Abstract
Heartbeats, muscle twitches, and lightning fast thoughts are all manifestations of bioelectricity and rely on the activity of a class of membrane proteins known as ion channels. The basic function of an ion channel can be distilled into: ‘The hole opens. Ions go through. The hole closes.’ Studies of the how the fundamental mechanisms by which this process happens and the consequences of such activity in the setting of excitable cells remains the central focus of much of the field. One might wonder after so many years of detailed poking at such a seemingly simple process, is there anything left to learn?
Ion channels are a concept that has fascinated scientists interested in the electrical function of the nervous system for at least the last 60 years (Catterall et al., 2012; Hille, 2001). Ion channel proteins form holes in membranes that open and close in response to various chemical and electrical stimuli. These structures allow cells to tap into the energy stored in transmembrane ionic gradients to generate the electrical signals that race through our nerves and muscles. In 1988 when Neuron launched, it published twenty-one papers devoted to some aspect of ion channel research in its first year. These covered topics spanning from basic channel biophysics to the behavior of channels in complex systems. In reflecting on the questions that motivated ion channel research 25 years ago it is striking that the spirit, if not the details, of the studies exemplified in Neuron’s inaugural year mark many of the same questions that occupy the field today. These include: What is the physical nature of a channel (Auld et al., 1988; Ballivet et al., 1988; Deneris et al., 1988; Levitan et al., 1988; Lotan et al., 1988; Rudy et al., 1988; Timpe et al., 1988)? How do ions and pharmacological tools interact with channel pores (MacKinnon et al., 1988; Miller, 1988; Miller et al., 1988)? Where are particular channels expressed (Harris et al., 1988; Siegel, 1988; Wang et al., 1988; Wisden et al., 1988; Wollner et al., 1988) and how is this regulated by development or electrical activity (Goldman et al., 1988; Hendry and Jones, 1988)? How do channels respond to manipulations in diverse types of excitable cells (Doerner and Alger, 1988; Haydon and Man-Son-Hing, 1988; Lechleiter et al., 1988; Lipscombe et al., 1988; Maricq and Korenbrot, 1988; Pfaffinger et al., 1988; Yakel and Jackson, 1988)? At the silver anniversary of the journal, we reflect on how much the field has changed, how certain classes of questions persist, and highlight some key open questions that rest upon the major achievements of the past quarter century but still represent areas of great opportunity for discovery.
The ion channel field is vast and it would take a book to do it justice. Great progress has been made in understanding how channels “gate” their pores. To capture some of this excitement in a short space, we focus on three areas of phenomenal advancement that frame key unaddressed problems: 1) the transformation from cartoon to three dimensions of our understanding of the molecular nature of channels, 2) a tale of one mechanism that is central to understanding neural signaling, voltage sensing, and 3) how the complicated, multicomponent protein complexes of channels are assembled and delivered to the right place in the cell. These basic issues permeate the biological functions of all ion channels and understanding such facets of channel biology remains critical for unraveling how channel operate in normal and disease states. Moreover, continued pursuit of these core issues is indispensible for developing new methods to manipulate channel function to drive both fundamental research and the development of new agents to treat diseases of the nervous system.
Ion channels as macromolecules
To understand an ion channel, how it works, how it is regulated, and how it interacts with pharmacological agents, one needs to know how the channel is built. Many of the core concepts about ion channel function have been developed through the study of two archetypal classes, voltage-gated channels permeable to either sodium or potassium that drive action potential propagation, and pentameric ligand gated ion channels from fast chemical synapses, typified by the nicotinic acetylcholine receptor. In this section, we focus voltage-gated ion channels and direct the reader to the following excellent reviews for a picture of the recent strides made in the ligand gated ion channel field (Changeux, 2012; Corringer et al., 2012; Unwin, 2013).
The basic concepts regarding the essential ion channel components, as exemplified by voltage-gated sodium and voltage-gated potassium channels, were in place 25 years ago and rested on exceptionally insightful biophysical and pharmacological studies of the representative channel types in native preparations (Hille, 1977a, 2001) (Figure 1A). The channel macromolecule was a protein that formed a “gated” pore having a large internal vestibule and a smaller external one. In between the vestibules lay a narrow ‘selectivity filter’ that allowed passage of certain ions. This filter was long enough to hold more than one ion at a time but so narrow that the ions needed to move through in single file. The gate was on the intracellular end of the pore and was controlled by a charged device embedded the membrane that sensed transmembrane membrane voltage. When Neuron started, the channel field was entering the molecular era. Many reports had to do with the identification and characterization of the genes for well-studied channels that turned out to be members of three channel superfamilies: voltage-gated ion channels (VGICs) (Auld et al., 1988; Catterall, 2000; Jan and Jan, 1997; Noda et al., 1986; Noda et al., 1984; Papazian et al., 1987; Tanabe et al., 1987; Tempel et al., 1987; Timpe et al., 1988), ligand gated ion channels (LGICs) (Ballivet et al., 1988; Corringer et al., 2000; Deneris et al., 1988; Mishina et al., 1984; Noda et al., 1982; Noda et al., 1983), and glutamate receptors (Hollmann and Heinemann, 1994; Hollmann et al., 1989). Such gene identification studies transformed the field as they enabled researchers to marshal the tools of site directed mutagenesis, functional studies, and chemical labeling (Karlin and Akabas, 1998) to take an activity that could only previously be studied in a native cell and manipulate it in ways that allowed them to assign particular amino acids to the function of crucial channel parts.
