Introduction
Recent studies have highlighted the role of the commensal microbiota in the control of natural killer T (NKT) cells and NKT cell-dependent inflammatory diseases at mucosal surfaces. In this review, we will provide a brief overview of the phenotype and function of NKT cells in immunity and will discuss the mechanisms and consequences of microbial regulation of NKT cells within the mucosa of the intestines and lungs.
CD1d and NKT cells
Natural killer T (NKT) cells are a subset of T cells, which respond to lipid antigens presented in the context of the atypical MHC class I molecule CD1d, which is expressed by rodents and humans [1,2]. NKT cells were initially identified as cells, which co-express NK and T cell markers and exhibit potent and innate-like, immediate secretion of Th1, Th2, and Th17 cytokines upon stimulation [3]. Later, it was demonstrated that NKT cells respond to lipid antigens presented by CD1d and depend on CD1d-restricted positive selection in the thymus, which is the criterion now typically used to define NKT cells and to delineate these cells from conventional peptide-reactive T cells co-expressing NK cell markers [3].
Two main subsets of NKT cells can be distinguished based on their T cell receptor (TCR) repertoire. Type I or invariant (i) NKT cells express a semi-invariant TCR composed of Vα14-Jα18 in mice and Vα24-Jα18 in humans, which pair with a restricted subset of Vβ chains [4]. iNKT cells can be specifically detected by CD1d tetramers loaded with the marine sponge glycosphingolipid α-galactosylceramide (α-GalCer), which binds to the iNKT TCR [5,6]. Type II, non-invariant or diverse NKT cells are similarly CD1d-restricted but express a less constrained TCR repertoire [7–9]. In line with a broader TCR repertoire, a lipid antigen universally recognized by all non-invariant NKT cells has not been described to date. Studies of non-invariant NKT cells have therefore relied on the characterization of CD1d-restricted, Vα14/Vα24-Jα18-negative T cells or on the study of a non-invariant NKT cell subset which recognizes sulfatide [7–10].
Invariant and non-invariant NKT cells are phenotypically and functionally distinct. iNKT cells are effector/memory cells, which exhibit baseline expression of activation markers such as CD69 and respond in an innate-like manner with immediate and substantial cytokine secretion upon activation [2]. iNKT cells can be activated by direct, CD1d-restricted presentation of self or microbial-derived lipid antigens [11]. In addition, toll-like receptor (TLR)- and dectin-1-dependent recognition of microbe-associated molecular patterns (MAMPS) by professional antigen presenting cells elicits secretion of cytokines such as IL-12, IL-18, and type I interferon, which indirectly activate iNKT cells in a process further enhanced by CD1d-restricted antigen presentation [12–15]. Indirect, cytokine-dependent iNKT cell activation provides an effective strategy for iNKT cell-dependent recognition of bacteria, viruses, and fungi devoid of lipid antigens and also contributes to iNKT cell activation in the context of bacteria containing CD1d-restricted lipid antigens [12–16]. In addition to cytokine-mediated effects, noradrenergic neurotransmitter-dependent stimulation of iNKT cells has recently been described as another indirect mechanism of iNKT cell activation [17]. In accordance with the variety of pathways to iNKT cell activation and their potent effects on other innate and adaptive immune cells, iNKT cells act as critical mediators at the interface between innate and adaptive immunity, where they regulate antimicrobial immunity, cancer immunosurveillance, and autoimmunity [2,18–21].
In contrast to iNKT cells, non-invariant NKT cells recognize distinct lipid antigens and resemble conventional T cells in that they lack constitutive expression of CD69 and are predominantly negative for the NK cell marker NK1.1 [7,10,22]. Non-invariant NKT cells are functionally diverse. A subset of non-invariant NKT cells including sulfatide-reactive type II cells exhibits regulatory functions in inflammatory disorders and inhibits tumor immunosurveillance in part through suppression of iNKT cells [10,18,22,23]. In contrast, in the context of human inflammatory bowel disease (IBD) and infectious hepatitis, non-invariant NKT cells actively contribute to inflammation [24,25]. These results suggest that functionally distinct subsets exist even within the group of non-invariant NKT cells.
