoglycemic activity in STZ and/ or alloxan diabetic animal models via inhibition of α-amylase and/or α-glucosidase or glucose absorption as plausible in vitro action mechanisms among many others (Table 1). On the other hand, neither in vivo nor in vitro bioactivity could be detected in antidiabetes pharmacology appraisals with Peganum harmala or Ferula persica. These findings strongly negate the claimed ethnotherapeutic uses promoted for these plant species. As for Varthemia iphionoides and Zizyphus spina-christi, the lack of complementary in vivo or in vitro testing necessitates further experimental design and verification on future accounts [56].
The hypoglycaemic properties of several classes of phytochemicals, including alkaloids, flavonoids, glycosides, glycolipids, polysaccharides, peptidoglycans, carbohydrates, amino acids, saponins, and terpenoids, have been exhaustively reported in the literature [37, 38, 57–60]. Additionally, it is well-accepted that certain herbs may alleviate considerably evident hyperglycaemia in clinical trials with well-characterised mechanisms of action [61, 62]; their test results, however, are subject to multiple factors. Among which, different parts of an herb may have different ingredient profiles or different extraction methodologies may yield diverse active ingredients. In addition, each plant species contains multiple compounds, only a few of which may be therapeutically effective either alone or acting in synergism [63, 64]. Hence, an urgent need exists for research proceedings in identifying the phytoconstituent(s) directly associated with hypoglycaemic/antihyperglycemic bioactivity with equivalent assessments of the intra- and inter-species variations in secondary metabolites. Future research directives may also incur extensive clinical population-based studies for selected species. Moreover, investigating the combination formulations of natural products with synthetic drugs of complementary pharmacologies may determine the optimal and cost-effective therapies. Additionally, as herb-drug interactions in diabetic treatments/supplements have not been well-evidenced or documented [65], it is warranted that follow-up studies on their long-term side-effects be conducted. Subsequently, this may invite the potential development of food products fortified with clinically safe and effective plant extracts and possible downstream planning and incorporation into diabetic diets [66].
In conclusion, the reported findings, uniquely indicating the potential use of medicinal plants as antidiabetic agents, are among the very few that explored Jordanian flora from semi-arid and arid bioclimatic areas for pharmaceutical leads. Comprehensive research aiming at fully exploiting any of the promising species from the Jordanian flora, either alone or in combination with existing therapies, might lead to discovery of new avenues for medicinal plants/natural compounds in reducing the major public health impact of diabetes. Characterization of molecular targets and elucidation of relevant mechanisms of action also stand for another set of plausible requirements. Then, despite modern medicine accessibility, traditional medicine can be propagated as a viable health alternative.
Footnotes
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- 1.Moore H, Summerbell C, Hooper L, Cruickshank K, Vyas A, Johnstone P, Ashton V, Kopelman P. Dietary advice for treatment of type 2 diabetes mellitus in adults. The Cochrane database of systemic reviews. 2004. p. CD004097. http://www.ncbi.nlm.nih.gov/pubmed/15106237. [DOI] [PubMed]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. http://dx.doi.org/10.2337/diacare27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Publications. 2011. Fact Sheet No 312. [Google Scholar]
- 4.Dababneh F, Asad M, Abu Diab A. Mortality data in Jordan 2007. 1. Ministry of Health; Jordan: 2010. Information and Research Newsletter. [Google Scholar]
- 5.International Diabetes Federation (IDF 2009) IDF Diabetes Atlas. Prevalence estimates of diabetes mellitus (DM), 2010 – MENA. 4th Edition. [Accessed on 10 April 2011]. IDF [Online]. http://www.diabetesatlas.org/content/prevalence-estimates-diabetes-mellitus-dm-2010.
- 6.Bulatova NR, Yousef AF, AbuRuz SM. Antiplatelet therapy for primary and secondary prevention in Jordanian patients with diabetes mellitus. Thromb Res. 2007;121:43–50. doi: 10.1016/j.thromres.2007.03.006. http://dx.doi.org/10.1016/j.thromres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Ajlouni K, Khader YS, Batieha A, Ajlouni H, El-Khateeb M. An increase of diabetes mellitus in Jordan over 10 years. J Diabetes Complicat. 2008;22:317–324. doi: 10.1016/j.jdiacomp.2007.01.004. http://dx.doi.org/10.1016/j.jdiacomp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Zindah M, Belbeisi A, Walke H, Mokdad AH. Obesity and diabetes in Jordan: Findings from the behavioral risk factor surveillance system, 2004. Prev Chronic Dis. 2008;5:1–8. http://www.ncbi.nlm.nih.gov/pubmed/18082006/ [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Eisawi D, El-Oqlah A, Al-Khader IA, editors. United Nations Environmental Program. Al-Rai Commercial Press; Amman: 2000. Jordan Country Study on Biological Diversity; pp. 7–11. [Google Scholar]
- 10.Al-Eisawi DM. List of Jordan vascular plants. Mitt Bot Staatssamml Münch. 1982;18:79–182. [Google Scholar]
- 11.Al-Eisawi DM. Vegetation of Jordan. UNESCO- Regional Office for Science and Technology for the Arab States; Cairo: 1996. p. 266. [Google Scholar]
- 12.Al-Eisawi DMH. Field guide to wild flowers of Jordan and neighbouring countries. Commercial Press, Jordan Press foundation Al-Rai; Amman: 1998. pp. 4–8. [Google Scholar]
- 13.Al-Khalil S. A survey of plants used in Jordanian traditional medicine. Int J Pharmacog. 1995;33:317–323. [Google Scholar]
- 14.Azaizeh H, Fulder S, Khalil K, Said O. Ethnobotanical knowledge of local Arab practitioners in the Middle Eastern region. Fitoterapia. 2003;74:98–108. doi: 10.1016/s0367-326x(02)00285-x. http://dx.doi.org/10.1016/S0367-326X(02)00285-X. [DOI] [PubMed] [Google Scholar]
- 15.Bonet MA, Valles J. Use of non-crop food vascular plants in Montseny biosphere reserve (Catalonia, Iberian Peninsula) Int J Food Sci Nutr. 2002;53:225–248. doi: 10.1080/09637480220132841. http://dx.doi.org/10.1080/09637480220132841. [DOI] [PubMed] [Google Scholar]
- 16.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. http://dx.doi.org/10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 17.Goldman P. Herbal medicines today and the roots of modern pharmacology. Annals Int Med. 2001;135:594–600. doi: 10.7326/0003-4819-135-8_part_1-200110160-00010. http://dx.doi.org/10.7326/0003-4819-135-8_Part_1-200110160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Phillipson JD. Phytochemistry and medicinal plants. Phytochemistry. 2001;56:237–243. doi: 10.1016/s0031-9422(00)00456-8. http://dx.doi.org/10.1016/S0031-9422(00)00456-8. [DOI] [PubMed] [Google Scholar]
- 19.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2001. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. http://dx.doi.org/10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Rabia A. Herbs as a food and medicine source in Palestine. Asian Pac J Cancer Prev. 2005;6:404–407. http://www.ncbi.nlm.nih.gov/pubmed/16236008. [PubMed] [Google Scholar]
- 21.Wazaify M, Afifi FU, El-Khateeb M, Ajlouni K. Complementary and alternative medicine use among Jordanian diabetes patients. Complement Ther Clin Pract. 2011;17:71–75. doi: 10.1016/j.ctcp.2011.02.002. http://dx.doi.org/10.1016/j.ctcp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Al-Aboudi A, Afifi FU. Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm Biol. 2011;49:221–239. doi: 10.3109/13880209.2010.501802. http://dx.doi.org/10.3109/13880209.2010.501802. [DOI] [PubMed] [Google Scholar]
- 23.Abu Irmaileh B, Afifi F. Treatment with medicinal plants in Jordan. Dirasat. 2000;27:53–74. [Google Scholar]
- 24.Oran SA, Al-Eisawi DM. Check list of medicinal plants in Jordan. Dirasat. 1998;25:84–112. [Google Scholar]
- 25.Hudaib M, Mohammad M, Bustanji Y, Tayyem R, Yousef M, Abuirjeie M, Aburjai T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J Ethnopharmacol. 2008;120:63–71. doi: 10.1016/j.jep.2008.07.031. http://dx.doi.org/10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Otoom SA, Al-Safi SA, Kerem ZK, Alkofahi A. The use of medicinal herbs by diabetic Jordanian patients. J Herb Pharmacother. 2006;6:31–41. http://dx.doi.org/10.1080/J157v06n02_03. [PubMed] [Google Scholar]
- 27.Said O, Khalil K, Fulder S, Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ehnopharmacol. 2002;83:251–265. doi: 10.1016/s0378-8741(02)00253-2. http://dx.doi.org/10.1016/S0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 28.Aburjai T, Hudaib M, Tayyem R. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Height region. J Ethnopharmacol. 2007;110:294–304. doi: 10.1016/j.jep.2006.09.031. http://dx.doi.org/10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Dafni A, Yaniv Z, Palevitch Z. Ethnobotanical survey of medicinal plants in northern Israel. J Ethnopharmacol. 1984;10:295–310. doi: 10.1016/0378-8741(84)90017-5. http://dx.doi.org/10.1016/0378-8741(84)90017-5. [DOI] [PubMed] [Google Scholar]
- 30.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharmacol. 2000;72:191–205. doi: 10.1016/s0378-8741(00)00230-0. http://dx.doi.org/10.1016/S0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 31.Camejo-Rodrigues J, Ascensao L, Bonet MA, Valles J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of “Serra de Sao Mamede” (Portugal) J Ethnopharmacol. 2003;89:199–209. doi: 10.1016/s0378-8741(03)00270-8. http://dx.doi.org/10.1016/S0378-8741(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 32.Jeambey Z, Johns T, Talhouk S, Batal M. Perceived health and medicinal properties of six species of wild edible plants in north-east Lebanon. Pub Health Nutr. 2009;12:1902–1911. doi: 10.1017/S1368980009004832. http://dx.doi.org/10.1017/S1368980009004832. [DOI] [PubMed] [Google Scholar]
- 33.Afifi-Yazar FU, Kasabri V, Abu-Dahab R. Medicinal plants from Jordan in the treatment of diabetes: Traditional uses vs. in vitro and in vivo evaluations; Mini-review. Planta Med. 2011;77:1210–1220. doi: 10.1055/s-0031-1279983. http://dx.doi.org/10.1055/s-0031-1279983. [DOI] [PubMed] [Google Scholar]
- 34.Aly HF, Ebrahim ME, Metawaa HM, Hosni EAA, Ebrahim FM. In vitro and in vivo evaluation of the antidiabetic effect of different extracts of Nepeta cataria in streptozotocin induced diabetic rats. J Am Sci. 2010;6:364–386. [Google Scholar]
- 35.Afifi FU, Abu Irmaileh B. Herbal medicine in Jordan with special emphasis on less commonly used medicinal herbs. J Ethnopharmacol. 2000;72:101–110. doi: 10.1016/s0378-8741(00)00215-4. http://dx.doi.org/10.1016/S0378-8741(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 36.Abu Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;89:193–197. doi: 10.1016/s0378-8741(03)00283-6. http://dx.doi.org/10.1016/S0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leeds from Indian medicinal plants with hypoglycaemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. http://dx.doi.org/10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Prabhakar PK, Doble M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr Diabetes Rev. 2008;4:291–308. doi: 10.2174/157339908786241124. http://dx.doi.org/10.2174/157339908786241124. [DOI] [PubMed] [Google Scholar]