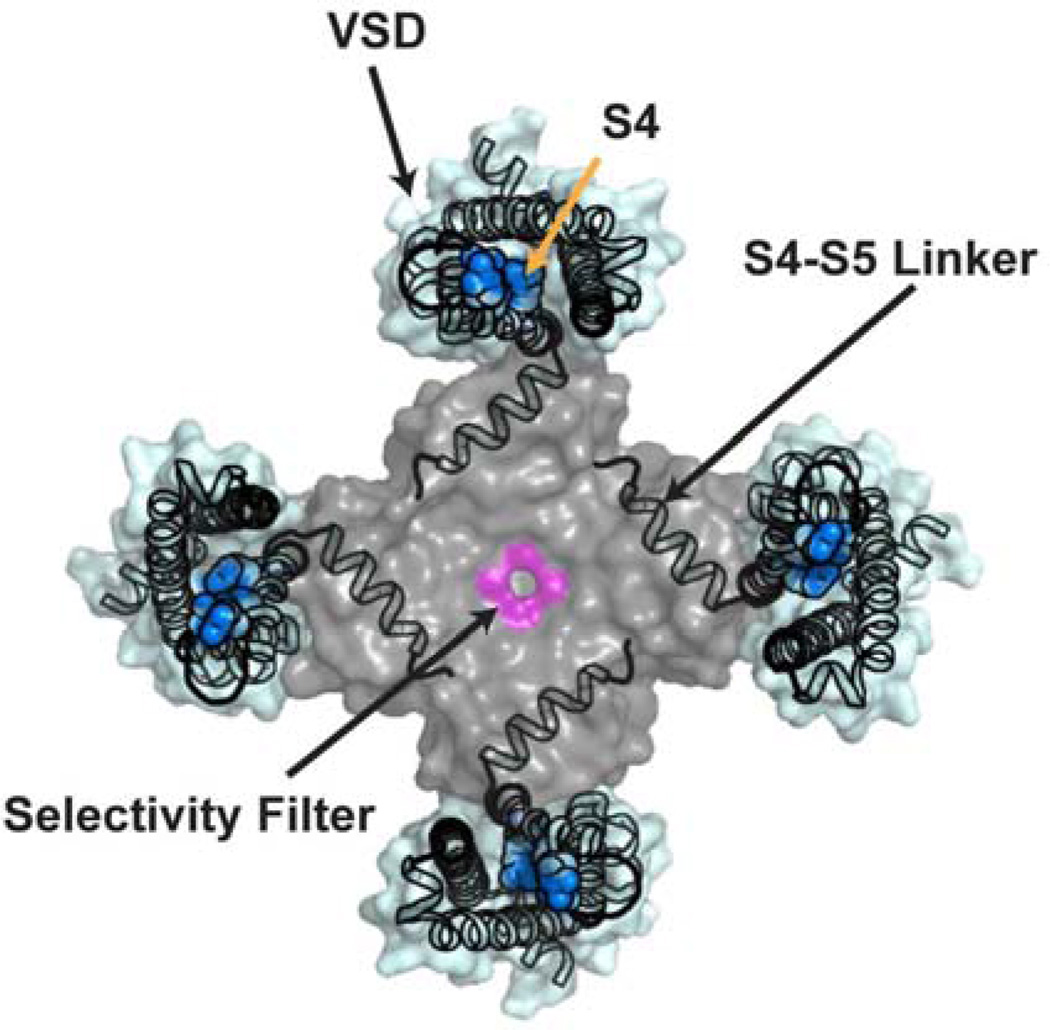
Figure 1.
Ion channels, from concept to structure. A, Cartoon model of an ion channel, based on studies of voltage-gated sodium and voltage gated potassium channels (after (Hille, 1977a)). This cartoon embodies the basic understanding of voltage-gated ion channels when ‘Neuron’ was launched. B, Unrooted tree depicting amino acid sequence relations of the minimal pore regions of VGIC superfamily members (from (Yu and Catterall, 2004)). Indicated subfamilies are (clockwise): voltage-gated calcium and sodium channels (CaV and NaV) two pore (TPC) and transient receptor potential (TRP) channels, inwardly rectifying potassium channels (Kir), calcium activated potassium channels (KCa), voltage-gated potassium channels (KV1–9), K2P channels, voltage-gated potassium channels from the EAG family (Kv10–12), cyclic nucleotide gated channels (CNG), and hyperpolarization activated channels (HCN). ‘R’ indicates recognizable regulatory domains. C, Ribbon diagram model of a bacterial sodium channel (BacNaV). With the exception of the intracellular domains, which are often sites of modulation by cellular factors and contain assembly domains, all key features in ‘A’ are present the models derived from crystallographic studies as of 2013. Model is a composite of the NaVAb (Payandeh et al., 2011) and ‘pore-only’ NaVAe1p (Shaya et al., 2013) structures. Elements from two membrane subunits and four cytoplasmic subunits are shown. Arginines in the S4 voltage-sensor are shown as space filling models. D, Illustration of PD-VSD domain swapping as seen from the extracellular side of a VGICs based on NaVAb (Payandeh et al., 2011). Individual subunits are colored, orange, cyan, yellow, and blue. Selectivity filter is violet and is indicated. Pore domain (PD) and voltage sensor domain (VSD) of the cyan subunit are indicated.
One of the most important principles that emerged from the surge of molecular identification efforts was that the transmembrane portions of voltage-gated sodium (NaV), voltage-gated potassium (KV), and voltage-gated calcium channel (CaV) pores were built from subunits that had essentially the same body plan of six segments (S1-S6) forming a single subunit in KVs or an array of four tandem repeats in NaVs and CaVs (Figure 1B). This architectural commonality provided a background for a host of mechanistic studies that defined pore lining residues (Liu et al., 1997; Ragsdale et al., 1994), selectivity filter elements (Backx et al., 1992; Ellinor et al., 1995; Heginbotham et al., 1994; Heinemann et al., 1992; Yang et al., 1993), and critical charges in the S4 segment of the voltage-sensor (Aggarwal and MacKinnon, 1996; Baker et al., 1998; Ji et al., 1996; Schoppa et al., 1992; Seoh et al., 1996; Stuhmer et al., 1989). These studies, and many others, inspired models that incorporated new ideas about the roles of particular amino acids and their possible locations within specific channel types. Because of the common core, despite idiosyncratic differences among KVs, NaVs, and CaVs in permeant ion type and in activation and inactivation properties, these details could still be discussed under the central paradigm of a gate, selectivity filter, and voltage sensor as outlined in Figure 1A.