The commensal microbiota regulates intestinal iNKT cell development and function
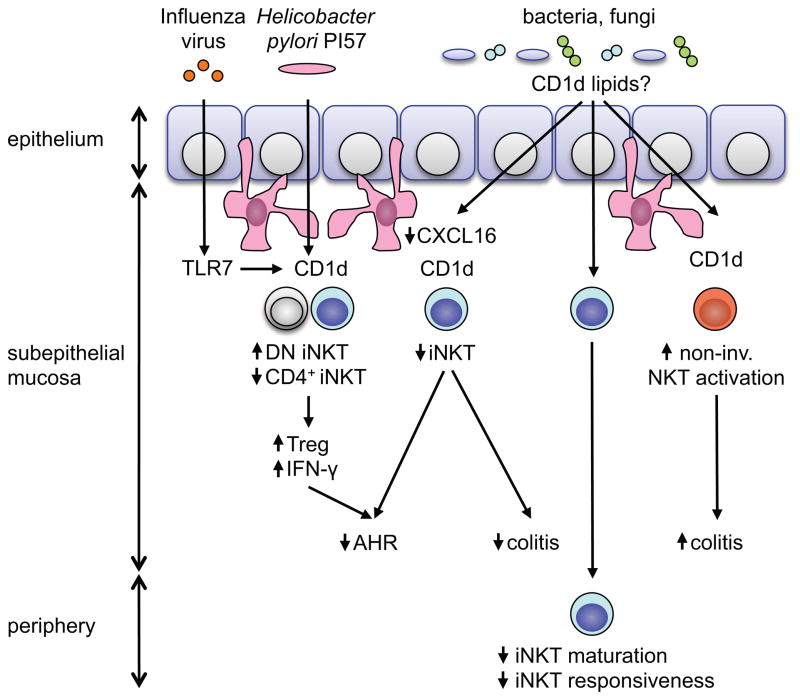
At the outer and inner surfaces of the body, NKT cells are in close contact with a rich microbiota colonizing the skin, the lung, and particularly the intestine [26–28]. This raises the question of whether microbial exposure at mucosal surfaces affects NKT cell development and function. Early work suggested that the frequency, phenotype, and function of NK1.1+ T cells in the thymus, spleen, liver, and bone marrow is unaltered in germfree (GF) mice [29]. Recent studies extended this work by utilizing CD1d tetramers instead of NK1.1 and through the analysis of iNKT cells at mucosal sites. These studies revealed that mutual pathways of regulation exist between the intestinal microbiota and NKT cells [30–33]. Thus, it was recently shown that GF mice exhibit increased relative and absolute numbers of iNKT cells in intestines, most prominently demonstrated in the colon, but not the thymus, spleen, and liver [31,33]. Mechanistic studies revealed that colonic iNKT cell levels are regulated during postnatal development in a manner dependent on CD1d and the chemokine CXCL16 and persist during life (Fig. 1) [31]. In the absence of the intestinal microbiota, a region 5′ of the Cxcl16 gene undergoes CpG hypermethylation, which is associated with increased CXCL16 expression and recruitment of iNKT cells to the colonic mucosa [31]. Importantly, microbial exposure during neonatal but not adult life restores intestinal iNKT cell levels, which demonstrates the presence of early and persistent effects of the intestinal microbiota on colonic iNKT cells [31]. Of note, even in GF mice, relative frequencies of iNKT cells in the intestine and the liver differed in a manner depending on the genetic background of mice, thus suggesting that the host genome and the intestinal microbiota independently regulate iNKT cell levels at peripheral sites [31].
Fig. 1. Graphical summary of mucosal NKT cell regulation.
At the mucosa of the large intestine and the lung, neonatal but not adult exposure to the microbiota is associated with a decrease in relative numbers of iNKT cells [31, 33]. This process is dependent on CXCL16 and CD1d and may potentially be regulated by microbiota-derived CD1d lipids [31]. As a consequence, neonatal exposure to the microbiota dampens iNKT cell-mediated intestinal inflammation and airway hyperreactivity (AHR) [31]. In addition, in neonatal but not adult mice, influenza A virus infection or exposure to PI57, a Helicobacter pylori-derived CD1d lipid, lead to expansion of double-negative (DN) iNKT cells, TH1 cytokine deviation, regulatory T cell (Treg) expansion, and protection from AHR [51]. At the intestinal mucosa, microbiota-dependent regulation of NKT cells differs between invariant and non-invariant (non-inv.) NKT cell subsets. While GF mice exhibit increased numbers of intestinal iNKT cells and severe iNKT cell-mediated inflammation [31], broad-spectrum antibiotic treatment dampens intestinal inflammation mediated by non-invariant NKT cells [41]. Finally, even within the invariant NKT cell subset, tissue- and potentially antigen-specific effects of the microbiota exist. In GF mice, iNKT cells at the intestinal mucosa exhibit increased activation and cytokine secretion in response to oxazolone [31], while iNKT cells in the spleen are hyporesponsive to α-GalCer stimulation [33].