- 39.Hui H, Tang G, Go VLW. Hypoglycaemic herbs and their action mechanisms. Chinese Med. 2009;4:11–21. doi: 10.1186/1749-8546-4-11. http://dx.doi.org/10.1186/1749-8546-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gharaibeh MN, Elayan HH, Salhab AS. Hypoglycaemic effects of Teucrium polium. J Ethnopharmacol. 1988;24:93–99. doi: 10.1016/0378-8741(88)90139-0. http://dx.doi.org/10.1016/0378-8741(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 41.Nusier MK, Bataineh HN, Bataineh ZM, Daradka HM. Effects of Ballota nigra on glucose and insulin in alloxan-diabetic albino rats. Neuro Endocrinol Lett. 2007;28:470–472. http://www.ncbi.nlm.nih.gov/pubmed/17627273. [PubMed] [Google Scholar]
- 42.Irshaid F, Mansi K, Aburjai T. Antidiabetic effect of essential oil from Artemisia sieberi growing in Jordan in normal and alloxan induced diabetic rats. Pak J Biol Sci. 2010;13:423–430. doi: 10.3923/pjbs.2010.423.430. http://dx.doi.org/10.3923/pjbs.2010.423.430. [DOI] [PubMed] [Google Scholar]
- 43.Al-Mustafa AH, Al-Thunibat OY. Antioxidant capacity of some Jordanian medicinal plants used traditionally for the treatment of diabetes. Pak J Biol Sci. 2008;1:351–358. doi: 10.3923/pjbs.2008.351.358. http://www.ncbi.nlm.nih.gov/pubmed/18817155. [DOI] [PubMed] [Google Scholar]
- 44.Qa’dan F, Verspohl EJ, Nahrstedt A, Petereit F, Matalka KZ. Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo. J Ethnopharmacol. 2009;124:224–227. doi: 10.1016/j.jep.2009.04.023. http://dx.doi.org/10.1016/j.jep.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Zaiton AS. Anti-diabetic activity of Ferula asafoetida extract in normal and alloxan-induced diabetic rats. Pak J Biol Sci. 2010;13:97–100. doi: 10.3923/pjbs.2010.97.100. http://dx.doi.org/10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 46.Hamdan II, Afifi FU. Studies on the in vitro and in vivo hypoglycaemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. J Ethnopharmacol. 2004;93:117–121. doi: 10.1016/j.jep.2004.03.033. http://dx.doi.org/10.1016/j.jep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Afifi F, Al-Khalidi B, Khalil E. Studies on the in vivo hypoglycaemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J Ethnopharmacol. 2005;100:314–318. doi: 10.1016/j.jep.2005.03.016. http://dx.doi.org/10.1016/j.jep.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Kasabri V, Afifi FU, Hamdan I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. J Ethnopharmacol. 2011a;133:888–896. doi: 10.1016/j.jep.2010.11.025. http://dx.doi.org/10.1016/j.jep.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Kasabri V, Afifi FU, Hamdan I. Evaluation of the acute antihyperglycemic effects of four selected indigenous plants from Jordan used in traditional medicine. Pharm Biol. 2011b;49:687–695. doi: 10.3109/13880209.2010.539619. http://dx.doi.org/10.3109/13880209.2010.539619. [DOI] [PubMed] [Google Scholar]
- 50.Rauter AP, Martins A, Borges C, Mota-Filipe H, Pinto R, Sepodes B, Justino J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother Res. 2010;24:S133–S138. doi: 10.1002/ptr.3017. http://dx.doi.org/10.1002/ptr.3017. [DOI] [PubMed] [Google Scholar]
- 51.Yazdanparast R, Ardestani A, Jamshini S. Experimental diabetes treated with Achillea santolina: Effects on pancreatic oxidative parameters. J Ethnopharmacol. 2007;112:13–18. doi: 10.1016/j.jep.2007.01.030. http://dx.doi.org/10.1016/j.jep.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 52.Srivatsan R, Das S, Gadde R, Manoj-Kumar K, Taduri S, Rao N, Ramesh B, Baharani A, Shah K, Kamireddy SC, Priyatham G, Balakumaran TA, Balakumaran SS, Kamath A, Rao A. Antioxidants and lipid peroxidation status in diabetic patients with and without complications. Arch Iran Med. 2009;12:121–127. [PubMed] [Google Scholar]
- 53.Ramalho-Santos J, Amaral S, Oliveira PJ. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4:46–54. doi: 10.2174/157339908783502398. http://dx.doi.org/10.2174/157339908783502398. [DOI] [PubMed] [Google Scholar]
- 54.Chung MJ, Cho SY, Bhuiyan MJ, Kim KH, Lee SJ. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. 2010;104:180–188. doi: 10.1017/S0007114510001765. http://dx.doi.org/10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]
- 55.Basak SS, Candan F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehn. essential oil. J Iran Chem Soc. 2010;7:216–226. http://dx.doi.org/10.1007/BF03245882. [Google Scholar]
- 56.Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. http://dx.doi.org/10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 57.Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diab Rev. 2010;6:247–254. doi: 10.2174/157339910791658826. http://dx.doi.org/10.2174/157339910791658826. [DOI] [PubMed] [Google Scholar]
- 58.Qi LW, Liu EH, Chu C, Peng YB, Cai HX, Li P. Anti-diabetic agents from natural products- an update from 2004–2009. Curr Top Med Chem. 2010;10:434–457. doi: 10.2174/156802610790980620. http://dx.doi.org/10.2174/156802610790980620. [DOI] [PubMed] [Google Scholar]
- 59.Bedekar A, Shah K, Koffas M. Natural products for type II diabetes treatment. Adv Appl Microbiol. 2010;71:21–73. doi: 10.1016/S0065-2164(10)71002-9. http://dx.doi.org/10.1016/S0065-2164(10)71002-9. [DOI] [PubMed] [Google Scholar]
- 60.Dinda B, Debnath S, Mohanta BC, Harigaya Y. Naturally occurring triterpenoid saponins. Chem Biodivers. 2010;7:2327–2580. doi: 10.1002/cbdv.200800070. http://dx.doi.org/10.1002/cbdv.200800070. [DOI] [PubMed] [Google Scholar]
- 61.Yin J, Zhang H, Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr Metab Immun Disord Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. http://dx.doi.org/10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuriyan R, Rajendran R, Bantwal G, Kurpad AV. Effect of supplementation of Coccinia cordifolia extract on newly defected diabetic patients. Diabetes Care. 2008;31:216–220. doi: 10.2337/dc07-1591. http://www.ncbi.nlm.nih.gov/pubmed/18000183. [DOI] [PubMed] [Google Scholar]
- 63.Angelova N, Kong HW, van der Heijden R, Yang SY, Choi YM, Kim HK, Wang M, Hankemeir, van der Greef J, Xu G, Verpoorte R. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. http://dx.doi.org/10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 64.Shan JJ, Rodgers K, Lai CT, Sutherland SK. Challenges in natural health product research: The importance of standardization. Proc West Pharmacol Soc. 2007;50:24–30. http://www.ncbi.nlm.nih.gov/pubmed/18605225. [PubMed] [Google Scholar]
- 65.Xu H, Williams KM, Liauw WS, Murray M, Day RO, McLachlan AJ. Effects of St John’s wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br J Pharmacol. 2008;153:1579–1586. doi: 10.1038/sj.bjp.0707685. http://dx.doi.org/10.1038/sj.bjp.0707685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mentreddy SR. Medicinal plant species with potential antidiabetic properties. J Sci Food Agric. 2007;87:743–750. http://dx.doi.org/10.1002/jsfa.2811. [Google Scholar]
- 67.Shalaby AF, Richter G. Chromatographic investigation of the essential oil of Achillea fragrantissima. J Pharm Sci. 1964;53:1502–1505. doi: 10.1002/jps.2600531215. http://dx.doi.org/10.1002/jps.2600531215. [DOI] [PubMed] [Google Scholar]
- 68.Shalaby AF, Steinegger E. [The phytochemical study Achillea fragrantissima (Forsk.) Sch. Bip] Pharm Acta Helv. 1964;39:756–761. http://www.ncbi.nlm.nih.gov/pubmed/14347174. [PubMed] [Google Scholar]
- 69.Shalaby AF, Tsingarida K, Steinegger E. [On the flavonoids from Achillea fragrantissima (Forsk.) Sch. Bip] Pharm Acta Helv. 1965;40:19–24. http://www.ncbi.nlm.nih.gov/pubmed/5858665. [PubMed] [Google Scholar]
- 70.el-Shazly AM, Hafez SS, Wink M. Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) from Egypt. Pharmazie. 2004;59:226–230. http://www.ncbi.nlm.nih.gov/pubmed/15074599. [PubMed] [Google Scholar]
- 71.Ageel AM, Mossa JS, Al-Yahya MA, Al-Said MS, Tariq M. Experimental studies on antirheumatic crude drugs used in Saudi traditional medicine. Drugs Exp Clin Res. 1989;15:369–372. http://www.ncbi.nlm.nih.gov/pubmed/2598777. [PubMed] [Google Scholar]
- 72.Barel S, Segal R, Yashphe J. The antimicrobial activity of the essential oil from Achillea fragrantissima. J Ethnopharmacol. 1991;33:187–191. doi: 10.1016/0378-8741(91)90177-f. http://dx.doi.org/10.1016/0378-8741(91)90177-F. [DOI] [PubMed] [Google Scholar]
- 73.Omar SS. Inhibitory effect of nisin and some of plant extracts against growth of Cronobacter sakazakii in reconstituted infant milk formula. Health Env Res Online. 2011;66:389–393. [Google Scholar]
- 74.Soltan MM, Zaki AK. Antiviral screening of forty-two Egyptian medicinal plants. J Ethnopharmacol. 2009;126:102–107. doi: 10.1016/j.jep.2009.08.001. http://dx.doi.org/10.1016/j.jep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Mustafa EH, Abu Zarga M, Abdalla S. Effects of cirsiliol, a flavone isolated from Achillea fragrantissima, on rat isolated ileum. Gen Pharmacol. 1992;23:555–560. doi: 10.1016/0306-3623(92)90127-6. http://dx.doi.org/10.1016/0306-3623(92)90127-6. [DOI] [PubMed] [Google Scholar]
- 76.Elmann A, Mordechay S, Erlank H, Telerman A, Rindner M, Ofir R. Anti-Neuroinflammatory effects of the extract of Achillea fragrantissima. BMC Complement Altern Med. 2011;11:98. doi: 10.1186/1472-6882-11-98. http://dx.doi.org/10.1186/1472-6882-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sathiyamoorthy P, Lugasi-Evgi H, Schlesinger P, Kedar J, Gopas J, Pollack Y, Golan-Goldhirsh A. Screening for cytotoxic and antimalarial activities in desert plants of the Negev and Bedouin market plant products. Pharm Biol. 1999;37:188–195. http://dx.doi.org/10.1076/phbi.37.3.188.6298. [Google Scholar]
- 78.Sathiyamoorthy P, Lugasi-Evgi H, Van-Damme P, Abu-Rabia A, Gopas J, Golan-Goldhrish A. Larvicidal activity in desert plants of the Negev and Bedouin market plant products. Pharm Biol. 1997;35:265–273. http://dx.doi.org/10.1076/phbi.35.4.265.13314. [Google Scholar]
- 79.Khafagy SM, Tosson S. Crystallographic, optical and chromatographic studies of judaicin, bitter principle of Artemisia judaica L. Planta Med. 1968;16:446–449. doi: 10.1055/s-0028-1099933. http://dx.doi.org/10.1055/s-0028-1099933. [DOI] [PubMed] [Google Scholar]
- 80.Urmanova FF, Komilov KM. Flavonoids of Achillea santolina. Chem Nat Comp. 1999;35:214. http://dx.doi.org/10.1007/BF02234939. [Google Scholar]
- 81.Ahmad VU, Khan MA, Baqal FT, Tareen RB. Santoflavone, A 5-deoxyflavonoid from Achillea santolina. Phytochemistry. 1995;38:1305–1307. http://dx.doi.org/10.1016/0031-9422(94)00785-R. [Google Scholar]
- 82.Balboul BAAA, Ahmed AA, Otsuka H, Bando M, Kido M, Takeda Y. A guaianolide and a germacranolide from Achillea santolina. Phytochemistry. 1997;46:1045–1049. http://dx.doi.org/10.1016/S0031-9422(97)00389-0. [Google Scholar]
- 83.Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. http://dx.doi.org/10.1016/j.foodchem.2006.10.066. [Google Scholar]
- 84.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Arch Med Sci. 2008;4:285–292. [Google Scholar]
- 85.Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10:9. doi: 10.1186/1472-6882-10-9. http://dx.doi.org/10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khalil A, Dababneh BF, Al-Gabbiesh AH. Antimicrobial activity against pathogenic microorganisms by extracts from herbal Jordanian plants. J Food Agric Env. 2009;7:103–106. [Google Scholar]
- 87.Zaringhalam J, Akbari A, Tekieh E, Manaheji H, Rezazadeh S. Achillea santolina reduces serum interleukin-6 level and hyperalgesia during complete Freund’s adjuvant-induced inflammation in male Wistar rats. Zhong Xi Jie He Xue Bao. 2010;8:1180–1189. doi: 10.3736/jcim20101211. http://dx.doi.org/10.3736/jcim20101211. [DOI] [PubMed] [Google Scholar]
- 88.Abdallah OM, Ali AA, Itokawa H. Cytotoxic activity of sesquiterpene lactones, isolated from Ambrosia maritima. Pharmazie. 1991;46:472. http://www.ncbi.nlm.nih.gov/pubmed/1763132. [PubMed] [Google Scholar]
- 89.Abdelgaleil SAM, Badawy MEI, Suganuma T, Kitahara K. Antifungal and biochemical effects of pseudoguaianolide sesquiterpenes isolated from Ambrosia maritima L. Afr J Micr Res. 2011;5:3385–3393. [Google Scholar]