Molecular identification of other channels revealed, unexpectedly, that the transmembrane scaffold comprising the VGIC core was found in a wide range channels that were not primarily gated by voltage, such as the large and diverse TRP channel family that has members that respond to temperature, irritants, and other sensory triggers (Nilius and Owsianik, 2011; Ramsey et al., 2006a). Moreover, two branches of the potassium channel family, inward rectifier (Kir) (Hibino et al., 2010) and two pore-domain (K2P) (Lesage and Barhanin, 2011) channels, lacked the S1-S4 segments and contained only transmembrane segments similar to the KV channel S5-S6 portion. These topology differences suggested a separation of function between the pore-forming and voltage-sensing domains and indicated a potential evolutionary route for how voltage gated channels might arise (Jan and Jan, 1994; Yu and Catterall, 2004). The later surprising discovery of two classes of membrane proteins that had S1-S4 voltage-sensor domains that were not connected to a pore module (Minor, 2006; Okamura et al., 2009), a voltage-sensitive phosphatase (Murata et al., 2005) and a proton channel (Ramsey et al., 2006b; Sasaki et al., 2006), further reinforced the idea that the core transmembrane elements of the VGIC family could have arisen by an evolutionary ‘assembly by pieces’ process. The presence such a modular structure within the membrane is now strongly supported by crystallographic studies of KVs (Long et al., 2005; Long et al., 2007) and bacterial NaVs (BacNaVs) (Payandeh et al., 2012; Payandeh et al., 2011; Zhang et al., 2012), which show largely structurally independent pore domains (PDs) and voltage sensor domains (VSDs), and protein dissection studies demonstrating that the PDs (McCusker et al., 2012; McCusker et al., 2011; Santos et al., 2012; Santos et al., 2008; Santos et al., 2006; Shaya et al., 2013; Shaya et al., 2011) and VSDs (Butterwick and MacKinnon, 2010; Chakrapani et al., 2010; Li et al., 2012) are capable of folding and operating separately. Although the modular design of soluble proteins is well known (Ye and Godzik, 2004), and is a clear principle underlying the nature of many channel extramembranous domains (Mayer, 2011; Minor, 2007), the parallel situation within the membrane portions of VGICs is striking. This modularity has been exploited to endow voltage-sensitivity onto channels that are not intrinsically voltage sensitive (Arrigoni et al., 2013; Lu et al., 2001b) and to deconstruct the action of toxins that target specific NaV VSDs (Bosmans et al., 2008). Further manipulation of this modular architecture holds great potential for engineering channels having novel properties and for developing a synthetic biology approach (Wang et al., 2013) to controlling the activity of neurons, muscle cells, and other excitable cell types.
In addition to the insights regarding the core function of a channel, which is to respond to a signal, open, and then let ions flow down their electrochemical gradients, the molecular description of the varied branches of VGIC superfamily tree revealed a striking diversification of intracellular elements attached to the core common transmembrane topology (Figure 1B). In some cases, these elements were found to have recognizable protein domains that sense metabolic signals such as cyclic nucleotides (Craven and Zagotta, 2006) or calcium (Contreras et al., 2013; Kovalevskaya et al., 2013) and help to integrate channel activity with cellular signaling events. Other intracellular domains have been shown to act in channel assembly (Haitin and Attali, 2008; Schwappach, 2008; Yi et al., 2001) and as sites for interaction with cytoplasmic subunits (Findeisen and Minor, 2010; Haitin and Attali, 2008; Pongs and Schwarz, 2010; Van Petegem et al., 2012). This molecular variation in extramembrane modules diversifies the functional properties of the basic transmembrane pore. Such architectural elaboration can endow a channel with sensitivity to multiple types of signals including calcium, phosphorylation, and protein-protein interactions. Figuring out how input signals are sensed by such modules and transmitted to the transmembrane portions of the channel remains an area filled with open questions. Additionally, many VGIC superfamily members have large regions that are not similar to known folds and that have yet undefined functions. Understanding such channel parts should inform our ability to connect the core activity of the transmembrane portion, controlling ion passage, with the more specialized activities necessary for channel function in particular cellular contexts. Focus on this integrative aspect of channel function will be essential for uncovering how the complex intracellular signaling network of a neuron, in which channels act in concert with many other signaling molecules, shapes dynamic changes in electrical activity.
Ion channel architecture in three dimensions
The molecular cloning era unveiled a VGIC superfamily that now constitutes the third largest family of signal transduction proteins, surpassed only by G-protein coupled receptors and kinases (Yu and Catterall, 2004). This molecular knowledge spurred a wealth of mutation-function studies that gave insights into the nature of the pore, selectivity filter, and gating mechanisms. Undoubtedly the remarkable cartographic power of such studies benefited from the fact that the probed areas were mostly confined to transmembrane portions that were under the strong constraint of being largely composed of helical segments. But as deeply insightful as these studies were, getting to the very essence of the macromolecular architecture responsible for channel function required direct structural studies. When understanding of channels was at the stage shown in Figure 1A it was recognized that field needed the tools of physical chemistry to understand channels better (Hille, 1977a). These tools have finally been unleashed in their full power as the molecular cloning era has given researchers the ability to make ion channels and channel domains in the amounts and of the quality required for X-ray crystallographic studies (Minor, 2007). Roughly ten years after the founding of Neuron, this still unrivaled mode of molecular characterization started to reveal the overall molecular construction underlying channels and channel domains. This information reveals the location of particular amino acids within the structure and greatly enhances the precision with which of the powerful analytical methods developed in the mutation-function era can be applied. Thus, now, with the architecture of a particular channel in full view, detailed mechanistic questions be addressed through studies that combine structural studies, functional experiments, and molecular simulations (Ostmeyer et al., 2013; Sauguet et al., 2013; Stansfeld and Sansom, 2011) and that start to realize the idea of understanding channel function from the fundamental level of physical chemistry.
The first structural breakthroughs at atomic resolution for full-length channels were enabled by the discovery of ion channels from bacteria and archaea that, to the surprise of many, possessed archetypal channels from the VGIC and LGIC families (Bocquet et al., 2007; Koishi et al., 2004; Ren et al., 2001; Schrempf et al., 1995; Tasneem et al., 2005) despite the fact that such organisms lack a nervous system. Similar to other realms of structural investigation, such bacterial and archaeal proteins proved invaluable for understanding the architecture and mechanisms behind the core functions of potassium channels (Doyle et al., 1998; Jiang et al., 2002; Zhou et al., 2001), NaVs (Payandeh et al., 2012; Payandeh et al., 2011; Zhang et al., 2012), and LGICs (Bocquet et al., 2009; Corringer et al., 2012; Hilf et al., 2010; Hilf and Dutzler, 2008, 2009). This principle of common mechanisms underlying basic biochemical functions has been fundamental to modern biochemistry (Kornberg, 2000; Monod, 1971) and should be kept in mind when questions arise regarding whether the structure or mechanistic features of a particular bacterial or archaeal channel is relevant for understanding its cousins from more ‘complex’ organisms such as humans. Although some details may be different, many features are likely conserved. Ironically, in a field that has been heavily driven by physiology, in nearly all cases, the biological role of such bacterial and archaeal channels remains a mystery.