In accordance with the concept of iNKT cell regulation by the intestinal microbiome, Wingender et al. reported that Vβ7 TCR usage and cytokine responses by iNKT cells differed in genetically identical mice obtained from different vendors and known to contain a distinct intestinal microbiota [33,34]. Indeed, differences in Vβ7 usage were lost upon cohousing in line with a transmissible factor responsible for these alterations [33]. Moreover, iNKT cells from GF as compared to SPF mice exhibited a reduction in Vβ7 usage as well as a less mature phenotype and were increased in relative numbers in the intraepithelial compartment and the lamina propria of the small and large intestine [33]. Together, these studies revealed profound effects of the intestinal microbiota on the phenotype and function of iNKT cells [33]. Interestingly, these studies also revealed potential organ- and/or antigen-dependent differences in iNKT cell responses in GF compared to SPF mice. As such, splenic iNKT cells of GF compared to SPF mice were hyporesponsive to α-GalCer and exhibited a less activated phenotype (Fig. 1) [33]. In contrast, intestinal iNKT cells of GF compared to SPF mice exhibited increased activation and cytokine secretion in the context of oxazolone-induced colitis [31]. These studies in GF mice indicate that, under physiologic circumstances, the intestinal microbiota may regulate iNKT cell responses in a tissue-specific manner. At mucosal sites, the microbiota appears to dampen the activity of iNKT cells and to decrease their numbers, whereas in other peripheral tissues, the microbiota may function in an opposite manner (Fig. 1).
The microbial elements which regulate intestinal NKT cells are currently unknown. iNKT cells not only respond to CD1d-restricted lipid antigens but also to cytokines such as IL-12 that are produced by dendritic cells upon TLR-mediated recognition of MAMPS [2,13]. However, mice with defects in TLR signaling due to genetic deficiency in the TLR adaptors MyD88 and Trif exhibit an unaltered phenotype and function of iNKT cells [31,33]. Moreover, exposure of GF mice to bacteria enriched in iNKT cell antigens such as Sphingomonas spp. restored CD69 expression by iNKT cells, while exposure to E. coli, a bacterium considered to be devoid of iNKT cell antigens, did not [33]. In line with these findings, mice bearing a restricted flora (RF) devoid of Sphingomonas spp. exhibited defects in iNKT cell numbers and CD69 expression [32,33]. These results, together with the observation of CD1d-dependent regulation of mucosal iNKT cell numbers [31], suggests that yet to be identified microbial lipid antigens may regulate the phenotype and function of mucosal iNKT cells in a CD1d-restricted manner.
Importantly, regulatory effects of the intestinal microbiota and the CD1d-iNKT cell axis are mutual. Thus, in addition to microbiota-dependent control of intestinal iNKT cells, CD1d and NKT cells regulate intestinal microbial colonization and the composition of the microbiota [30]. GF CD1d-deficient mice exhibit enhanced intestinal monocolonization as a consequence of defects in CD1d-mediated release of antimicrobial peptides from small intestinal Paneth cells [30]. Moreover, CD1d deficiency is associated with intestinal dysbiosis in accordance with the regulation of the intestinal microbiota by CD1d and NKT cells [30]. Similarly, CD1d-deficient mice are susceptible to wide variety of mucosal pathogens [35]. In conclusion, mucosal iNKT cells and the intestinal microbiota cross-regulate each other in a bidirectional manner.