- 90.Mahmoud AA, Ahmed AA, El Bassuony AA. A new chlorosesquiterpene lactone from Ambrosia maritima. Fitoterapia. 1999;70:575–578. http://dx.doi.org/10.1016/S0367-326X(99)00091-X. [Google Scholar]
- 91.AbouZid S, Orihara Y. Biosynthesis of polyacetylenes in Ambrosia maritima hairy roots. Planta Med. 2007;73:1327–1329. doi: 10.1055/s-2007-981616. http://dx.doi.org/10.1055/s-2007-981616. [DOI] [PubMed] [Google Scholar]
- 92.Abdel-Hamid AZ. Development of bait formulations for control of intermediate hosts of African schistosome species. J Appl Toxicol. 1997;17:391–395. doi: 10.1002/(sici)1099-1263(199711/12)17:6<391::aid-jat456>3.0.co;2-i. http://dx.doi.org/10.1002/(SICI)1099-1263(199711/12)17:6<391::AID-JAT456>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 93.El-Ansary A, Mohamed AM, Mahmoud SS, El-Bardicy S. On the pathogenicity of attenuated Schistosoma mansoni cercariae released from metabolically disturbed Biomphalaria alexandrina. J Egypt Soc Parasitol. 2003;33:777–794. [PubMed] [Google Scholar]
- 94.Shams KA, Abou-Setta LM, Radwan HM. Molluscicidal activity and screening of forty Egyptian medicinal plants and determination of the active fractions. Asian J Chem. 2012;24:3548–3552. [Google Scholar]
- 95.Greerts S, Van Blerk K, Triest L. Effect of Ambrosia maritima on Anopheles stephensi and Aedes aegypti. J Ethnopharmacol. 1994;42:7–11. doi: 10.1016/0378-8741(94)90016-7. http://dx.doi.org/10.1016/0378-8741(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 96.Greerts S, Belot J, Sabbe F, Triest L, Sidhom M. Ambrosia maritima: effects on molluscs and non-target organisms. J Ethnopharmacol. 1991;33:1–12. doi: 10.1016/0378-8741(91)90153-5. http://dx.doi.org/10.1016/0378-8741(91)90153-5. [DOI] [PubMed] [Google Scholar]
- 97.El-Ela NA, Talha M, Al-Aziz AA. Response and effect of two plant crude extracts on mosquito larvae Culex pipiens. J Egypt Public Health Assoc. 1998;73:649–665. http://www.ncbi.nlm.nih.gov/pubmed/17217029. [PubMed] [Google Scholar]
- 98.Barakat R, Farghaly A, el-Sawy MF, Soliman NK, Duncan J, Miller FD. An epidemiological assessment of Ambrosia maritima on the transmission of schistosomiasis in the Egyptian Nile Delta. Trop Med Parasitol. 1993;44:181–186. http://www.ncbi.nlm.nih.gov/pubmed/8256093. [PubMed] [Google Scholar]
- 99.Ahmed MB, Khater MR. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen- induced liver damage. J Ethnopharmacol. 2001;75:169–174. doi: 10.1016/s0378-8741(00)00400-1. http://dx.doi.org/10.1016/S0378-8741(00)00400-1. [DOI] [PubMed] [Google Scholar]
- 100.Saleh MM, Rizk AM. Flavonoids and coumarins of Anthemis pseudocotiola. Planta Med. 1974;25:60–62. doi: 10.1055/s-0028-1097913. http://dx.doi.org/10.1055/s-0028-1097913. [DOI] [PubMed] [Google Scholar]
- 101.Kilic O, Kocak A, Bagci E. Composition of the volatile oils of two Anthemis L. taxa from Turkey. Z Naturforsch C. 2011;66:535–540. doi: 10.1515/znc-2011-11-1201. http://dx.doi.org/10.5560/ZNC.2011.66c0535. [DOI] [PubMed] [Google Scholar]
- 102.El-Ela MA, Jakupovic J, Bohlmann F, Ahmed AA, Seif El-Din A, Khafagi S, Sabri N, El- Ghazouly M. Seco-germacranolides from Anthemis pseudocotula. Phytochemistry. 1990;29:2704–2706. http://dx.doi.org/10.1016/0031-9422(90)85221-Z. [Google Scholar]
- 103.Vukovic I, Vujisic L, Vajs V, Tesevic V, Macura S, Janackovic P, Milosavljevic S. Sesquiterpene lactones from the aerial parts of Anthemis arvensis L. Biochem Syst Ecol. 2006;34:303–309. http://dx.doi.org/10.1016/j.bse.2005.09.007. [Google Scholar]
- 104.Al-Dabbas MM, Hashinaga F, Abdelgaleil SA, Suganuma T, Akiyama K, Hayashi H. Antibacterial activity of an eudesmane sesquiterpene isolated from common Varthemia, Varthemia iphionoides. J Ethnopharmacol. 2005;97:237–240. doi: 10.1016/j.jep.2004.11.007. http://dx.doi.org/10.1016/j.jep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Al-Dabbas MM, Suganuma T, Kitahara K, Hou DX, Fujii M. Cytotoxic, antioxidant and antibacterial activities of Varthemia iphionoides Boiss. extracts. J Ethnopharmacol. 2006;108:287–293. doi: 10.1016/j.jep.2006.05.006. http://dx.doi.org/10.1016/j.jep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Afifi FU, Al-Khalil S, Abdul-Haq BK, Mahasneh A, Al-Eisawi DM, Sharaf M, Wong LK, Schiff PL., Jr Antifungal flavonoids from Varthemia iphionoides. Phytother Res. 1991;5:173–175. http://dx.doi.org/10.1002/ptr.2650050407. [Google Scholar]
- 107.Al-Dabbas MM, Al-Ismail K, Abu-Taleb R, Hashimoto F, Rabah IO, Kitahara K, Fujita K, Suganuma T. Chemistry and antiproliferative activities of 3-methoxyflavones isolated from Varthemia iphionoides. Chem Nat Comp. 2011;47:17–21. http://dx.doi.org/10.1007/s10600-011-9821-8. [Google Scholar]
- 108.Avato P, Raffo F, Aldouri NA, Vartanian ST. Essential oils of Varthemia iphionoides from Jordan. Flav Frag J. 2004;19:559–561. http://dx.doi.org/10.1002/ffj.1351. [Google Scholar]
- 109.Tamir H, Satovic Z, Gorelick J, Gorelick J, Danin A, Fischer R, Chaimovitsh D, Dudai N. Intraspecific variation of Chiliadenus iphionoides essential oil in Israel. Chem Biodivers. 2011;8:1065–1082. doi: 10.1002/cbdv.201000335. http://dx.doi.org/10.1002/cbdv.201000335. [DOI] [PubMed] [Google Scholar]
- 110.Al-Dabbas MM, Kitahara K, Suganuma T, Hashimoto F, Tadera K. Antioxidant and alpha-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci Biotechnol Biochem. 2006;70:2178–2184. doi: 10.1271/bbb.60132. http://dx.doi.org/10.1271/bbb.60132. [DOI] [PubMed] [Google Scholar]
- 111.Afifi F, Saket M, Jaghabir M, Al-Eisawi D. Effect of Varthemia iphionoides on blood glucose level of normal rats and rats with streptozocin-induced diabetes mellitus. Curr Ther Res. 1997;58:888–892. http://dx.doi.org/10.1016/S0011-393X(97)80055-0. [Google Scholar]
- 112.Gorelick J, Kitron A, Pen S, Rosenzweig T, Madar Z. Anti-diabetic activity of Chiliadenus iphionoides. J Ethnopharmacol. 2011;137:1245–1249. doi: 10.1016/j.jep.2011.07.051. http://dx.doi.org/10.1016/j.jep.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 113.Afifi F, Aburjai T. Antiplatelet activity of Varthenmia iphionoides. Fitoterapia. 2004;75:629–633. doi: 10.1016/j.fitote.2004.04.014. http://dx.doi.org/10.1016/j.fitote.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 114.Al-Abbas MM, Al-Ismail K, Al-Qudah YH. Antioxidant activity of different extracts from Varthemia iphionoides. Rivista Italiana delle Sostanze Grasse. 2010;87:243–249. [Google Scholar]
- 115.Harlev E, Nevo E, Lansky EP, Lansky S, Bishayee A. Anticancer attributes of desert plants: a review. Anticancer Drugs. 2012;23:255–271. doi: 10.1097/CAD.0b013e32834f968c. http://dx.doi.iorg/10.1097/CAD.0b013e32834f968c. [DOI] [PubMed] [Google Scholar]
- 116.Abu-Hijleh A, Jarrar N, Adwan K. Antibacterial activity of common Varthemia, Varthemia iphionoides ethanol extract alone and in combination with cefotaxine. Adv Biol Res. 2009;3:144–147. [Google Scholar]
- 117.Fushiya S, Kishi Y, Hattori K, Batkhuu J, Takano F, Singab AN, Okuyama T. Flavonoids from Cleome droserifolia suppress NO production in activated macrophages in vitro. Planta Med. 1999;65:404–407. doi: 10.1055/s-1999-14084. http://dx.doi.org/10.1055/s-1999-14084. [DOI] [PubMed] [Google Scholar]
- 118.El-Askary HI. Terpenoids from Cleome droserifolia (Forssk.) Del. Molecules. 2005;31:971–977. doi: 10.3390/10080971. http://www.ncbi.nlm.nih.gov/pubmed/18007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdel-Kader MS, Alqasoumi SI, Al-Taweel AM. Hepatoprotective constituents from Cleome droserifolia. Chem Pharm Bull (Tokyo) 2009;57:620–624. doi: 10.1248/cpb.57.620. http://dx.doi.org/10.1248/cpb.57.620. [DOI] [PubMed] [Google Scholar]
- 120.Aboushoer MI, Fathy HM, Abdel-Kader MS, Goetz G, Omar AA. Terpenes and flavonoids from an Egyptian collection of Cleome droserifolia. Nat Prod Res. 2010;24:687–696. doi: 10.1080/14786410903292433. http://dx.doi.org/10.1080/14786410903292433. [DOI] [PubMed] [Google Scholar]
- 121.El Naggar EMB, Bartošíková L, Žemlička M, Švajdlenka E, Rabišková M, Strnadová V, Nečas J. Antidiabetic Effect of Cleome droserifolia Aerial Parts: Lipid peroxidation-induced oxidative stress in diabetic rats. Acta Vet. Brno. 2005;74:347. http://dx.doi.org/10.2754/avb200574030347. [Google Scholar]
- 122.Ezzat SM, Motaal AA. Isolation of new cytotoxic metabolites from Cleome droserifolia growing in Egypt. Z Naturforsch C. 2012;67:266–274. doi: 10.1515/znc-2012-5-605. http://dx.doi.org/10.5560/ZNC.2012.67c0266. [DOI] [PubMed] [Google Scholar]
- 123.Nicola WG, Ibrahim KM, Mikhail TH, Girgis RB, Khadr ME. Role of the hypoglycemic plant extract Cleome droserifolia in improving glucose and lipid metabolism and its relation to insulin resistance in fatty liver. Boll Chim Farm. 1996;135:507–517. http://www.ncbi.nlm.nih.gov/pubmed/9035562. [PubMed] [Google Scholar]
- 124.El-Khawaga OY, Abou-Seif MA, El-Waseef A, Negm AA. Hypoglycemic, hypolipidemic and antioxidant activities of Cleome droserifolia in streptozotocin-diabetic rats. J Stress Phys Biochem. 2010;6:28–41. [Google Scholar]
- 125.El-Shenawy NS, Abdel-Nabi IM. Hypoglycemic effect of Cleome droserifolia ethanolic leaf extract in experimental diabetes, and on non-enzymatic antioxidant, glycogen, thyroid hormone and insulin levels. Diabetologia Croat. 2006;35:15–22. [Google Scholar]
- 126.El-Shenawy NS, Soliman MF, Abdel-Nabi IM. Does Cleome droserifolia have anti-schistosomiasis mansoni activity? Rev Inst Med Trop Sao Paulo. 2006;48:223–228. doi: 10.1590/s0036-46652006000400010. http://dx.doi.org/10.1590/S0036-46652006000400010. [DOI] [PubMed] [Google Scholar]
- 127.Saad B, Dakwar S, Said O, Abu-Hijleh G, Al Battah F, Kmeel A, Aziazeh H. Evaluation of medicinal plant hepatotoxicity in co-cultures of hepatocytes and monocytes. Evid Based Complement Alternat Med. 2006;3:93–98. doi: 10.1093/ecam/nel002. http://dx.doi.org/10.1093/ecam/nel002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Villaseñor IM, Lemon P, Palileo A, Bremner JB. Antigenotoxic spinasterol from Cucurbita maxima flowers. Mutat Res. 1996;360:89–93. doi: 10.1016/0165-1161(95)00071-2. http://dx.doi.org/10.1016/0165-1161(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 129.Azevedo-Meleiro CH, Rodriguez-Amaya DB. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J Agric Food Chem. 2007;55:4027–4033. doi: 10.1021/jf063413d. http://dx.doi.org/10.1021/jf063413d. [DOI] [PubMed] [Google Scholar]
- 130.Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T, Inglett GE. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem. 2007;55:4005–4013. doi: 10.1021/jf0706979. http://dx.doi.org/10.1021/jf0706979. [DOI] [PubMed] [Google Scholar]
- 131.Rezig L, Chouaibi M, Msaada K, Hamdi S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind Crop Prod. 2012;37:82–87. http://dx.doi.org/10.1016/j.indcrop.2011.12.004. [Google Scholar]
- 132.Srbinoska M, Hrabovski N, Rafajlovska V, Sinadinovic-Fiser S. Characterisatin of the seed and seed extracts of the pumpkins Cucurbita maxima D. and Cucurbita pepo L. from Macedonia. Macedon J Chem Chemical Eng. 2012;31:65–78. [Google Scholar]
- 133.Andjelkovic M, Van Camp J, Trawka A, Verhe R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur J Lipid Sci Tech. 2010;112:208–217. http://dx.doi.org/10.1002/ejlt.200900021. [Google Scholar]
- 134.Nara K, Yamaguchi A, Maeda N, Koga H. Antioxidative activity of water soluble polysaccharide in pumpkin fruits (Cucurbita maxima Duchesne) Biosci Biotechnol Biochem. 2009;73:1416–1418. doi: 10.1271/bbb.80529. http://dx.doi.org/10.1271/bbb.80529. [DOI] [PubMed] [Google Scholar]
- 135.Bowman T, Barringer S. Analysis of factors affecting volatile compound formation in roasted pumpkin seeds with selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) and sensory analysis. J Food Sci. 2012;77:C51–C60. doi: 10.1111/j.1750-3841.2011.02465.x. http://dx.doi.org/10.1111/j.1750-3841.2011.02465.x. [DOI] [PubMed] [Google Scholar]