In addition to the strides made using bacterial and archaeal channels as robust model systems for defining core channel mechanisms (ex. (Cuello et al., 2010a; Cuello et al., 2010b)), advancements in the ability to produce eukaryotic membrane proteins for crystallography has yielded structures of homomeric representatives from three of the eukaryotic potassium channel branches, KV (Long et al., 2005; Long et al., 2007), Kir (Tao et al., 2009; Whorton and MacKinnon, 2011), and K2P (Brohawn et al., 2012; Miller and Long, 2012) channels. The era of three-dimensional definition of channels has only just started. We can expect many more breakthroughs as we gain the ability to produce complicated multiprotein complexes of channels that act as heteromeric complexes, such as Kv7 channels (Soldovieri et al., 2011) and the NMDA receptor (Mayer, 2011), and multicomponent complexes such as CaVs (Findeisen and Minor, 2010) and KATP (Proks and Ashcroft, 2009).
Channels in three dimensions - Plus ça change, Plus c’est la meme chose?
Structures of bacterial, archaeal, and eukaryotic VGIC family members have revealed a wealth of information that has helped refine concepts about gating, voltage-sensor movement (Vargas et al., 2012), and ion selectivity (Alam and Jiang, 2011; Nimigean and Allen, 2011; Roux et al., 2011). Yet, if one compares overall picture of a VGIC from the pre-molecular era (Figure 1A) and that of a BacNaV from the post-structural era (Payandeh et al., 2012; Payandeh et al., 2011; Shaya et al., 2013; Zhang et al., 2012) (Figure 1C), one could come away with the impression that little has changed. The key concepts, while now defined in atomic detail, appear the same: the central pore, the narrow selectivity filter on the extracellular side, the interior aqueous cavity, the intracellular gate, and the voltage sensor bearing charged residues. Remarkably, as channels have changed from cartoon depictions to real three-dimensional structures, many of the main questions about how these various parts function remain incompletely answered and are beset by a host of new ones arising from unanticipated aspects of the channel architecture.
For example, how a VGIC senses voltage changes and how these changes cause the channel to gate remains incompletely answered (Chowdhury and Chanda, 2012). All VGIC structures to date have the voltage sensors in a conformation that is thought to represent an activated state, as it would be when the membrane is depolarized. Despite this activated position the pore domain conformations among the structures are very different. The eukaryotic KV structures have an open intracellular gate (Long et al., 2005; Long et al., 2007), whereas the full-length BacNaV structures show a closed intracellular gate that cannot allow ions to pass (Payandeh et al., 2012; Payandeh et al., 2011; Zhang et al., 2012). How can this be? These striking differences indicate that our understanding of the coupling between voltage sensor movement and channel gating is still imperfect (Chowdhury and Chanda, 2012). Moreover, we remain unclear on how much the context of the bilayer influences channel conformation. Such issues underscore the challenges in working with proteins that respond to voltage and highlight a need to develop reagents that can be used to isolate important states in the functional cycle of a VGIC.
There are at least three basic states for most channels: closed, open, and inactivated. What one would like to develop are tools for trapping such states so that representative structures of each could be obtained. For comparison, it is interesting to contrast the VGIC situation with that of another class of membrane proteins that move ions, ATP-based pumps. Thanks to the rich array of ATP analogs and other pharmacological tools, structural studies of ATP-based pumps have mapped nearly all of the major conformational intermediates of the transport cycle (Moller et al., 2010). The hope is that structural understanding of the VGIC superfamily can attain this level of description within the next decade. Moreover, even though there appears to be a common core for the transmembrane parts of VGIC family, given the shear diversity of gating inputs, which include voltage, temperature, small molecules, and lipids, there are bound to be unexpected variations in structural transitions and a lush conformational diversity that will come to light only with structural descriptions of many VGIC subtypes in different states. Developing new molecules to control channel function and obtaining structures of complexes with such modulators drive mechanistic understanding and, importantly, provide new tools for forging connections with the underlying biological functions.
From the standpoint of the Figure 1A cartoon there are two other prominent unexpected features revealed by VGIC superfamily structural studies. All of the full-length VGIC structures display a domain swapped architecture in which the pore from one subunit is next to the VSD from its neighbor rather than its own VSD (Figures 1D and 2). Such a domain-swapped architecture must impart some level of cooperativity among the channel subunits as the movement of one VSD could directly impact two PDs, the one that it contacts directly and the one it is connected to by virtue of the S4-S5 linker. Domain swapping is common in diverse soluble proteins and most often occurs in hinge-loop regions that bridge larger domains (Bennett and Eisenberg, 2004; Liu and Eisenberg, 2002; Rousseau et al., 2012), which is exactly the situation present how the S4-S5 linker bridges the VSD and PD. Both the origins and the consequences for function of this swapped topology remain unclear. Further, how is this domain swapping played out VGIC superfamily members such as CaVs, NaVs, and TPCs in which the subunits have covalent constraints between the six transmembrane blocks (Figure 1B) is not known. Domain swapping is not unique to voltage-gated channels. K2P (Brohawn et al., 2013) and Glutamate Receptor (Sobolevsky et al., 2009) structures reveal domain swapping in the membrane and extramembranous domains, respectively. Clearly, such a quirky topology, particularly within the KV, BacNaV, and K2P membrane domains, poses new challenges for how we think about biogenesis of these proteins. Not only is there a question about what the disparate pore domains do while waiting for the other three during protein synthesis, but how do they then assemble into these interlocked structures? Are there chaperones that act within the plane of the membrane to guide such processes and prevent misfolding events?
Figure 2.