Microbial regulation of NKT cell-dependent intestinal inflammation
NKT cells play critical roles in human IBD and its murine models [36,37]. Studies by Heller et al. revealed that intestinal inflammation in oxazolone colitis, a mouse model of human ulcerative colitis (UC), is dependent on CD1d and NKT cells [38]. IL-13 plays a dominant role in this process and is secreted by an IL-17BR+ Th2 subset of iNKT cells as well as group 2 innate lymphoid cells, both of which respond to epithelial-derived IL-25 [39]. Similar findings were made in human UC, where key pathogenic cytokines such as IL-13 were produced by CD161+ T cells in a manner dependent on CD1d implying an NKT cell-dependent process [25]. Interestingly, however, iNKT cell were suggested to be critical mediators of oxazolone colitis, while non-invariant NKT cells contributed to pathogenic cytokine production in human UC [25,38]. More recent studies have provided insight into the basis underlying these apparent species-specific effects. First, while protection from oxazolone colitis in mice deficient in the iNKT cell receptor Jα18 chain (Traj18−/− mice) suggested pathogenic roles of iNKT cells in oxazolone colitis [38], Traj18−/− mice were recently shown to not only lack iNKT cells but to also exhibit substantial defects in the conventional TCR repertoire [40]. As a consequence, protection from oxazolone colitis in Traj18−/− mice may relate to defects in iNKT cells as well as in conventional T cell subsets. Morever, mice with transgenic expression of a non-invariant NKT cell receptor (Vα3.2, Vβ9; hereafter, 24αβTg mice) developed spontaneous intestinal inflammation, which was further exacerbated in the presence of transgenic CD1d expression [41]. Together, these studies suggest a central role of NKT cells in Th2-mediated intestinal inflammation in humans and mice. In contrast, murine Th1 models of IBD revealed protective roles of iNKT cells likely through secretion of Th2 cytokines, while CD161+ T cells did not contribute to Th1 cytokine secretion in human Crohn’s disease (CD) [25,42–44]. Thus, in contrast to Th2-mediated colitis and human UC, a clear picture of the contribution of NKT cells to human CD and murine Th1-mediated disease has not emerged.
The intestinal microbiota controls the postnatal development of intestinal iNKT cells [31,33]. This raises the question of whether microbial elements also regulate iNKT cell-dependent inflammatory processes within the intestinal mucosa. Indeed, colonization of GF mice with SPF flora in neonatal but not adult mice was shown to be associated with protection from morbidity and mortality in the oxazolone colitis model, in a manner dependent on CD1d and iNKT cells (Fig. 1) [31]. These findings are in line with the hygiene hypothesis and thus the concept that microbial exposure during early life reduces the susceptibility to inflammatory diseases such as IBD and asthma [45–47]. Moreover, these results suggest that the regulation of intestinal iNKT cells by the microbiota contributes to mucosal immune homeostasis. Intriguingly, the outcome of microbial exposure is dependent on the type of NKT cell involved and the timing of microbial influences. Thus, while early postnatal exposure to the microbiota results in persistent defects in iNKT cells, microbial exposure in adult mice is not associated with homeostatic regulation of intestinal iNKT cell abundance and function [31]. In addition, neither the microbiota nor its TLR-dependent recognition are required for iNKT cell-mediated intestinal inflammation in oxazolone colitis as the inflammation is observed when the animals are GF, which suggests that the inflammatory target in this model of human UC is derived from non-microbial self elements in the sense of a bona fide autoimmune response [31,48]. In contrast, spontaneous intestinal inflammation mediated by non-invariant NKT cells in 24αβTg mice is significantly reduced upon antibiotic treatment thus demonstrating an essential role of the microbiota in this process (Fig. 1) [41].
In conclusion, the intestinal microbiota plays both protective and pathogenic roles in NKT cell-mediated intestinal inflammation, which are dependent on the time of microbial exposure and the type of NKT cell involved. Further work is required to elucidate whether the regulation of mucosal NKT cell abundance and function in health and disease is related to specific microbial species and to delineate antigen- and cytokine-dependent pathways of NKT cell activation. Moreover, it remains an intriguing question as to whether specific alterations found in the intestinal microbiota of patients with IBD [49] contribute to intestinal inflammation via regulation and activation of NKT cells.
Microbial regulation of NKT cell-dependent immune responses in the lung
Similar to the intestines, the lung forms a large internal surface of the body, which is in close contact with a variety of immune cells. Moreover, while the lower respiratory tract has traditionally been seen as a sterile environment, recent culture-independent approaches revealed a microbial density of an estimated 2,000 bacterial genomes per cm2 [27]. These results suggest that interactions between the commensal microbiota and mucosal NKT cells may also exist in the lung. Indeed, recent research by us and others demonstrated that the principles which govern commensal microbial regulation of iNKT cells in the intestine also apply to the lung. As such, GF mice harbor increased numbers of iNKT cells in the lung in a process dependent on Cxcl16 hypermethylation and increased expression of CXCL16 protein (Fig. 1) [31]. These alterations were associated with increased airway resistance, eosinophil infiltration, and proinflammatory cytokine production in an ovalbumin (OVA)-driven asthma model [31]. In addition, similar to observations in the intestine, alterations in mucosal iNKT cell abundance and susceptibility to iNKT cell-dependent airway hyperreactivity were prevented by neonatal but not adult microbial colonization of GF mice (Fig. 1) [31]. These studies suggest that inadequate microbial colonization in early life leads to increased quantities and environmental sensitivity of iNKT cells in lungs leading to susceptibility to asthma. Consistent with this, early life, but not late life, exposure to antibiotics results in increased susceptibility to experimental asthma [50].