- 136.de Cerqueira PM, Freitas MCJ, Pumar M, Santangelo SB. The pumpkin (Cucurbita maxima, L.) seed flour effect on the rat glucose and lipid metabolism. Braz J Nutr. 2008;21:129–136. [Google Scholar]
- 137.Rózycki J, Kupryszewski G, Rolka K, Ragnarsson U, Zbyryt T, Krokoszyńska I, Wilusz T. New active analogues of Cucurbita maxima trypsin inhibitor III (CMTI-III) modified in the non-contact region. Biol Chem Hoppe Seyler. 1994;375:21–23. doi: 10.1515/bchm3.1994.375.1.21. http://dx.doi.org/10.1515/bchm3.1994.375.1.21. [DOI] [PubMed] [Google Scholar]
- 138.Shan J, Baguinon M, Zheng L, Krishnamoorthi R. Expression, refolding, and activation of the catalytic domain of human blood coagulation factor XII. Protein Expr Purif. 2003;27:143–149. doi: 10.1016/s1046-5928(02)00608-3. http://dx.doi.org/10.1016/S1046-5928(02)00608-3. [DOI] [PubMed] [Google Scholar]
- 139.Mullai K, Jebanesan A. Larvicidal, ovicidal and repellent activities of the leaf extract of two cucurbitacious plants against filarial vector Culex quinquefasciatus (Say) (Diptera: Culicidae) Trop Biomed. 2007;24:1–6. http://www.ncbi.nlm.nih.gov/pubmed/17568371. [PubMed] [Google Scholar]
- 140.Cairnes DA, Ekundayo O, Kingston DG. Plant anticancer agents. X. Lignans from Juniperus phoenicea. J Nat Prod. 1980;43:495–497. doi: 10.1021/np50010a010. http://dx.doi.org/10.1021/np50010a010. [DOI] [PubMed] [Google Scholar]
- 141.Comte G, Chulia AJ, Vercauteren J, Allais DP. Phenylpropane glycosides from Juniperus phoenicea. Planta Med. 1996;62:88–89. doi: 10.1055/s-2006-957817. http://dx.doi.org/10.1055/s-2006-957817. [DOI] [PubMed] [Google Scholar]
- 142.Rezzi S, Cavaleiro C, Bighelli A, Salgueiro L, da Cunha AP, Casanova J. Intraspecific chemical variability of the leaf essential oil of Juniperus phoenicea subsp. turbinata from Corsica. Biochem Syst Ecol. 2001;29:179–188. doi: 10.1016/s0305-1978(00)00044-2. http://dx.doi.org/10.1016/S0305-1978(00)00044-2. [DOI] [PubMed] [Google Scholar]
- 143.Angioni A, Barra A, Russo MT, Coroneo V, Dessi S, Cabras P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J Agric Food Chem. 2003;51:3073–3078. doi: 10.1021/jf026203j. http://dx.doi.org/10.1021/jf026203j. [DOI] [PubMed] [Google Scholar]
- 144.Cosentino S, Barra A, Pisano B, Cabizza M, Pirisi FM, Palmas F. Composition and antimicrobial properties of Sardinian Juniperus essential oils against foodborne pathogens and spoilage microorganisms. J Food Prot. 2003;66:1288–1291. doi: 10.4315/0362-028x-66.7.1288. http://www.ncbi.nlm.nih.gov/pubmed/12870766. [DOI] [PubMed] [Google Scholar]
- 145.Barrero AF, Herrador MM, Arteaga R, del Moral JFQ, Sanchez-Fernandez E, Akssira M, Aitigri M, Mellouki F, Akkad S. Chemical composition of the essential oil from the leaves of Juniperus phoenicea L. from North Africa. J Ess Oil. 2006;18:168–169. http://dx.doi.org/10.1080/10412905.2006.9699057. [Google Scholar]
- 146.Dob T, Dahmane D, Chelghoum C. Chemical composition of the essential oil of Juniperus phoenicea L. from Algeria. J Ess Oil. 2008;20:15–20. http://dx.doi.org/10.1080/10412905.2008.9699410. [Google Scholar]
- 147.Yvon Y, Raoelison EG, Razafindrazaka R, Randriantsoa A, Romdhane M, Chabir N, Mkaddem MG, Bouajila J. Relationship between chemical composition or antioxidant activity and antihypertensive activity for six essential oils. J Food Sci. 2012;77:H184–H191. doi: 10.1111/j.1750-3841.2012.02812.x. http://dx.doi.org/10.1111/j.1750-3841.2012.02812.x. [DOI] [PubMed] [Google Scholar]
- 148.Tavares L, McDougall GJ, Fortalezas S, Stewart D, Ferreira RB, Santos CN. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. 2012;135:562–570. doi: 10.1016/j.foodchem.2012.05.023. http://dx.doi.org/10.1016/j.foodchem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 149.Achak N, Romane A, Alifriqui M, Adams RP. Chemical studies of leaf essential oils of three species of Juniperus from Tensift Al Haouz-Marrakech Region (Morocco) J Ess Oil Res. 2009;21:337–341. http://dx.doi.org/10.1080/10412905.2009.9700185. [Google Scholar]
- 150.Derwich E, Benziane Z, Boukir A. Chemical composition of leaf essential oil of Juniperus phoenicea and evaluation of its antibacterial activity. Int J Agric Biol. 2010;12:199–204. [Google Scholar]
- 151.Mazari K, Bendimerad N, Bekhechi C, Fernandez X. Chemical composition and antimicrobial activity of essential oils isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. J Med Plant Res. 2010;4:959–964. [Google Scholar]
- 152.Ennajar M, Afloulous S, Romdhane M, Ibrahim H, Cazaux S, Abderraba M, Raies A, Bouajila J. Influence of the process, season, and origin on volatile composition and antioxidant activity of Juniperus phoenicea L. leaves essential oils. J Food Sci. 2011;76:C224–C230. doi: 10.1111/j.1750-3841.2010.01995.x. http://dx.doi.org/10.1111/j.1750-3841.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 153.Fouad B, Abderrahmane R, Youssef A, Rajae H, El Fels MA. Chemical composition and antibacterial activity of the essential oil of Moroccan Juniperus phoenicea. Nat Prod Commun. 2011;6:1515–1518. http://www.ncbi.nlm.nih.gov/pubmed/22164797. [PubMed] [Google Scholar]
- 154.Ait-Ouazzou A, Lorán S, Arakrak A, Laglaoui A, Rota C, Herrera A, Pagán R, Conchello P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res Int. 2012;45:313–319. http://dx.doi.org/10.1016/j.foodres.2011.09.004. [Google Scholar]
- 155.Vourlioti-Arapi F, Michaelakis A, Evergetis E, Koliopoulos G, Haroutounian SA. Essential oils of indigenous in Greece six Juniperus taxa Chemical composition and larvicidal activity against the West Nile virus vector Culex pipiens. Parasitol Res. 2012;110:1829–1839. doi: 10.1007/s00436-011-2706-8. http://dx.doi.org/10.1007/s00436-011-2706-8. [DOI] [PubMed] [Google Scholar]
- 156.Barrero AF, Quilez del Moral JF, Herrador MM, Akssira M, Bennamara A, Akkad S, Aitigri M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry. 2004;65:2507–2515. doi: 10.1016/j.phytochem.2004.07.021. http://dx.doi.org/10.1016/j.phytochem.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 157.el-Sawi SA, Motawae HM, Ali AM. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. grown in Egypt. Afr J Tradit Complement Altern Med. 2007;4:417–426. doi: 10.4314/ajtcam.v4i4.31236. http://www.ncbi.nlm.nih.gov/pubmed/20161910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Medini H, Elaissi A, Farhat F, Khouja ML, Chemli R, Harzallah-Skhiri F. Seasonal and geographical influences on the chemical composition of Juniperus phoenicea L. essential oil leaves from the Northern Tunisia. Chem Biodivers. 2009;6:1378–1387. doi: 10.1002/cbdv.200800202. http://dx.doi.org/10.1002/cbdv.200800202. [DOI] [PubMed] [Google Scholar]
- 159.Ennajar M, Bouajila J, Lebrihi A, Mathieu F, Abderraba M, Raies A, Romdhane M. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees) J Food Sci. 2009;74:M364–M371. doi: 10.1111/j.1750-3841.2009.01277.x. http://dx.doi.org/10.1111/j.1750-3841.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 160.Ennajar M, Bouajila J, Lebrihi A, Mathieu F, Savagnac A, Abderraba M, Raies A, Romdhane M. The influence of organ, season and drying method on chemical composition and antioxidant and antimicrobial activities of Juniperus phoenicea L. essential oils. J Sci Food Agric. 2010;90:462–470. doi: 10.1002/jsfa.3840. http://dx.doi.org/10.1002/jsfa.3840. [DOI] [PubMed] [Google Scholar]
- 161.Nasri N, Tlili N, Elfalleh W, Cherif E, Ferchichi A, Khaldi A, Triki S. Chemical compounds from Phoenician juniper berries (Juniperus phoenicea) Nat Prod Res. 2011;25:1733–1742. doi: 10.1080/14786419.2010.523827. http://dx.doi.org/10.1080/14786419.2010.523827. [DOI] [PubMed] [Google Scholar]
- 162.Medini H, Elaissi A, Larbi Khouja M, Piras A, Porcedda S, Falconieri D, Marongiu B, Chemli R. Chemical composition and antioxidant activity of the essentail oil of Juniperus phoenicea L. Nat Prod Res. 2011;25:1695–1706. doi: 10.1080/14786419.2010.535168. http://dx.doi.org/10.1080/14786419.2010.535168. [DOI] [PubMed] [Google Scholar]
- 163.Mansouri N, Satrani B, Ghanmi M, El Ghadraoui L, Aafi A. Chemical and biological study of essential oils of Moroccan Juniperus phoenicea ssp lycia and Juniperus phoenicea ssp turbinata. Biotech Agron Soc Env. 2011;15:415–424. [Google Scholar]
- 164.Hayouni E, Abedrabba M, Bouix M, Hamdi M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007;105:1126–1134. http://dx.doi.org/10.1016/j.foodchem.2007.02.010. [Google Scholar]
- 165.Cosentino S, Barra A, Pisano B, Cabizza M, Pirisi FM, Palmas F. Composition and antimicrobial properties of Sardinian Juniperus essential oils against foodborne pathogens and spoilage microorganisms. J Food Prot. 2003;66:1288–1291. doi: 10.4315/0362-028x-66.7.1288. http://www.ncbi.nlm.nih.gov/pubmed/12870766. [DOI] [PubMed] [Google Scholar]
- 166.Ozturk M, Tumen I, Ugur A, Aydogmus-Ozturk F, Topcu G. Evaluation of fruit extracts of six Turkish Juniperus species for their antioxidant, anticholinesterase and antimicrobial activities. J Food Agric Sci. 2011;91:867–876. doi: 10.1002/jsfa.4258. http://dx.doi.org/10.1002/jsfa.4258. [DOI] [PubMed] [Google Scholar]
- 167.Samoylenko V, Dunbar DC, Gafur MA, Khan SI, Ross SA, Mossa JS, El-Feraly FS, Tekwani BL, Bosselaers J, Muhammad I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother Res. 2008;22:1570–1576. doi: 10.1002/ptr.2460. http://dx.doi.org/10.1002/ptr.2460. [DOI] [PubMed] [Google Scholar]
- 168.Garboui SS, Borg-Karlson AK, Palsson K. Tick repellent properties of three Libyan plants. J Med Entomol. 2009;46:1415–1419. doi: 10.1603/033.046.0623. http://dx.doi.org/10.1603/033.046.0623. [DOI] [PubMed] [Google Scholar]
- 169.Ali SA, Rizk MZ, Ibrahim NA, Abdallah MS, Sharara HM, Moutafa MM. Protective role of Juniperus phoenicea and Cupressus sempervirens against CCl4. World J Gastrointest Pharmacol Ther. 2010;1:123–131. doi: 10.4292/wjgpt.v1.i6.123. http://dx.doi.org/10.4292/wjgpt.v1.i6.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abdel-Kader MS, Alqasoumi SI. Efficacy and safety of Juniperus phoenicea extracts useed as as remedy for liver in Saudi Arabia. Planta Med. 2008;74:PA171. http://dx.doi.org/10.1055/s-0028-1084169. [Google Scholar]
- 171.Tumen I, Suntar I, Keles H, Kupeil Akkol E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid Based Complement Alternat Med. 2012;2012:728281. doi: 10.1155/2012/728281. http://dx.doi.org/10.1155/2012/728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ito H, Yamaguchi K, Kim TH, Khennouf S, Gharzouli K, Yoshida T. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J Nat Prod. 2002;65:339–345. doi: 10.1021/np010465i. http://dx.doi.org/10.1021/np010465i. [DOI] [PubMed] [Google Scholar]
- 173.Khennouf S, Benabdallah H, Gharzouli K, Amira S, Ito H, Kim TH, Yoshida T, Gharzouli A. Effect of tannins from Quercus suber and Quercus coccifera leaves on ethanol-induced gastric lesions in mice. J Agric Food Chem. 2003;51:1469–1473. doi: 10.1021/jf020808y. http://dx.doi.org/10.1021/jf020808y. [DOI] [PubMed] [Google Scholar]
- 174.Ormeno E, Fernandez C, Mevy JP. Plant coexistence alters terpene emission and content of Mediterranean species. Phytochemistry. 2007;68:840–852. doi: 10.1016/j.phytochem.2006.11.033. http://dx.doi.org/10.1016/j.phytochem.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 175.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–345. doi: 10.1016/s0378-8741(98)00126-3. http://dx.doi.org/10.1016/S0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 176.Manolaraki F, Sotiraki S, Stefanakis A, Skampardonis V, Volanis M, Hoste H. Anthelmintic activity of some Mediterranean browse plants against parasitic nematodes. Parasitology. 2010;137:685–696. doi: 10.1017/S0031182009991399. http://dx.doi.org/10.1017/S0031182009991399. [DOI] [PubMed] [Google Scholar]