The five pores of a VGIC. Extracellular view of Figure 1D. Pore domain is grey and shown as semi-transparent to reveal positions of the S4-S5 linkers. Voltage sensor domains (VSD) are light blue. S4 segments are colored blue. S4 arginine side chains, which are shown as space filling, occupy the cation selective ‘gating pore’ or ‘omega pore’ within each VSD. The selectivity filter is violet and is indicated.
The last surprise highlighted here is the way in which lipids from the bilayer seem to play a role in walling off part of the internal pore. Both BacNaVs (McCusker et al., 2012; Payandeh et al., 2012; Payandeh et al., 2011; Shaya et al., 2013; Zhang et al., 2012) and K2Ps (Brohawn et al., 2013; Brohawn et al., 2012; Miller and Long, 2012) have interior cavities in which the pore forming segments are arranged in a way that opens lateral portals into the bilayer (Brohawn et al., 2012). Studies from the Figure 1A era had proposed that hydrophobic channel blockers, such as anesthetics, might enter the channel pore from the bilayer (Hille, 1977b, 2001). These side portals now suggest a physical means for such a process. And while it should be of no surprise that a channel domain bathed in lipids might have important interactions with particular parts of the surrounding bilayer, such features do open new questions including: how might modulators move through such portals? and do the size and shape of these lateral access pathways change as the channel passes through its conformational cycle? Addressing the issue of lipid structure around a channel and its influence on structure remains challenging and an important area for further inquiry.
Thus, even though things have changed greatly as channels have progressed from cartoon conceptions to fully developed three-dimensional molecules, the basic questions of what opens the hole?, how does the hole open?, where is the gate?, and what goes through the hole? remain central to the field. These questions are not only central to studies of channels form ‘classically’ investigated types such as VGICs, LGICs, and Glutamate receptors, but are even more pressing less well understood channels built on alternative trimeric scaffolds such as P2X receptors (Jiang et al., 2013) and ASICs (Wemmie et al., 2013), and channels that respond to temperature (Nilius and Owsianik, 2011) and mechanical force (Coste et al., 2012; Kim et al., 2012).
Stops and starts on the way to understanding voltage sensing
A beautiful illustration of the iterative nature of scientific progress on ion channels and of the way that new methods enable definitive experiments to be done is the story of voltage sensing. Having invented the voltage clamp, in the 1940s and 1950s Hodgkin and Huxley simulated the complex dynamics by which the conducting devices of the squid giant axon membrane turn on and off to generate the action potential (Hodgkin and Huxley, 1952). They recognized that to sense voltage the devices needed to have a charge (perhaps an ion captured from solution) in the plane of the membrane that would be displaced inward and outward by changes in the membrane electric field. It took 20 years until Armstrong and Bezanilla measured the very small current that is generated by the motion of this “gating charge” (Armstrong and Bezanilla, 1973). While evidence accumulated over the years that the conducting devices are made of protein, it took the invention of single channel patch clamp recording (Hamill et al., 1981) to show that the mechanism of conduction through the best known conductors was too fast for a transporter and must be flux through a pore (Hille, 2001).
The cloning of the first voltage-gated sodium and potassium channels in the mid- to late-1980s led to the discovery of the strange S4 segment, the only sequence motif similarity between sodium and potassium channels: a repeat with several arginines residues spaced at intervals of three, interspersed with hydrophobic amino acids. Perhaps this was the voltage sensor? It would mean that S4 would have to sit in the membrane and slide through it in response to voltage change. The few structures available for membrane proteins at the time had shown that membrane segments tended to be α-helices oriented perpendicular to the membrane plane. These examples led Catterall, Guy, and Seetharamulu to postulate that the arginine side chains of S4 curl around the helix with the pitch similar to the red stripe on a barbershop pole. This arrangement would then allow S4 would to turn a screw-like motion as it ratcheted through the membrane and permit each arginine to replace its predecessor as the S4 helix traversed the membrane (Catterall, 1986; Guy and Seetharamulu, 1986).
Neuron was born when site-directed mutagenesis and functional analysis promised to nail down the molecular mechanism of voltage sensing. The obvious first thing to try was to neutralize S4 arginines. This was done in sodium channel (Stuhmer et al., 1989) and potassium channels (Liman et al., 1991; Papazian et al., 1991) and leading to massive shifts in the voltage dependence of gating. Case closed? Well not quite. These studies also found that substitutions of arginine with lysine produced similar shifts, despite preserving the charge.
Moreover, so did hydrophobic for hydrophobic mutations in the residues between the arginines (Lopez et al., 1991). Clearly, another approach was needed to test the contribution of the arginines to gating charge.
If only one could measure the amount of gating charge per channel directly, determine if S4 is really a transmembrane segment, and, if so, see if it moves in and out through the membrane. In 1996 two groups measured the total gating charge in a cell expressing wildtype or arginine neutralized potassium channels and divided the value by the number of channels on the cell membrane determined either using a radio-labeled blocking toxin or noise analysis (Aggarwal and MacKinnon, 1996; Seoh et al., 1996). The results closely agreed: each of the 4 subunits of the channel had 3–4 gating charges, corresponding to the first 4 arginines of S4. At about the same time, cysteine accessibility analysis in both KVs and NaVs showed that S4 does indeed span the membrane and that it moves outward with membrane depolarization by an amount that displaces the same first 4 arginines through a narrow passage, thereby accounting for the transfer of about 3 charges per subunit (Larsson et al., 1996; Yang et al., 1996; Yang and Horn, 1995). The agreement between the studies was remarkable. But one was left hankering for a real-time measure of S4 motion. Voltage clamp fluorometry made this possible, showing that the voltage-dependence and kinetics of S4 displacement precisely match the displacement of gating charge (Cha and Bezanilla, 1997; Larsson et al., 1996; Mannuzzu et al., 1996; Yang et al., 1996; Yang and Horn, 1995).