Studies by Umetsu and colleagues further demonstrated that influenza A virus infection in suckling but not adult mice protects from allergen-induced airway reactivity later in life [51]. The mechanistic basis for these observation relied in TLR7- and T-bet-dependent expansion of a double-negative iNKT cell subset, which produced a Th1-skewed cytokine profile conferring protection against Th2-based airway hyperreactivity (Fig. 1) [51]. Moreover, in accordance with the hygiene hypothesis and the related observation of an inverse correlation between Helicobacter pylori colonization and asthma [52], a single administration of a cholesteryl α-glucoside (PI57) derived from H. pylori was able to achieve protection from OVA-induced airway hyperreactivity (Fig. 1) [51]. Specificity was demonstrated by the fact that a Sphingomonas-derived α-glucuronylceramide did not confer protection and that injection of the Th1-skewing glycosphingolipid α-C-GalCer, but not the Th1- and Th2-inducing α-GalCer prevented airway hyperreactivity [51]. Importantly, PI57 acted as a microbial antigen with direct, CD1d-dependent activation of double-negative iNKT cells in a manner independent of TLR-induced, cytokine-mediated effects on iNKT cells [51]. Finally, in addition to direct effects on iNKT cells, microbial elements were shown to suppress pathogenic actions of iNKT cells in allergic airway disease through induction of regulatory T (Treg) cells. Thus, Streptococcus pneumoniae-derived type-3-polysaccharide (T3P) and pneumolysoid induces Tregs, which suppress iNKT cell-dependent airway hyperreactivity [53].
Together, these studies elegantly illustrate how interactions between iNKT cells and a single virus or bacterial-derived lipid antigen may profoundly shape the mucosal immune system of the host leading to persistent, lifelong effects on immunity. It will consequently be critical to investigate whether alterations found in the lung microbiome of patients with asthma directly contribute to disease pathogenesis through the regulation of mucosal NKT cells [27].
Future perspective
Several recent studies have highlighted the role of commensal bacteria in the control of NKT cells at mucosal surfaces. Future studies will be required to delineate whether a similar regulation is observed at the skin, where a rich microbiota is in close contact with NKT cells and CD1a-restricted, lipid-reactive T cells [26,54]. Moreover, future work will be critical to delineate potential species- and antigen-specific effects of the microbiota on NKT cells and to investigate the roles of viruses and fungi in this process. Finally, it will be of major clinical interest to identify strategies for therapeutic manipulation of iNKT cells in inflammatory diseases at mucosal surfaces. While current knowledge suggests that protective influences of the microbiota on NKT cells are limited to early postnatal development, future efforts should focus on the development of strategies that may reinduce plasticity within the iNKT cell compartment as a pathway to mucosal homeostasis.
*Highlights.
Mucosal NKT cells are regulated by the commensal microbiota.
Microbiota-dependent iNKT modulation occurs during early postnatal development.
Antigen-dependent and -independent mechanisms contribute to iNKT cell regulation.
Microbial exposure leads to protection from iNKT cell-mediated inflammation.