- 177.Peterson A, Machmudah S, Roy BC, Goto M, Sasaki M, Hirose T. Extraction of essential oil from geranium (Pelargonium graveolens) with supercritical carbon dioxide. J Chem Tech Biotech. 2006;81:167–172. http://dx.doi.org/10.1002/jctb.1375. [Google Scholar]
- 178.Rao BRR. Chemical composition and uses of Indian rose-scented geranium (Pelargonium species) essential oil - A review. J Ess Oil-Bear Plant. 2009;12:381–394. http://dx.doi.org/10.1080/0972060X.2009.10643735. [Google Scholar]
- 179.Seo SM, Kim J, Lee SG, Shin CH, Shin SC, Park IK. Fumigant antitermitic activity of plant essential oils and components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), Geranium (Pelargonium graveolens), and Litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe) J Agric Food Chem. 2009;57:6596–6602. doi: 10.1021/jf9015416. http://dx.doi.org/10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 180.Bayoub K, Baibai T, Mountassif D, Retmane A, Soukri A. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Afr J Biotech. 2010;9:4251–4258. [Google Scholar]
- 181.Misra A, Srivastava NK. Value addition of essential monoterpene oil(s) in Geranium (Pelargonium graveolens) on leaf positions for commercial exploitation. Afr J Agric Res. 2010;5:2077–2079. [Google Scholar]
- 182.Cavar SE, Maksimovic M. Antioxidant activity of essential oil and aqueous extract of Pelargonium graveolens L’Her. Food Cont. 2012;23:263–267. http://dx.doi.org/10.1016/j.foodcont.2011.07.031. [Google Scholar]
- 183.Pohlit AM, Lopes NP, Gama RA, Tadei WP, Neto VF. Patent literature on mosquito repellent inventions which contain plant essential oils—a review. Planta Med. 2011;77:598–617. doi: 10.1055/s-0030-1270723. http://dx.doi.org/10.1055/s-0030-1270723. [DOI] [PubMed] [Google Scholar]
- 184.Dorman HJ, Dean SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oil. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. http://dx.doi.org/10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 185.Rosato A, Vitali C, De Laurentis N, Armenise D, Antonietta Milillo M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14:727–732. doi: 10.1016/j.phymed.2007.01.005. http://dx.doi.org/10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 186.Jaenson TG, Carboui S, Palsson K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2006;43:731–736. doi: 10.1603/0022-2585(2006)43[731:rooole]2.0.co;2. http://dx.doi.org/10.1603/0022-2585(2006)43[731:ROOOLE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 187.Zhuang SR, Chiu HF, Chen SL, Tsai JH, Lee MY, Lee HS, Shen YC, Yan YY, Shane GT, Wang CK. Effects of a Chinese medical herbs complex on cellular immunity and toxicity-related conditions of breast cancer patients. Br J Nutr. 2012;107:712–718. doi: 10.1017/S000711451100345X. http://dx.doi.org/10.1017/S000711451100345X. [DOI] [PubMed] [Google Scholar]
- 188.Bondi ML, Al-Hillo MRY, Lamara K, Ladjel S, Bruno M, Piozzi F, Simmonds MSJ. Occurrence of the antifeedant 14, 15-dihydroajugapitin in the aerial parts of Ajuga iva from Algeria. Biochem Syst Eco. 2000;28:1023–1025. doi: 10.1016/s0305-1978(00)00012-0. http://dx.doi.org/10.1016/S0305-1978(00)00012-0. [DOI] [PubMed] [Google Scholar]
- 189.Sabri NN, Asaad A, Khafagy SM. Isolation of four ecdysones from Ajuga iva roots and a rapid semiquantitative method for ecdysone determination. Planta Med. 1981;42:293–295. doi: 10.1055/s-2007-971644. http://dx.doi.org/10.1055/s-2007-971644. [DOI] [PubMed] [Google Scholar]
- 190.Coll J. New minor ecdysteroids from Ajuga iva (Labiatae) and complete H-1-NMR assignment of cyasteronee. Afinidad. 2007;64:242–250. [Google Scholar]
- 191.Hamden K, Ayadi F, Jamoussi K, Masmoudi H, Elfeki A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33:165–175. doi: 10.1002/biof.5520330302. http://dx.doi.org/10.1002/biof.5520330302. [DOI] [PubMed] [Google Scholar]
- 192.Bouderbala S, Prost J, Lacaille-Dubois MA, Bouchenak M. Iridoid extracts from Ajuga iva increase the antioxidant enzyme activities in red blood cells of rats fed a cholesterol-rich diet. Nutr Res. 2010;30:358–365. doi: 10.1016/j.nutres.2010.05.004. http://dx.doi.org/10.1016/j.nutres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 193.Dinda B, Debnath S, Banik R. Naturally occurring iridoids and secoiridoids. An updated review, Part 4. Chem Pharm Bull. 2011;59:803–833. doi: 10.1248/cpb.59.803. http://dx.doi.org/10.1248/cpb.59.803. [DOI] [PubMed] [Google Scholar]
- 194.El-Hilay J, Lyoussi B. Hypoglycaemic effect of the lyophilized aqueous extract of Ajuga iva in normal and streptozocin diabetic rats. J Ethnopharmacol. 2002;80:109–113. doi: 10.1016/s0378-8741(01)00407-x. http://dx.doi.org/10.1016/S0378-8741(01)00407-X. [DOI] [PubMed] [Google Scholar]
- 195.El-Hilay J, Tahraoui A, Ismaili ZH, Lyoussi B. Acute hypoglycaemic, hypocholesterolemic and hypotriglyceridemic effects of continuous intravenous infusion of a lyophilised aqueous extract of Ajuga iva L. Schreber whole plant in streptozotocin-induced diabetic rats. Pak J Pharm Sci. 2007;20:261–268. http://www.ncbi.nlm.nih.gov/pubmed/17604246. [PubMed] [Google Scholar]
- 196.El-Hilaly J, Lyoussi B, Wibo M, Morel N. Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J Ethnopharmacol. 2004;93:69–74. doi: 10.1016/j.jep.2004.03.020. http://dx.doi.org/10.1016/j.jep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 197.El-Hilay J, Tahraoui A, Ismaili ZH, Lyoussi B. Hypolipidemic effects of acute and sub-chronic administration of an aqueous extract of Ajuga iva L. whole plant in normal and diabetic rats. J Ethnopharmacol. 2006;105:441–448. doi: 10.1016/j.jep.2005.11.023. http://dx.doi.org/10.1016/j.jep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 198.Bouderbala S, Lamri-Senhadji M, Prost J, Lacaille-Dubois MA, Bouchenak M. Changes in antioxidant defense status in hypercholesterolemic rats treated with Ajuga iva. Phytomedicine. 2008;15:453–461. doi: 10.1016/j.phymed.2007.10.001. http://dx.doi.org/10.1016/j.phymed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 199.Taleb-Senouci D, Lacaille-Dubois MA, Bouchenak M. Ajuga iva aqueous extract improves reverse cholesterol transport in streptozotocin-induced diabetic rat. J Pharm Pharmacol. 2012;64:1188–1194. doi: 10.1111/j.2042-7158.2012.01501.x. http://dx.doi.org/10.1111/j.2042-7158.2012.01501.x. [DOI] [PubMed] [Google Scholar]
- 200.Chenni A, Yahia DA, Boukortt FO, Prost J, Lacaille-Dubois MA, Bouchenak M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. J Ethnopharmacol. 2007;109:207–213. doi: 10.1016/j.jep.2006.05.036. http://dx.doi.org/10.1016/j.jep.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 201.Taleb-Senouci D, Ghomari H, Krouf D, Bouderbala S, Prost J, Lacaille-Dubois MA, Bouchenak M. Antioxidant effect of Ajuga iva aqueous extract in streptozotocin-induced diabetic rats. Phytomedicine. 2009;16:623–631. doi: 10.1016/j.phymed.2008.12.004. http://dx.doi.org/10.1016/j.phymed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 202.Beghalia M, Ghalem S, Allalia H, Belouatek A, Marouf A. Effects of Atriplex halimus and Ajuga iva L. Schreb on calcium oxalate Uurolithiasis risk in vitro. Asian J Chem. 2009;21:1119–1125. [Google Scholar]
- 203.Jbilou R, Amri H, Bouayad N, Ghailani N, Ennabili A, Sayah F. Insecticidal effects of extracts of seven plant species on larval development, alpha-amylase activity and offspring production of Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae) Bioresour Technol. 2008;99:959–964. doi: 10.1016/j.biortech.2007.03.017. http://dx.doi.org/10.1016/j.biortech.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 204.Jbilou R, Ennabili A, Sayah F. Insecticidal activity of four medicinal plant extracts against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) Afr J Biotech. 2006;5:936–940. [Google Scholar]
- 205.Ahmad S, Ahmad I, Saleem M, Jabbar A, Nisar-Ur-Rehman, Saeed-Ul-Hassan S, Akhtar KS, Choudhary MI. Secondary Metabolites from Alhagi maurorum. J Chem Soc Pak. 2009;31:960–963. [Google Scholar]
- 206.Ahmad S, Riaz N, Saleem M, Jabbar A, Nisar-Ur-Rehman, Ashraf M. Antioxidant flavonoids from Alhagi maurorum. J Asian Nat Prod Res. 2010;12:138–143. doi: 10.1080/10286020903451724. http://dx.doi.org/10.1080/10286020903451724. [DOI] [PubMed] [Google Scholar]
- 207.Laghari AH, Ali Memon A, Memon S, Nelofar A, Khan KM, Yasmin A. Determination of free phenolic acids and antioxidant capacity of methanolic extracts obtained from leaves and flowers of camel thorn (Alhagi maurorum) Nat Prod Res. 2012;26:173–176. doi: 10.1080/14786419.2010.538846. http://dx.doi.org/10.1080/14786419.2010.538846. [DOI] [PubMed] [Google Scholar]
- 208.Shaker E, Mahmoud H, Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn) Food Chem Toxicol. 2010;48:2785–2790. doi: 10.1016/j.fct.2010.07.007. http://dx.doi.org/10.1016/j.fct.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 209.Laghari AH, Memon S, Nelofar A, Khan KM, Yasmin A, Syed MN, Aman A. A new flavanenol with urease-inhibition activity isolated from roots of manna plant camelthorn (Alhagi maurorum) J Mol Struct. 2010;965:65–67. http://dx.doi.org/10.1016/j.molstruc.2009.11.039. [Google Scholar]
- 210.Laghari AH, Memon S, Nelofar A, Khan KM. Alhagi maurorum: A convenient source of lupeol. Ind Crop Prod. 2011;34:1141–1145. http://dx.doi.org/10.1016/j.indcrop.2011.03.031. [Google Scholar]
- 211.Abu-Taleb AM, El-Deeb K, Al-Otibi FO. Bioactivity of some plant extracts against Drechslera biseptata and Fusarium solani. J Food Agric Env. 2011;9:769–774. [Google Scholar]
- 212.Oskoueian A, Oskoueian E, Rudi H, Farida Z, Fatemeh S, Karimi E. Antioxidant activity and bioactive compounds in Alhagi maurorum. Clin Biochem. 2011;44:S343–S344. http://dx.doi.org/10.1016/j.clinbiochem.2011.08.856. [Google Scholar]
- 213.Awaad AS, El-Meligy RM, Qenawy SA, Atta AH, Soliman GA. Anti-inflammatory, antinociceptive and antipyretic effects of some desert plants. J Saudi Chem Soc. 2011;15:367–373. http://dx.doi.org/10.1016/j.jscs.2011.02.004. [Google Scholar]
- 214.Shaker E, Mahmoud H, Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn) Food Chem Toxicol. 2010;48:2785–2790. doi: 10.1016/j.fct.2010.07.007. http://dx.doi.org/10.1016/j.fct.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 215.Awaad Amani AS, Maitland DJ, Soliman GA. Antiulcerogenic activity of Alhagi maurorum. Pharm Biol. 2006;44:292–296. http://dx.doi.org/10.1080/13880200600714160. [Google Scholar]
- 216.Naseri MKG, Mard SA. Gastroprotective effect of Alhagi maurorum on exprimental gastric ulcer in rats. Pak J Med Sci. 2007;23:570–573. [Google Scholar]
- 217.Atta AH, Abo EL-Sooud K. The antinociceptive effect of some Egyptian medicinal plant extracts. J Ethnopharmacol. 2004;95:235–238. doi: 10.1016/j.jep.2004.07.006. http://dx.doi.org/10.1016/j.jep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 218.Atta AH, Mouneir SM. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J Ethnopharmacol. 2004;92:303–309. doi: 10.1016/j.jep.2004.03.017. http://dx.doi.org/10.1016/j.jep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 219.Marashdah MS, Farraj AI. Pharmacological activity of 2% aqueous acetic acid extract of Alhagi maurorum rootst. J Saudi Chem Soc. 2010;14:247–250. http://dx.doi.org/10.1016/j.jscs.2010.02.015. [Google Scholar]
- 220.Marashdah MS, Al-Hazimi HM. Pharmacological activity of ethanolic extract of Alhagi maurorum roots. Arab J Chem. 2010;3:39–42. http://dx.doi.org/10.1016/j.arabjc.2009.12.007. [Google Scholar]
- 221.Ashraf M, Ahmad K, Ahmad I, Ahmad S, Arshad S, Shah SMA, Nasim FUH. Acetylcholinesterase and NADH oxidase inhibitory activity of some medicinal plants. J Med Plant Res. 2011;5:2086–2089. [Google Scholar]
- 222.Rahman SMA, Abd-Ellatif SA, Deraz SF, Khalil AA. Antibacterial activity of some wild medicinal plants collected from western Mediterranean coast, Egypt: Natural alternatives for infectious disease treatment. Afr J Biotech. 2011;10:10733–10743. [Google Scholar]