One still had some explaining to do. How does one accommodate charged arginines in a hydrophobic membrane? Conserved negatively charged residues in the S2 and S3 membrane segments were shown to electrostatically interact with S4 arginines (Papazian et al., 1995) and these could accommodate the two arginines at a time that entered the inaccessible pathway in the span of the membrane (Baker et al., 1998). Moreover, evidence was obtained that suggested that S4 does actually turn when it moves outward (Cha and Bezanilla, 1997; Glauner et al., 1999), supporting the helical screw model. But what was the nature of this pathway? A major kerfuffle followed the publication of the first structure of an archaeal voltage-gated channel, KvAP, which was caught in a conformation in which S4 lay at the interface between the membrane and the internal solution, leading to the proposal that the voltage sensing motion moved it into the membrane with the arginines remaining at the periphery facing the lipid (Jiang et al., 2003). This radical notion was supported by modeling that suggested that the dislocated charge of the arginine side chain may not be as adverse to a lipid environment as previously thought (Freites et al., 2005). However, disulfide bridging indicated that S4 borders the pore in both the resting and activated states (Gandhi et al., 2003) and subsequent structures of a mammalian potassium channel (Long et al., 2005) confirmed the intimate electrostatic pairing between S4 arginines and acidic residues in S2 and S3 shown earlier by Papazian.
The nature of the S4 arginine “conduction pathway” remained to be explained. Substitution of arginine with histidine converted the pathway to either a proton pore or pump (Starace and Bezanilla, 2004). So was this a pore of the kind through which sodium or potassium ions permeate? Or was it a narrow crevice that only could accommodate protons? More radical mutations of arginine that further reduced side chain bulk were found to turn the VSD of a potassium channel into a non-selective cation channel that “opens” when that arginine position enters the narrow pathway in the membrane (Tombola et al., 2005). Subsequent work showed that a potassium channel has five pores: one signature central pore that is selective for potassium and four peripheral gating pores or “omega pores”, one in each VSD (Tombola et al., 2007) (Figure 2). This “five-hole” architecture was present in NaVs too, where naturally occurring mutations of S4 arginines were found to cause disease (Sokolov et al., 2007; Struyk and Cannon, 2007). Striking too, the proton conducting pore of the voltage-gated Hv1 channel, which lacks a pore domain (Ramsey et al., 2006b; Sasaki et al., 2006), is located in its VSD and has been proposed to be gated by movement of S4 into a position that allows omega pore-like conductance (Koch et al., 2008; Lee et al., 2009; Tombola et al., 2008).
So, has the mechanism of voltage sensing been cracked? One could find affirmation to this question in the striking agreement between recent molecular dynamics simulation of potassium channel gating motions (Jensen et al., 2012) and 24 years of experimentation in the Neuron era. However, much remains to be explained. The “consensus model” of voltage sensing (Vargas et al., 2012) still has substantial discrepancies between KVs and NaVs channels that could indicate functional divergence or incomplete accounting of the process. Even more curious is the fact that CNG, TRP and SK channels that are not sensitive to voltage contain VSDs. Why should a channel need a VSD if it is not voltage-sensitive? Moreover, one wants to know if the peripheral location of the VSD makes it a hotspot for lipid modulation or for regulation by auxiliary subunits (Gofman et al., 2012; Nakajo and Kubo, 2011). While contact between the internal loop following S4 and a piece of the S6 intracellular gate could explain the voltage control of one aspect of gating, how is S4 motion “communicated” to the inactivation gate in the selectivity filter? Indeed, because prolonged times at zero voltage lead to slow inactivation, concern has been raised that the structures obtained to date reflect the C-type inactivated state – a state that alters S4 motion and possibly its posture. If so, not only have we never “seen” S4 at rest, but we may also not know its activated conformation. Finally, how does S4’s control of gating interact with parallel control by ion or cyclic nucleotide binding domains? So yes. In answer to the question: voltage sensing has been cracked open. Now it just needs to be closed up.
Channel biogenesis: RNA processing; channel assembly and traffic
The intricacy of the inner workings of ion channels discussed thus far makes one wonder how channels form, how they are positioned at just the right place for their physiological functions, and how they may be subjected to modulation at various stages of their biogenesis and trafficking. Inquiries about the origin and trajectory of ion channels inside the cell have revealed the great lengths and ingenious ways taken by cells to ensure that channels can their job properly. These studies have shown that right from the birth of a channel protein, neurons use a wide range of interventions to shape the properties and destinations of particular channel molecules.
RNA processing: RNA editing, alternative splicing, dendritic targeting and silencing
The first step in making a protein is to produce the mRNA that codes for it. This initial step in the life of a channel can be altered in ways that profoundly affect its function, location, and expression. Sometimes a gene sequence does not specify the protein sequence. This violation of the central dogma of molecular biology, known as ‘RNA editing’ in which particular adenosines within an mRNA are converted to inosine and change codon meaning, provides a layer of modulation that dramatically reshapes protein function and that is found in a large number of channel types (Hoopengardner et al., 2003; Huang et al., 2012; Rosenthal and Seeburg, 2012) Glutamate receptors, the first eukaryotic gene products found to be modifiable by RNA editing, exemplify the involvement of RNA editing (Lomeli et al., 1994) as well as alternative splicing (Mosbacher et al., 1994) in the regulation of channel function, assembly, and dendritic mRNA targeting (Greger et al., 2003; Isaac et al., 2007; La Via et al., 2013; Penn and Greger, 2009).
One of the best-studied RNA editing events changes an amino acid within the pore of AMPA receptors from glutamine to arginine. The resultant physiochemical change of neutral to basic side chain has a profound impact on the core function of the channel making the channel impermeable to calcium ions and lowering overall ion permeability. The importance of this editing process is highlighted by the observation that knockout of the RNA editing enzyme ADAR2 (Adenosine Deaminase Acting on RNA 2) is lethal to mice and is attributed to the critical RNA editing of glutamate receptors at the Q/R site for restricting calcium permeability (Higuchi et al., 2000). RNA editing also modifies KV, NaVs, CaVs, and LGICs (Hoopengardner et al., 2003; Huang et al., 2012). The presence and level of edited transcripts may allow excitable cells to change their electrical properties as a consequence of activity or environmental factors (Rosenthal and Seeburg, 2012). A striking example of this effect is the observation of differential RNA editing of the Kv1.1 voltage-gated potassium channel in polar, temperate, and tropical octopi at a site in the S6 segment of the pore that changes a single amino acid from isoleucine to valine and accelerates channel inactivation. This change may enables polar dwelling octopi to maintain rapid action potential firing in cold conditions (Garrett and Rosenthal, 2012).