Acknowledgments
Experimental studies by the authors were supported by: The Deutsche Forschungsgemeinschaft (DFG) (SZ814/1-1, 2-1, 4-1; DFG Cluster “Inflammation at Interfaces”) (S.Z.); NIH grants DK044319, DK051362, DK053056, DK088199, the Harvard Digestive Diseases Center (HDDC) (DK0034854) (R.S.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 4.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8− T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A. 2010;107:10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SH, Weiss A, Benlagha K, Kyin T, Teyton L, Bendelac A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193:893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, Chiba A, Fuchs BB, Mylonakis E, Besra GS, Levitz SM, et al. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–450. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 14.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 15.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 16.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 18.Berzofsky JA, Terabe M. The contrasting roles of NKT cells in tumor immunity. Curr Mol Med. 2009;9:667–672. doi: 10.2174/156652409788970706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinjo Y, Kronenberg M. Detection of microbes by natural killer T cells. Adv Exp Med Biol. 2009;633:17–26. doi: 10.1007/978-0-387-79311-5_3. [DOI] [PubMed] [Google Scholar]
- 21.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 22.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 24.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Program NCS, Bouffard GG, Blakesley RW, Murray PR, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur J Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, Brugman S, Yamaguchi K, Ishikawa H, Aiba Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–1250. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. This study demonstrates that neonatal exposure to the intestinal microbiota has persistent effects on iNKT cells and protects from intestinal inflammation during adulthood in a manner dependent on epigenetic modification of the Cxcl16 locus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, Kronenberg M, Braun J. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–1226. doi: 10.4049/jimmunol.0902620. Wei et al. show that germfree mice as well as those carrying a restricted intestinal flora exhibit alterations in the frequency of iNKT cells in spleen, liver, and thymus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, Braun J, Mazmanian SK, Kronenberg M. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. This study demonstrates that relative iNKT cell numbers are increased in GF mice in the small and large intestine. Moreover, splenic iNKT cells of germfree mice exhibited a less mature phenotype and hyporesponsiveness to lipid antigen-mediated activation. Phenotypic alterations of iNKT cells were restored by exposure to bacteria containing iNKT cell-activating lipids but not in response to bacteria devoid of such lipids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 36.Liao CM, Zimmer MI, Wang CR. The Functions of Type I and Type II Natural Killer T Cells in Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2013;19:1330–1338. doi: 10.1097/MIB.0b013e318280b1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeissig S, Kaser A, Dougan SK, Nieuwenhuis EE, Blumberg RS. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1101–1105. doi: 10.1152/ajpgi.00342.2007. [DOI] [PubMed] [Google Scholar]
- 38.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 39.Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH, Pannell R, Gao W, Wrigley K, Sprenkle J, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol. 2012;47:1198–1211. doi: 10.1007/s00535-012-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, Gapin L. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13:705–706. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Liao CM, Zimmer MI, Shanmuganad S, Yu HT, Cardell SL, Wang CR. dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142:326–334. e321–322. doi: 10.1053/j.gastro.2011.10.030. This study demonstrates that transgenic expression of a type II NKT cell receptor in the presence of transgenic expression of CD1d results in spontaneous intestinal inflammation in a manner dependent on the intestinal microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ, et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119–128. doi: 10.1053/gast.2000.9114. [DOI] [PubMed] [Google Scholar]
- 43.Shibolet O, Kalish Y, Klein A, Alper R, Zolotarov L, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Adoptive transfer of ex vivo immune-programmed NKT lymphocytes alleviates immune-mediated colitis. J Leukoc Biol. 2004;75:76–86. doi: 10.1189/jlb.0703351. [DOI] [PubMed] [Google Scholar]
- 44.Ueno Y, Tanaka S, Sumii M, Miyake S, Tazuma S, Taniguchi M, Yamamura T, Chayama K. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflamm Bowel Dis. 2005;11:35–41. doi: 10.1097/00054725-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R, von Mutius E, Group GTS. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein CN. Epidemiologic clues to inflammatory bowel disease. Curr Gastroenterol Rep. 2010;12:495–501. doi: 10.1007/s11894-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 48.Schiechl G, Bauer B, Fuss I, Lang SA, Moser C, Ruemmele P, Rose-John S, Neurath MF, Geissler EK, Schlitt HJ, et al. Tumor development in murine ulcerative colitis depends on MyD88 signaling of colonic F4/80+CD11b(high)Gr1(low) macrophages. J Clin Invest. 2011;121:1692–1708. doi: 10.1172/JCI42540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **51.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. The authors demonstrate that infection of suckling mice with influenza A virus or exposure to a glycolipid antigen derived from Helicobacter pylori protects against allergen-induced airway hyperreactivity in adulthood in a manner dependent on CD4−CD8− NKT cells. This study is the first to demonstrate long-lasting, iNKT cell-mediated protection against immune-mediated diseases through early microbial exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. J Immunol. 2012;188:4611–4620. doi: 10.4049/jimmunol.1101299. [DOI] [PubMed] [Google Scholar]
- 54.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]