- 223.Sadaqa EA, Bawazir AA, Qasem JR. Allelopathic activity of some common weeds species in onion fields. Allelopathy J. 2010;26:175–184. [Google Scholar]
- 224.Allerdings E, Ralph J, Steinhart H, Bunzel M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry. 2006;67:1276–1286. doi: 10.1016/j.phytochem.2006.04.018. http://dx.doi.org/10.1016/j.phytochem.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 225.Suzuki R, Okada Y, Okuyama T. A new flavone C-glycoside from the style of Zea mays L. with glycation inhibitory activity. Chem Pharm Bull (Tokyo) 2003;51:1186–1188. doi: 10.1248/cpb.51.1186. http://dx.doi.org/10.1248/cpb.51.1186. [DOI] [PubMed] [Google Scholar]
- 226.Suzuki R, Iijima M, Okada Y, Okuyama T. Chemical constituents of the style of Zea mays L. with glycation inhibitory activity. Chem Pharm Bull (Tokyo) 2007;55:153–155. doi: 10.1248/cpb.55.153. http://dx.doi.org/10.1248/cpb.55.153. [DOI] [PubMed] [Google Scholar]
- 227.Ranilla LG, Apostolidis E, Genovese MI, Lajolo FM, Shetty K. Evaluation of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J Med Food. 2009;12:704–713. doi: 10.1089/jmf.2008.0122. http://dx.doi.org/10.1089/jmf.2008.0122. [DOI] [PubMed] [Google Scholar]
- 228.Barros-Rios J, Malvar RA, Jung HJ, Santiago R. Cell wall composition as a maize defense mechanism against corn borers. Phytochemistry. 2011;72:365–371. doi: 10.1016/j.phytochem.2011.01.004. http://dx.doi.org/10.1016/j.phytochem.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 229.Li J, Lim SS, Lee JY, Kim JK, Kang SW, Kim JL, Kang YH. Purple corn anthocyanins dampened high-glucose-induced mesangial fibrosis and inflammation: possible renoprotective role in diabetic nephropathy. J Nutr Biochem. 2012;23:320–331. doi: 10.1016/j.jnutbio.2010.12.008. http://dx.doi.org/10.1016/j.jnutbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 230.Suzuki R, Okada Y, Okuyama T. The favorable effect of style of Zea mays L. on streptozotocin induced diabetic nephropathy. Biol Pharm Bull. 2005;28:919–920. doi: 10.1248/bpb.28.919. http://dx.doi.org/10.1248/bpb.28.919. [DOI] [PubMed] [Google Scholar]
- 231.Miao MS, Zhang GL, Miao YY, Shi JJ, Liu HL. Influence of Zea mays L. saponin (ZMLS) on ultrastructure of kidney and pancreas in diabetes rats induced by streptozocin. Zhongguo Zhong Yao Za Zhi. 2008;33:1179–1183. [PubMed] [Google Scholar]
- 232.Lee CH, Garcia HS, Parkin KL. Bioactivities of kernel extracts of 18 strains of maize (Zea mays) J Food Sci. 2010;75:C667–C672. doi: 10.1111/j.1750-3841.2010.01784.x. http://dx.doi.org/10.1111/j.1750-3841.2010.01784.x. [DOI] [PubMed] [Google Scholar]
- 233.Rau O, Wurglics M, Dingermann T, Abdel-Tawab M, Schubert-Zsilavecz M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Pharmazie. 2006;61:952–956. http://www.ncbi.nlm.nih.gov/pubmed/17152989. [PubMed] [Google Scholar]
- 234.Li W, Wei CV, White PJ, Beta T. High-amylose corn exhibits better antioxidant activity than typical and waxy genotypes. J Agric Food Chem. 2007;55:291–298. doi: 10.1021/jf0622432. http://dx.doi.org/10.1021/jf0622432. [DOI] [PubMed] [Google Scholar]
- 235.Meiouet F, El Kabbaj S, Daudon M. In vitro study of the litholytic effects of herbal extracts on cystine urinary calculi. Prog Urol. 2011;21:40–47. doi: 10.1016/j.purol.2010.05.009. http://dx.doi.org/10.1016/j.purol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 236.Baytop T. Therapy with Medicinal Plants in Turkey. 2nd Edition. Nobel Tip Kitabevleri; Istanbul: 1999. pp. 319–320. [Google Scholar]
- 237.Alaadin AM, Al-Khateeb EH, Jager AK. Antibacterial activity of the Iraqi Rheum ribes root. Pharm Biol. 2007;45:688–690. http://dx.doi.org/10.1080/13880200701575049. [Google Scholar]
- 238.Ozcan MM, Dursun N, Arslan D. Some nutritional properties of Prangos ferulacea (L.) Lindl and Rheum ribes L. stems growing wild in Turkey. Int J Food Sci Nutr. 2007;58:162–167. doi: 10.1080/09637480601154145. http://dx.doi.org/10.1080/09637480601154145. [DOI] [PubMed] [Google Scholar]
- 239.Ozturk M, Aydozmus-Ozturk F, Duru ME, Topcu G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007;103:623–630. http://dx.doi.org/10.1016/j.foodchem.2006.09.005. [Google Scholar]
- 240.Naqishbani AM, Josefsen K, Pedersen ME, Jager AK. Hypoglycaemic activity of Iraqi Rheum ribes root extract. Pharm Biol. 2009;47:380–383. http://dx.doi.org/10.1080/13880200902748478. [Google Scholar]
- 241.Ozbek H, Ceylan E, Kara M, Ozgokce F, Koyuncu M. Hypoglycaemic effect of Rheum ribes roots in alloxan induced diabetic and normal mice. Scand J Lab Animal Sci. 2004;31:113–115. [Google Scholar]
- 242.Hudson JB, Lee MK, Sener B, Erdemoglu N. Antiviral activities in extracts of Turkish medicinal plants. Pharm Biol. 2000;38:171–175. doi: 10.1076/1388-0209(200007)3831-SFT171. http://dx.doi.org/10.1076/1388-0209(200007)3831-SFT171. [DOI] [PubMed] [Google Scholar]
- 243.Bazzaz BSF, Khajehkaramadin M, Shokooheizadeh HR. In vitro antibacterial activity of Rheum ribes extract obtained from various plant parts against clinical isolates of gram negative pathogens. Iran J Pharm Res. 2005;2:87–91. [Google Scholar]
- 244.Oktay M, Yildirim A, Bilaloglu V, Gulcin I. Antioxidant activity of different parts of isgin (Rheum ribes L.) Asian J Chem. 2007;19:3047–3055. [Google Scholar]
- 245.Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Proc. 2011;89:217–233. http://dx.doi.org/10.1016/j.fbp.2010.04.008. [Google Scholar]
- 246.Sardari S, Shokrgozar MA, Ghavami G. Cheminformatics based selection and cytotoxic effects of herbal extracts. Toxicol In Vitro. 2009;23:1412–1421. doi: 10.1016/j.tiv.2009.07.011. http://dx.doi.org/10.1016/j.tiv.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 247.Ismaeil N, Hidayat H. The cytotoxic effect of the crude extracts of local rhubarb Rheum ribes L. in Kurdistan-Iraq on normal and cancer cell lines in vitro. Basic Clin Pharm Toxicol. 2009;105:56–57. [Google Scholar]
- 248.Sindhu RK, Kumar P, Kumar J, Kumar A, Arora S. Investigations into the anti-ulcer activity of Rheum ribes Linn leaves extracts. Int J Pharm Pharmaceut Sci. 2010;2:90–92. [Google Scholar]
- 249.Sayyah M, Boostani H, Pakseresh S, Malayeri A. Efficacy of hydroalcoholic extract of Rheum ribes L. in treatment of major depressive disorder. J Med Plant Res. 2009;3:573–575. [Google Scholar]
- 250.El-Din Hussein Mahran G, Glombitza KW, Mirhom YW, Hartmann R, Michel CG. Novel saponins from Zizyphus spina-christi growing in Egypt. Planta Med. 1996;62:163–165. doi: 10.1055/s-2006-957842. http://dx.doi.org/10.1055/s-2006-957842. [DOI] [PubMed] [Google Scholar]
- 251.Abdel-Zaher AO, Salim SY, Assaf MH, Abdel-Hady RH. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharmacol. 2005;101:129–138. doi: 10.1016/j.jep.2005.04.007. http://dx.doi.org/10.1016/j.jep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 252.Pordel Shahri M, Shadizadeh SR, Jamialahmadi M. A new type of surfactant for enhanced oil recovery. Petrol Sci Tech. 2012;30:585–593. http://dx.doi.org/10.1080/10916466.2010.489093. [Google Scholar]
- 253.Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Berghe DV, Vlietinck AJ. Chemical and biological investigations on Zizyphus spina-christi L. Phytother Res. 2001;15:593–597. doi: 10.1002/ptr.883. http://dx.doi.org/10.1002/ptr.883. [DOI] [PubMed] [Google Scholar]
- 254.Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009;112:858–862. http://dx.doi.org/10.1016/j.foodchem.2008.06.053. [Google Scholar]
- 255.Ghannadi A, Tavakoli N, Mehri-Ardestani M. Volatile constituents of the leaves of Ziziphus spinachristi (L.) Willd. from Bushehr, Iran. J Ess Oil Res. 2003;15:191–192. http://dx.doi.org/10.1080/10412905.2003.9712109. [Google Scholar]
- 256.Said A, Fawzy G, Abu Tabl ESA, Tzakou O. Volatile constituents of Zizyphus jujuba aerial parts and Zizyphus spina-christii fruits from Egypt. J Ess Oil-Bear Plant. 2010;13:170–174. http://dx.doi.org/10.1080/0972060X.2010.10643807. [Google Scholar]
- 257.Nazif NM. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Chem. 2002;76:77–81. http://dx.doi.org/10.1016/S0308-8146(01)00243-6. [Google Scholar]
- 258.Amoo IA, Atasie VN. Nutritional and functional properties of Tamarindus indica pulp and Zizyphus spina-christi fruit and seed. J Food Agric Env. 2012;10:16–19. [Google Scholar]
- 259.Nesseem DI, Michel CG, Sleem AA, Al-Alfy TS. Formulation and evaluation of antihyperglycemic leaf extracts of Zizyphus spina-christi (L.) Wild. Pharmazie. 2009;64:104–109. http://www.ncbi.nlm.nih.gov/pubmed/19320283. [PubMed] [Google Scholar]
- 260.Michel CG, Nesseem DI, Ismail MF. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. J Ethnopharmacol. 2011;133:53–62. doi: 10.1016/j.jep.2010.09.001. http://dx.doi.org/10.1016/j.jep.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 261.Avizeh R, Najafzadeh H, Pourmandi M, Mirzaee M. Effect of glibenclamide and fruit extract of Zizyphus spina-christi on alloxan- induced diabetic dogs. Int J Appl Res Vet Med. 2010;8:109–113. [Google Scholar]
- 262.Abdel-Wahhab MA, Omara EA, Abdel-Galil MM, Hassan NS, Nada SA, Saeed A, el-Sayed MM. Zizyphus spina-christi extract protects against aflatoxin B1-initiated hepatic carcinogenicity. Afr J Tradit Complement Altern Med. 2007;4:248–256. [PMC free article] [PubMed] [Google Scholar]
- 263.Abdel-Wahhab M, Abdel-Galil MM, Hassan AM, Hassan NS, Nada SA, Saeed A. Zizyphus spina-christi extract protects against aflatoxin B-1-initiated hepatic carcinogenicity. Toxicol Lett. 2005;158:S68–S68. [PMC free article] [PubMed] [Google Scholar]
- 264.Amin A, Mahmoud-Ghoneim D. Zizyphus spina-christi protects against carbon tetrachloride-induced liver fibrosis in rats. Food Chem Toxicol. 2009;47:2111–2119. doi: 10.1016/j.fct.2009.05.038. http://dx.doi.org/10.1016/j.fct.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 265.Godini A, Kazem M, Naseri G, Badavi M. The effect of Ziziphus spina-christi leaf extract on isolated rat aorta. J Pak Med Assoc. 2009;53:537–539. http://www.ncbi.nlm.nih.gov/pubmed/19757700. [PubMed] [Google Scholar]
- 266.Hadizadeh I, Peivastegan B, Kolahi M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak J Biol Sci. 2009;12:58–63. doi: 10.3923/pjbs.2009.58.63. http://dx.doi.org/10.3923/pjbs.2009.58.63. [DOI] [PubMed] [Google Scholar]
- 267.Adzu B, Amos S, Wambebe C, Gamaniel K. Antinociceptive activity of Zizyphus spina-christi root bark extract. Fitoterapia. 2001;72:344–350. doi: 10.1016/s0367-326x(00)00289-6. http://dx.doi.org/10.1016/S0367-326X(00)00289-6. [DOI] [PubMed] [Google Scholar]
- 268.Adzu B, Haruna AK. Studies on the use of Zizyphus spina-christi against pain in rats and mice. Afr J Biotech. 2007;6:1317–1324. [Google Scholar]
- 269.Adzu B, Amos S, Amizan MB, Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 2003;87:245–250. doi: 10.1016/s0001-706x(03)00114-1. http://dx.doi.org/10.1016/S0001-706X(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 270.Adzu B, Amos S, Dzarma S, Wambebe C, Gamaniel K. Effect of Zizyphus spina-christi Willd aqueous extract on the central nervous system in mice. J Ethnopharmacol. 2002;79:13–16. doi: 10.1016/s0378-8741(01)00348-8. http://dx.doi.org/10.1016/S0378-8741(01)00348-8. [DOI] [PubMed] [Google Scholar]
- 271.Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother Res. 2006;20:961–965. doi: 10.1002/ptr.1985. http://dx.doi.org/10.1002/ptr.1985. [DOI] [PubMed] [Google Scholar]
- 272.Borodin YI, Selyatitskaya VG, Obukhova LA, Pal’chikova NA, Odintsov SV, Kukushkina TA. Effects of polyphenol compounds from Alchemilla vulgaris on morphofunctional state of thyroid gland in rats exposed to low temperature. Bull Exp Biol Med. 1999;127:637–639. http://dx.doi.org/10.1007/BF02433301. [PubMed] [Google Scholar]