Where the transcript goes and how it is translated is also a point of modulation that also impacts channel function. For instance, dendritic targeting and local translation of glutamate receptor mRNA is regulated by neuronal activity (Aoto et al., 2008; Grooms et al., 2006; Ju et al., 2004; Maghsoodi et al., 2008; Smith et al., 2005) and may involve RNA binding proteins such as FMRP (Fragile X Mental Retardation Protein)(Muddashetty et al., 2007; Schutt et al., 2009; Soden and Chen, 2010) and CPEB3 (Cytoplasmic Polyadenylation Element Binding protein 3)(Huang et al., 2006; Pavlopoulos et al., 2011). It is remarkable that dendritically targeted GluA1 and GluA2 mRNAs correspond to the unedited flip isoform (La Via et al., 2013), which matures more rapidly in the ER (Penn and Greger, 2009) and thus may lead to formation of AMPA receptors that permeate calcium ions (Seeburg et al., 1998). This finding raises intriguing questions about the dynamics of local production of glutamate receptors and how receptor composition and hence channel properties such as calcium permeability and kinetics may vary with neuronal activity.
As yet another example of channel modulation at the RNA level, targeting Kv1.2 mRNA via a long noncoding RNA that is upregulated by nerve injury may account for the increased excitation of dorsal root ganglion sensory neurons and neuropathic pain (Zhao et al., 2013). Thus intra- and inter-RNA duplex formation during and shortly after transcription appears to launch a variety of channel RNA processing with profound influence over whether and where a channel will be made, as well as the subunit composition and channel properties.
Channel assembly: folding and assembly of homomeric or heteromeric channels
Many ion channels are assembled from multiple transmembrane subunits, including every member of the potassium channel subfamilies from the VGIC superfamily (Figure 1B). Hence, how a channel is made and checked for proper folding and assembly by the cell is the critical first step in its lifecycle. Studies of archetypes from the VGIC (Schwappach, 2008), LGIC (Tsetlin et al., 2011; Valles and Barrantes, 2012), and GluR (Hansen et al., 2010; Sukumaran et al., 2012) superfamilies have begun to outline the role of key domains in guiding assembly and heteromeric assembly specificity, and have started to map the importance of interaction with quality control elements of the ER and Golgi.
One of the best studied examples is in Kv channels where the cytoplasmic N-terminal tetramerization domain facilitates assembly of subunits within the same subfamily (Covarrubias et al., 1991; Kreusch et al., 1998; Li et al., 1992); replacing this T1 domain with an artificial tetramerization domain supports channel assembly but alters channel kinetics whereas removal of T1 domain drastically reduces surface expression of functional channels (Deutsch, 2002; Minor et al., 2000; Zerangue et al., 2000). Thus, whereas the T1 domain acquires its tertiary structure shortly after emerging from the ribosomal exit tunnel (Kosolapov et al., 2004) and enables interactions among Kv subunits still attached to ribosomes (Lu et al., 2001a), there are other subunit interactions that mediate channel assembly. This notion is echoed by the finding of reduced surface expression of GluA2 lacking its amino terminal domain (ATD) for dimerization (Kumar et al., 2011). Dimerization of dimers is another common theme for Kv channels (Tu and Deutsch, 1999) and glutamate receptors (Kumar et al., 2011).
Studies of Kv channel biogenesis illustrate how a monomeric channel subunit first acquires secondary structure within the ribosome and then folds into a membrane protein in the ER. Most of the helical segments that span the membrane or reside in sequences connecting transmembrane segments adopt their compact structures within a permissive vestibule in the ribosomal tunnel near the exit port (Tu and Deutsch, 2010). This is thought to followed by concerted insertion of the VSD, linker, and PD (Sato et al., 2002; Tu et al., 2000). Remarkably, despite the fact that many parts of the PD make subunit-subunit interactions within a fully assembled channel, the PD portion of a single Kv subunit appears to be able to adopt its tertiary fold in the absence of other Kv subunits (Gajewski et al., 2011). This finding suggests that each subunit takes on a fairly mature appearance prior to tetramerization and raises questions about what happens to the polar elements of the transmembrane portions, which face the ion conducing portions of the selectivity filter and central pore in the fully assembled channels, while they are waiting to interact with the other three members required to make a functioning pore.
The exposure of polar residues within the transmembrane domain is likely to facilitate ER retention or retrieval of monomeric channel subunits and partially folded or assembled channel complexes via ER quality control machineries involving proteins like Rer1 (Sato et al., 2003; Sato et al., 2004), as shown for muscle acetylcholine receptor subunits (Valkova et al., 2011). Inefficient folding as demonstrated for CFTR chloride channels and squid KVs (Liu et al., 2001; Ward et al., 1995) and exposure of charged residues on voltage sensors to the membrane could lead to the association of Rer1 and calnexin to NaVs due to their affinity for transmembrane segments that contain charged residues, causing channels to be chaperoned to various trafficking pathways (Li et al., 2010).
There are some common themes for channel biogenesis shared by tetrameric VGICs and the pentameric LGICs. Surface expression of nicotinic acetylcholine receptors and GABAA receptors depends on the evolutionarily conserved ER membrane complex (EMC) that regulates protein folding and ER-associated degradation (Richard et al., 2013). Unlike dimers lacking GABAA receptor a or β subunit that are retained in the ER, assembly of heterodimers of a and β subunits involves calnexin and the immunoglobulin heavy chain binding protein BiP (Bradley et al., 2008; Connolly et al., 1996; Luscher et al., 2011). In addition to ER chaperones such as BiP/GRP78, calnexin and ERp57 (Blount and Merlie, 1991; Colombo et al., 2013; Gelman et al., 1995; Paulson et al., 1991; Wanamaker and Green, 2007), the ER membrane protein RIC-3 regulates acetylcholine receptor assembly and ER dwell time (Alexander et al., 2010).