- 273.Oktyabrsky O, Vysochina G, Muzyka N, Samoilova Z, Kukushkina T, Smirnova G. Assessment of anti-oxidant activity of plant extracts using microbial test systems. J Appl Micr. 2009;106:1175–1183. doi: 10.1111/j.1365-2672.2008.04083.x. http://dx.doi.org/10.1111/j.1365-2672.2008.04083.x. [DOI] [PubMed] [Google Scholar]
- 274.D’Agostino M, Dini I, Ramundo E, Senatore F. Flavonoid glycosides of Alchemilla vulgaris L. Phyther Res. 1998;12(Suppl 1):S162–S163. http://dx.doi.org/10.1002/(SICI)1099-1573(1998)12:1+3.0.CO;2-P. [Google Scholar]
- 275.Condrat D, Crisan F, Szabo MR, Chambree DR, Lupea AX. Flavonoids in Angiospermatophyta and Spermatophyta species and their antioxidant activity. Revista de Chimie. 2009;60:1129–1134. [Google Scholar]
- 276.Smolyakova IM, Andreeva VY, Kalinkina GI, Avdeenko SN, Shchetinin PP. Development of extraction techniques and standardization methods for a common lady’s mantle (Alchemilla vulgaris) extract. Pharm Chem J. 2012;45:675–678. http://dx.doi.org/10.1007/s11094-012-0702-7. [Google Scholar]
- 277.Djipa CD, Delmee M, Quetin-Leclercq J. Antimicrobial activity of bark extracts of Syzygium jambos (L.) Alston (Myrtaceae) J Ethnopharmacol. 2000;71:307–313. doi: 10.1016/s0378-8741(99)00186-5. http://dx.doi.org/10.1016/S0378-8741(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 278.Condrat D, Mosoarca C, Zamfir AD, Crisan F, Szabo MR, Lupea AX. Qualitative and quantitative analysis of gallic acid in Alchemilla vulgaris, Allium ursinum, Acorus calamus and Solidago virga-aurea by chip-electrospray ionization mass spectrometry and high performance liquid chromatography. Centr Eur J Chem. 2010;8:530–535. http://dx.doi.org/10.2478/s11532-010-0012-4. [Google Scholar]
- 279.Said O, Saad B, Fulder S, Khalil K, Kassis E. Weight loss in animals and humans treated with ‘Weighlevel’, a combination of four medicinal plants used in traditional Arabic and Islamic medicine. Evid Based Complement Alternat Med. 2011;24:1–6. doi: 10.1093/ecam/nen067. http://dx.doi.org/10.1155/2011/207034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Swanston-Flat SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–464. doi: 10.1007/BF00405106. http://dx.doi.org/10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 281.Shrivastava R, John GW. Treatment of aphthous stomatitis with topical Achemilla vulgaris in glycerine. Clin Drug Investig. 2006;26:567–573. doi: 10.2165/00044011-200626100-00003. http://dx.doi.org/10.2165/00044011-200626100-00003. [DOI] [PubMed] [Google Scholar]
- 282.Shrivastava R, Cucuat N, John GW. Effects of Alchemilla vulgaris and glycerine on epithelial and myofibroblast cell growth and cutaneous lesion healing in rats. Phytother Res. 2007;21:369–373. doi: 10.1002/ptr.2060. http://dx.doi.org/10.1002/ptr.2060. [DOI] [PubMed] [Google Scholar]
- 283.Condrat D, Szabo MR, Radu D, Lupea AX. Plant spices from the Angiospermatophyta and Spermatophyta genus with antiradical and antimicrobial activity. Oxid Commun. 2009;32:924–929. [Google Scholar]
- 284.Keskin D, Oskay D, Oskay M. Antimicrobial activity of selected plant species marketed in the West Anatolia. Int J Agric Biol. 2010;12:916–920. [Google Scholar]
- 285.Sufka KJ, Roach JT, Chambliss WG, Broom SL, Feltenstein MW, Wyandt CM, Zeng L. Anxiolytic properties of botanical extracts in the chick social separation-stress procedure. Psychopharmacol. 2001;153:219–224. doi: 10.1007/s002130000571. http://dx.doi.org/10.1007/s002130000571. [DOI] [PubMed] [Google Scholar]
- 286.Reher G, Reznicek G, Baumann A. Triterpenoids from Sarcopoterium spinosum and Sanguisorba minor. Planta Med. 1991;57:506. doi: 10.1055/s-2006-960189. http://dx.doi.org/10.1055/s-2006-960189. [DOI] [PubMed] [Google Scholar]
- 287.Sarikaya BB, Kayalar H. Quantitative determination of α-tocopherol and quality control studies in Sarcopoterium spinosum L. Marmara Pharm J. 2011;15:2–10. [Google Scholar]
- 288.Reher G. Proanthocyanidins from Poterium spinosum (Rosaceae) Pharm Weekbl Sci. 1987;9:243–243. [Google Scholar]
- 289.Bachrach ZY. Ethnobotanical studies of Sarcopoterium spinosum in Israel. Israel J Plant Sci. 2007;55:111–114. http://dx.doi.org/10.1560/IJPS.55.1.111. [Google Scholar]
- 290.Schlutz GO, Venulet J. [Poterium spinosum and its influence on the blood sugar level in rabbits] Experientia. 1964;15:78–79. doi: 10.1007/BF02151250. http://www.ncbi.nlm.nih.gov/pubmed/5852158. [DOI] [PubMed] [Google Scholar]
- 291.Mishkinsky J, Menczel E, Sulman FG. Hypoglycaemic effect of Poterium spinosum L. (Rosaceae) Arch Int Pharmacodyn Ther. 1966;161:306–313. http://www.ncbi.nlm.nih.gov/pubmed/5926873. [PubMed] [Google Scholar]
- 292.Shani J, Joseph B, Sulman FG. Fluctuations in the hypoglycaemic effect of Poterium spinosum L. (Rosaceae) Arch Int Pharmacodyn Ther. 1970;185:344–349. http://www.ncbi.nlm.nih.gov/pubmed/5473794. [PubMed] [Google Scholar]
- 293.Rosenzweig T, Abitbol G, Taler D. Evaluating the anti-diabetic effects of Sareopoterium spinosum extracts in vitro. Israel J Plant Sci. 2007;55:103–109. http://dx.doi.org/10.1560/IJPS.55.1.103. [Google Scholar]
- 294.Smirin P, Taler D, Abitbol G, Brutman-Barazani T, Kerem Z, Sampson SR, Rosenzweig T. Sarcopoterium spinosum extract as an antidiabetic agent: in vitro and in vivo study. J Ethnopharmacol. 2010;129:10–17. doi: 10.1016/j.jep.2010.02.021. http://dx.doi.org/10.1016/j.jep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 295.Auclair MC, Lemeignan M, Rodallec A. Action potential changes induced by a polyflavane on normal or hypoxic guinea pig myocardial strips. C R Acad Sci Hebd Seances Acad Sci D. 1976;283:695–698. [PubMed] [Google Scholar]
- 296.Durodola J. Tumour inhibitory effects of crude extracts from Poterium spinosum. Planta Med. 1975;27:231–234. doi: 10.1055/s-0028-1097791. http://dx.doi.org/10.1055/s-0028-1097791. [DOI] [PubMed] [Google Scholar]
- 297.Shahverdi AR, Saadat F, Khorramizadeh MR, Iranshahi M, Khoshayand MR. Two matrix metalloproteinases inhibitors from Ferula persica var. persica. Phytomedicine. 2006;13:712–717. doi: 10.1016/j.phymed.2006.01.003. http://dx.doi.org/10.1016/j.phymed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 298.Iranshahi M, Amin GR, Jalalizadeh H, Shafiee A. New germacrane derivative from Ferula persica. Pharm Biol. 2003;41:431–433. http://dx.doi.org/10.1076/phbi.41.6.431.17834. [Google Scholar]
- 299.Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch C. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. http://www.ncbi.nlm.nih.gov/pubmed/15813369. [DOI] [PubMed] [Google Scholar]
- 300.Mirjani R, Shahverdi AR, Iranshahi M, Amin G, Shafiee A. Identification of antifungal compounds from Ferula persica var. persica. Pharm Biol. 2005;43:293–295. doi: 10.1080/13880200590951658. http://dx.doi.org/10.1080/13880200590951658. [DOI] [PubMed] [Google Scholar]
- 301.Iranshahi M, Noroozi S, Behravan J, Karimi G, Schneider B. Persicasulfide C, a new sulphur-containing derivative from Ferula persica. Nat Prod. Res. 2009;23:1584–1588. doi: 10.1080/14786410802393571. http://dx.doi.org/10.1080/14786410802393571. [DOI] [PubMed] [Google Scholar]
- 302.Iranshahi M, Askari M, Sahebkar A, Hadjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. Daru. 2009;17:99–103. [Google Scholar]
- 303.Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev. 2009;18:412–415. doi: 10.1097/CEJ.0b013e32832c389e. http://dx.doi.org/10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- 304.Iranshahi M, Amin G, Shafie A. A new coumarin from Ferula persica. Pharm Biol. 2004;42:440–442. http://dx.doi.org/10.1080/13880200490886102. [Google Scholar]
- 305.Hanafi-Bojd MY, Iranshahi M, Mosaffa F, Tehrani SO, Kalalinia F, Behravan J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011;77:1590–1593. doi: 10.1055/s-0030-1270987. http://dx.doi.org/10.1055/s-0030-1270987. [DOI] [PubMed] [Google Scholar]
- 306.Iranshahi M, Mojarab M, Sadeghian H, Hanafi-Bojd MY, Schneider B. Polar secondary metabolites from Ferula persica roots. Phytochemistry. 2008;69:473–478. doi: 10.1016/j.phytochem.2007.08.001. http://dx.doi.org/10.1016/j.phytochem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 307.Javidnia K, Miri R, Kamalinejad M, Edraki N. Chemical composition of Ferula persica Wild. essential oil from Iran. Flav Frag J. 2005;20:605–606. http://dx.doi.org/10.1002/ffj.1496. [Google Scholar]
- 308.Iranshahi M, Amin G, Sourmaghi MS, Shafiee A, Hadjiakhoondi A. Sulphur-containing compounds in the essential oil of the root of Ferula persica Willd. var. Persica. Flav Frag J. 2006;21:260–261. http://dx.doi.org/10.1002/ffj.1574. [Google Scholar]
- 309.Asili J, Sahebkar A, Bazzaz BSF, Sharifi S, Iranshahi M. Identification of essential oil components of Ferula badrakema fruits by GC-MS and C-13-NMR methods and evaluation of its antimicrobial activity. J Ess Oil-Bear Plants. 2009;12:7–15. http://dx.doi.org/10.1080/0972060X.2009.10643685. [Google Scholar]
- 310.Sahebkar A, Iranshahi M. Volatile constituents of the genus Ferula(Apiaceae): A review. J Ess Oil-Bear Plants. 2011;14:504–531. http://dx.doi.org/10.1080/0972060X.2011.10643969. [Google Scholar]
- 311.Noroozi S, Mosaffa F, Soltani F, Iranshahi M, Karimi G, Malekaneh M, Haghighi F, Behravan J. Antigenotoxic effects of the disulfide compound persicasulfide A (PSA) on rat lymphocytes exposed to oxidative stress. Planta Med. 2009;75:32–36. doi: 10.1055/s-0028-1088360. http://dx.doi.org/10.1055/s-0028-1088360. [DOI] [PubMed] [Google Scholar]
- 312.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48:242–246. doi: 10.3109/13880200903081796. http://dx.doi.org/10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 313.Ziai SA, Gholami O, Iranshahi M, Zamani AH, Jeddi-Tehrani M. Umbelliprenin induces apoptosis in CLL cell lines. Iran J Pharm Res. 2012;11:653–659. [PMC free article] [PubMed] [Google Scholar]
- 314.Pinelli P, Leri F, Vignolini P, Bacci L, Baronti S, Romani A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica. J Agric Food Chem. 2008;56:9127–9132. doi: 10.1021/jf801552d. http://dx.doi.org/10.1021/jf801552d. [DOI] [PubMed] [Google Scholar]
- 315.Moldovan L, Gaspar A, Toma L, Craciunescu O, Saviuc C. Comparison of polyphenolic content and antioxidant capacity of five Romanian traditional medicinal plants. Revista di Chimie. 2011;62:299–303. [Google Scholar]
- 316.Otles S, Yalcin B. Phenolic compounds analysis of root, stalk, and leaves of nettle. ScientificWorldJournal. 2012;2012:564367. doi: 10.1100/2012/564367. http://dx.doi.org/10.1100/2012/564367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Akbay P, Basaran AA, Undeger U, Basaran N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytotherapy Res. 2003;17:34–37. doi: 10.1002/ptr.1068. http://dx.doi.org/10.1002/ptr.1068. [DOI] [PubMed] [Google Scholar]
- 318.Basaran AA, Akbay P, Undeger U, Basaran N. In vitro immunomodulatory and mutagenic activity of the flavonoid glycosides from Urtica dioica L. Toxicology. 2001;164:S171–S172. doi: 10.1002/ptr.1068. [DOI] [PubMed] [Google Scholar]
- 319.Ozcan C, Dilgin Y, Yaman M. Determination of quercetin in medicinal plants such as rose hip (Rosa canina), nettle (Urtica dioica), terebinth (Terebinthina chica) and purslane (Portulace oleracea) using HPLC-MS method. Asian J Chem. 2012;24:3396–3400. [Google Scholar]
- 320.Ilies T, Radulescu V. Chemical composition of the essential oil of Urtica dioica Ilies, DC. Chem Nat Comp. 2012;48:506–507. http://dx.doi.org/10.1007/s10600-012-0291-4. [Google Scholar]
- 321.Gül S, Demirci B, Baser KHC, Akpulat HA, Aksu P. Chemical composition and in vitro cytotoxic, genotoxic effects of essential oil from Urtica dioica L. Bull Env Contamin Toxicol. 2012;88:666–671. doi: 10.1007/s00128-012-0535-9. http://dx.doi.org/10.1007/s00128-012-0535-9. [DOI] [PubMed] [Google Scholar]
- 322.Kraus R, Spiteller G. [Lignan glucosides from roots of Urtica dioica] Liebigs Ann Chem. 1990. pp. 1205–1213. http://dx.doi.org/10.1002/jlac.1990199001218.
- 323.Rohricht C. Yield and constituents of greater nettle strains (Urtica dioica L.) Z Arznei Gewürzpflanzen. 2007;12:193–195. [Google Scholar]
- 324.Sasan TA, Goodarzi MT, Jamshid K, Panah MH. Antidiabetic effects of the aqueous extract of Urtica dioica on high-fructose fed rats. Clin Biochem. 2011;44:S332. http://dx.doi.org/10.1016/j.clinbiochem.2011.08.821. [Google Scholar]
- 325.Nickavar B, Yousefian N. Evaluation of alpha-amylase inhibitory activities of selected antidiabetic medicinal plants. J Verbr Lebensm. 2011;6:191–195. http://dx.doi.org/10.1007/s00003-010-0627-6. [Google Scholar]
- 326.Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74:677–681. doi: 10.1016/s0367-326x(03)00182-5. http://dx.doi.org/10.1016/S0367-326X(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 327.Farzami B, Ahmadvand D, Vardasbi S, Majin FJ, Khaghani S. Induction of insulin secretion by a component of Urtica dioica leave extract in perifused Islets of Langerhans and its in vivo effects in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2003;89:47–53. doi: 10.1016/s0378-8741(03)00220-4. http://dx.doi.org/10.1016/S0378-8741(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 328.Onal S, Timur S, Okutucu B, Zihniogku F. Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol. 2005;35:29–36. doi: 10.1081/PB-200041438. http://dx.doi.org/10.1081/PB-200041438. [DOI] [PubMed] [Google Scholar]
- 329.Golalipour MJ, Khori V. The protective activity of Urtica dioica leaves on blood glucose concentration and beta-cells in streptozotocin-diabetic rats. Pak J Biol Sci. 2007;10:1200–1204. doi: 10.3923/pjbs.2007.1200.1204. http://dx.doi.org/10.3923/pjbs.2007.1200.1204. [DOI] [PubMed] [Google Scholar]
- 330.Golalipour MJ, Ghafari S, Kouri V, Kestkar AA. Proliferation of the beta-cells of pancreas in diabetic rats treated with Urtica dioica. Int J Morphol. 2010;28:399–404. [Google Scholar]
- 331.Domola MS, Vu V, Robson-Doucette CA, Sweeney G, Wheeler MB. Insulin mimetics in Urtica dioica: Structural and computational analyses of Urtica dioica extracts. Phytotherapy Res. 2010;24:S175–S182. doi: 10.1002/ptr.3062. http://dx.doi.org/10.1002/ptr.3062. [DOI] [PubMed] [Google Scholar]
- 332.Bnouham M, Merhfour FZ, Ziyyat A, Aziz M, Legssyer A, Mekhfi H. Antidiabetic effect of some medicinal plants of Oriental Morocco in neonatal non-insulin-dependent diabetes mellitus rats. Hum Exp Toxicol. 2010;29:865–871. doi: 10.1177/0960327110362704. http://dx.doi.org/10.1177/0960327110362704. [DOI] [PubMed] [Google Scholar]
- 333.Rau O, Wurglics M, Dingermann T, Abdel-Tawab M, Schubert-Zsilavecz M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Pharmazie. 2006;61:952–956. http://www.ncbi.nlm.nih.gov/pubmed/17152989. [PubMed] [Google Scholar]
- 334.Golalipour MJ, Ghafari S, Afshar M. Protective role of Urtica dioica L. (Urticaceae) extract on hepatocytes morphometric changes in STZ diabetic Wistar rats. Turk J Gastroenter. 2010;21:262–269. doi: 10.4318/tjg.2010.0098. http://www.ncbi.nlm.nih.gov/pubmed/20931430. [DOI] [PubMed] [Google Scholar]
- 335.Fazeli SA, Gharravi AM, Ghafari S, Jahanshahi M, Golalipour MJ. Effects of Urtica dioica extract on CA3 hippocampal pyramidal cell loss in young diabetic rats. Neur Regen Res. 2010;5:901–905. [Google Scholar]
- 336.Gulcin I, Kufrevioglu OI, Oktay M, Buyukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. http://dx.doi.org/10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 337.Turker AU, Usta C. Biological screening of some Turkish medicinal plant extracts for antimicrobial and toxicity activities. Nat Prod Res. 2008;22:136–146. doi: 10.1080/14786410701591663. http://dx.doi.org/10.1080/14786410701591663. [DOI] [PubMed] [Google Scholar]
- 338.Toldy A, Atalay M, Stadler K, Sasvari M, Jakus J, Jung KJ, Chung HY, Nyakas C, Radak Z. The beneficial effects of nettle supplementation and exercise on brain lesion and memory in Rat. J Nutr Biochem. 2009;20:974–981. doi: 10.1016/j.jnutbio.2008.09.001. http://dx.doi.org/10.1016/j.jnutbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 339.Modaresi M, Golchoobian H. Effect of hydro-alcoholic extract of nettle on immune system in mice. Asian J Chem. 2012;24:2339–2341. [Google Scholar]
- 340.Testai L, Chericoni S, Calderone V, Nencioni G, Nieri P, Morelli I, Martinotti E. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J Ethnopharmacol. 2002;81:105–109. doi: 10.1016/s0378-8741(02)00055-7. http://dx.doi.org/10.1016/S0378-8741(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 341.Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5:1–11. http://dx.doi.org/10.1080/J157v05n04_01. [PubMed] [Google Scholar]
- 342.El Haouari M, Bnouham M, Bendahou M, Aziz M, Ziyyat A, Legssyer A, Mekhfi H. Inhibition of rat platelet aggregation by Urtica dioica leaves extracts. Phytotherapy Res. 2006;20:568–572. doi: 10.1002/ptr.1906. [DOI] [PubMed] [Google Scholar]
- 343.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684–6688. doi: 10.3748/wjg.v11.i42.6684. http://www.ncbi.nlm.nih.gov/pubmed/16425366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kandis H, Karapolat S, Yildirim U, Saritas A, Gezer S, Memisogullari R. Effects of Urtica dioica on hepatic ischemia-reperfusion injury in rats. Clinics. 2010;65:1357–1361. doi: 10.1590/S1807-59322010001200021. http://dx.doi.org/10.1590/S1807-59322010001200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Denzler KL, Waters R, Jacobs BL, Rochon Y, Langland JO. Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals. PLoS One. 2010;5:e12561. doi: 10.1371/journal.pone.0012561. http://dx.doi.org/10.1371/journal.pone.0012561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gansser D, Spiteller GGG. Aromatase inhibitors from Urtica diodica roots. Planta Med. 1995;61:138–140. doi: 10.1055/s-2006-958033. http://dx.doi.org/10.1055/s-2006-958033. [DOI] [PubMed] [Google Scholar]
- 347.Sharaf M, el-Ansari MA, Matlin SA, Saleh NA. Four flavonoid glycosides from Peganum harmala. Phytochemistry. 1997;44:533–536. doi: 10.1016/s0031-9422(96)00531-6. http://dx.doi.org/10.1016/S0031-9422(96)00531-6. [DOI] [PubMed] [Google Scholar]
- 348.Sobhani AM, Ebrahimi SA, Mahmoudian M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J Pharm Pharm Sci. 2002;5:19–23. http://www.ncbi.nlm.nih.gov/pubmed/12042115. [PubMed] [Google Scholar]
- 349.Hussain Z, Waheed A, Qureshi RA, Burdi DK, Verspohl EJ, Khan N, Hasan M. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother Res. 2004;18:73–77. doi: 10.1002/ptr.1372. http://dx.doi.org/10.1002/ptr.1372. [DOI] [PubMed] [Google Scholar]
- 350.Shapira Z, Terkel J, Egozi Y, Nyska A, Friedman J. Abortifacient potential for the epigeal parts of Peganum harmala. J Ethnopharmacol. 1989;27:319–325. doi: 10.1016/0378-8741(89)90006-8. http://dx.doi.org/10.1016/0378-8741(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 351.Singh AB, Khaliq T, Chaturvedi JP, Narender T, Srivastava AK. Anti-diabetic and anti-oxidative effects of 4-hydroxypipecolic acid in C57BL/KsJ-db/db mice. Human Exp Toxicol. 2012;31:57–65. doi: 10.1177/0960327111407227. http://dx.doi.org/10.1177/0960327111407227. [DOI] [PubMed] [Google Scholar]
- 352.Astulla A, Zaima K, Matsuno Y, Hirasawa Y, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H. Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med. 2008;62:470–472. doi: 10.1007/s11418-008-0259-7. http://dx.doi.org/10.1007/s11418-008-0259-7. [DOI] [PubMed] [Google Scholar]
- 353.Misra P, Khaliq T, Dixit A, SenGupta S, Samant M, Kumari S, Kumar A, Kushawaha PK, Majumder HK, Saxena AK, Narender T, Dube A. Antileishmanial activity mediated by apoptosis and structure-based target study of peganine hydrochloride dihydrate: an approach for rational drug design. J Antimicrob Chemother. 2008;62:998–1002. doi: 10.1093/jac/dkn319. http://dx.doi.org/10.1093/jac/dkn319. [DOI] [PubMed] [Google Scholar]
- 354.Rahimi-Moghaddam P, Ebrahimi SA, Ourmazdi H, Selseleh M, Karjalian M, Haj-Hassani G, Alimohammadian MH, Mahmoudian M, Shafiei M. In vitro and in vivo activities of Peganum harmala extract against Leishmania major. J Res Med Sci. 2011;16:1032–1039. http://www.ncbi.nlm.nih.gov/pubmed/22279479. [PMC free article] [PubMed] [Google Scholar]
- 355.Farouk L, Laroubi A, Aboufatima R, Benharref A, Chait A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J Ethnopharmacol. 2008;115:449–454. doi: 10.1016/j.jep.2007.10.014. http://dx.doi.org/10.1016/j.jep.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 356.Bremner P, Rivera D, Calzado MA, Obon C, Inocencio C, Beckwith C, Fiebich BL, Munoz E, Heinrich M. Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J Ethnopharmacol. 2009;124:295–305. doi: 10.1016/j.jep.2009.04.035. http://dx.doi.org/10.1016/j.jep.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 357.Im JH, Jin YR, Lee JJ, Yu JY, Han XH, Im SH, Hong JT, Yoo HS, Pyo MY, Yun YP. Antiplatelet activity of beta-carboline alkaloids from Perganum harmala: a possible mechanism through inhibiting PLCgamma2 phosphorylation. Vascul Pharmacol. 2009;50:147–152. doi: 10.1016/j.vph.2008.11.008. http://dx.doi.org/10.1016/j.vph.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 358.Bahmani M, Rafieian-Kopaei M, Parsaei P, Mohsenzadegan A. The anti-leech effect of Peganum harmala L. extract and some anti-parasite drugs on Limnatis nilotica. Afr J Micro Res. 2012;6:2586–2090. [Google Scholar]
- 359.Abbasipour H, Mahmoudvand M, Rastegar F, Basij M. Insecticidal activity of Peganum harmala seed extract against the diamondback moth Plutella xylostella. Bull Insect. 2010;63:259–263. [Google Scholar]
- 360.Zeng Y, Zhang Y, Weng Q, Hu M, Zhong G. Cytotoxic and insecticidal activities of derivatives of harmine, a natural insecticidal component isolated from Peganum harmala. Molecules. 2010;15:7775–7791. doi: 10.3390/molecules15117775. http://dx.doi.org/10.3390/molecules15117775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–782. doi: 10.1016/j.fitote.2010.04.004. http://dx.doi.org/10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 362.Diba K, Shoar MG, Shabatkhori M, Khorshivand Z. Antifungal activity of alcoholic extract of Peganum harmala seeds. J Med Plant Res. 2011;5:5550–5554. [Google Scholar]
- 363.Darabpour E, Bavi AP, Motamedi H, Nejad SMS. Antibacterial activity of different parts of Peganum harmala L. growing in Iran against multi-drug resistant bacteria. Exp Clin Sci. 2011;10:252–263. [PMC free article] [PubMed] [Google Scholar]
- 364.Sarpeleh A, Sharifi K, Sonbolkar A. Evidence of antifungal activity of wild rue (Peganum harmala L.) on phytopathogenic fungi. J Plant Dis Protect. 2009;116:208–213. [Google Scholar]
- 365.Hashem M. Antifungal Properties of Crude Extracts of Five Egyptian Medicinal Plants Against Dermatophytes and Emerging Fungi. Mycopathologia. 2011;172:37–46. doi: 10.1007/s11046-010-9390-6. http://dx.doi.org/10.1007/s11046-010-9390-6. [DOI] [PubMed] [Google Scholar]
- 366.Hayet E, Maha M, Mata M, Mighri Z, Laurent G, Mahjoub A. Biological activities of Peganum harmala leaves. Afr J Biotec. 2010;9:8199–8205. [Google Scholar]
- 367.Adhami HR, Farsam H, Krenn L. Screening of medicinal plants from Iranian traditional Mmdicine for acetylcholinesterase inhibition. Phytotherapy Res. 2011;25:1148–1152. doi: 10.1002/ptr.3409. http://dx.doi.org/10.1002/ptr.3409. [DOI] [PubMed] [Google Scholar]
- 368.Zheng XY, Zhang L, Cheng XM, Zhang ZJ, Wang CH, Wang ZT. Identification of Acetylcholinesterase Inhibitors from Seeds of Plants of Genus Peganum by Thin-Layer Chromatography-Bioautography. J Planar Chromatogr Modern TLC. 2011;24:470–474. http://dx.doi.org/10.1556/JPC.24.2011.6.3. [Google Scholar]
- 369.Herraiz T, Gonxalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. Beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem Toxicol. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. http://dx.doi.org/10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 370.El Gendy MAM, Soshilov AA, Denison MS, El-Kad AOS. Harmaline and harmalol inhibit the carcinogen-activating enzyme CYP1A1 via transcriptional and posttranslational mechanisms. Food Chem Toxicol. 2012;50:353–362. doi: 10.1016/j.fct.2011.10.052. http://dx.doi.org/10.1016/j.fct.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lamchouri F, Settaf A, Cherrah Y, Hassar M, Zemzami M, Atif N, Nadori EB, Zaid A, Lyoussi B. In vitro cell-toxicity of Peganum harmala alkaloids on cancerous cell-lines. Fitoterapia. 2000;71:50–54. doi: 10.1016/s0367-326x(99)00117-3. http://dx.doi.org/10.1016/S0367-326X(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 372.Hamsa TP, Kuttan G. Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol. 2010;649:64–73. doi: 10.1016/j.ejphar.2010.09.010. http://dx.doi.org/10.1016/j.ejphar.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 373.Sodaeizadeh H, Rafieiolhossaini M, Van Damme P. Herbicidal activity of a medicinal plant, Peganum harmala L., and decomposition dynamics of its phytotoxins in the soil. Ind Crop Prod. 2010;31:385–394. http://dx.doi.org/10.1016/j.indcrop.2009.12.006. [Google Scholar]