One striking finding is that often, interaction with small molecules, including the natural ligand of a channel, can influence biogenesis. Not only does glutamate act as a chemical chaperone in the biogenesis of glutamate receptors (Penn and Greger, 2009), but GABA may be an intracellular chaperone for GABAA receptor biogenesis (Eshaq et al., 2010) and nicotine may act in a similar way for nascent α4β2 and α3β4 nicotinic acetylcholine receptors in the ER to enhance their surface expression (Colombo et al., 2013; Govind et al., 2012; Mazzo et al., 2013; Miwa et al., 2011; Sallette et al., 2005; Srinivasan et al., 2011). Similar mechanisms may be involved in the rescue of deficient trafficking of a mutant HERG potassium channel in human long QT syndrome by HERG channel blockers (Rajamani et al., 2002; Zhou et al., 1999), and the ability of sulfonylureas to function as chemical chaperones to rescue the trafficking defects of ATP-sensitive potassium channels bearing certain mutations that cause congenital hyperinsulinism (Yan et al., 2004). Together, these observations suggest that a better understanding of ion channel biogenesis should enlighten understanding of basic issues about membrane protein folding and may also yield new means to intervene in cases where channel activity has gone wrong in disease states.
Channel traffic: early internal decisions regulating channel density and composition
Insuring that only properly folded and assembled channels make it to plasma membrane is important as channels that lack key elements of regulation, could cause serious dysfunction. Starting with ER quality control, proteins that reside in the ER or Golgi shuttle channel complexes between these intracellular compartments before mature channels proceed in forward traffic to reach the cell membrane (Colombo et al., 2013; Dancourt and Barlowe, 2010; Deutsch, 2003; Luscher et al., 2011; Schwappach, 2008). Curiosity about the remarkable ability of a cell to enforce octomeric assembly of ATP-sensitive potassium (KATP) channels containing four Kir6.1/2 subunits of the Kir family and four SUR1/2 subunits of an equally ancient transporter family led to the finding that COPI recognition of arginine-based motifs on these a and b subunits in partially assembled KATP channel complexes causes their retrieval from the Golgi back to the ER (Heusser et al., 2006; Yuan et al., 2003; Zerangue et al., 1999). Similar arginine-based ER retrieval motifs have been found in TASK channels (O'Kelly et al., 2002), sodium channels (Zhang et al., 2008), glutamate receptors (Horak et al., 2008; Nasu-Nishimura et al., 2006; Ren et al., 2003; Scott et al., 2001; Vivithanaporn et al., 2006; Xia et al., 2001), acetylcholine receptors (Keller et al., 2001; Srinivasan et al., 2011) and the ER resident CALF-1 (Calcium Channel Localization Factor-1) that promotes surface expression of calcium channels (Saheki and Bargmann, 2009).
Short traffic motifs have also been found to facilitate ER exit and forward trafficking of channels, such as diacidic motifs in potassium channels (Ma et al., 2001; Mikosch and Homann, 2009; Mikosch et al., 2006; Zuzarte et al., 2007) via potentially cooperative interactions with Sec24 cargo receptors of COPII vesicles (Mikosch et al., 2009; Sieben et al., 2008), and the I/LXM motif in the acetylcholine receptor β4 subunit that binds to Sec24D/C but not Sec24A/B cargo receptors (Mancias and Goldberg, 2008). These diverse interactions exemplify the distinct cargo-binding capacities of Sec24 paralogs (Dong et al., 2012; Lord et al., 2013; Miller and Schekman, 2013).
Not only do the Sec24 cargo receptors in the pre-budding Sec23-Sec24-Sar1 complex serve evolutionarily conserved functions for forward trafficking of various ion channels, the cornichon family of proteins that may interact with both cargos and the Sec23-Sec24-Sar1 complex for incorporation into COPII vesicles could also function as cargo receptors in organisms ranging from yeast to mammals. Drosophila Cornichon is a cargo receptor for ER export of the TGFα-like growth factor Gurken (Bokel et al., 2006). In yeast, the cornichon homologs Erv14p and Erv15p are cargo receptors for membrane proteins important for yeast budding and sporulation (Nakanishi et al., 2007; Powers and Barlowe, 2002). Erv14p is also crucial for functional expression of mammalian potassium channels in yeast (Haass et al., 2007). Mammalian cornichon homologs 2 and 3 (CNIH-2/-3) that associate with AMPA receptors in central neurons can increase their surface expression and alter channel properties in expression systems (Gill et al., 2011; Kato et al., 2010; Schwenk et al., 2009). Conditional knockout of these mouse cornichon homologs reduces surface expression of heteromeric AMPA receptors that contain GluA1 and GluA2; the selectivity for GluA1 is attributed to the ability of TARP (Transmembrane AMPA receptor Regulatory Protein) -8 to prevent functional association of cornichons with non-GluA1 AMPA subunits (Herring et al., 2013). These examples illustrate how cargo receptors and dedicated auxiliary subunits may regulate channel traffic thereby controlling channel density and composition.
As channels assemble in the ER and traffic through the secretory pathway and endosomal pathway, they are exposed to different chaperones and modifying enzymes as well as different pHs ranging from pH 7.2 in the ER lumen, to pH to 6.0–6.7 in the Golgi, and 5.5 in secretory vesicles (Mindell, 2012; Stauber and Jentsch, 2013). Retrieval of channels from the cell surface for recycling or degradation also takes channels from a neutral to a low pH environment on the extracellular/luminal side. The sensitivity of various channels to pH on the extracellular and luminal side of the membrane may be one of the mechanisms to modulate channel activity in different intracellular compartments and seems to be a fundamental property of the channel life cycle that deserves increased scrutiny.
What next?
In the final paragraphs of this Perspective, we offer some thoughts for key challenges that remain for the field:
Channels as macromolecular complexes in motion
Since the first characterization of the squid axonal sodium and potassium conductances and their voltage dependence sixty years ago by Hodgkin and Huxley (Hodgkin and Huxley, 1952), a desire to understand the nature and mechanics of ion channels has driven the field to devise novel approaches, such as the patch clamp (Hamill et al., 1981), and to harness challenging technologies including crystallography and real time monitoring of channel conformational changes, in order to study how ion channels work and how they mediate neuronal signaling. These studies have uncovered the molecular motions of sensing and gating most completely in voltage-gated (Chowdhury and Chanda, 2012; Tombola et al., 2006; Vargas et al., 2012), acetylcholine-gated (Changeux, 2012; Corringer et al., 2012; Unwin, 2013), and glutamate-gated (Mayer, 2011; Paoletti et al., 2013) channels, and revealed the modular construction of many channel types, both within the membrane portions (Minor, 2006; Yu and Catterall, 2004) and in the extramembranous parts (Mayer, 2011; Minor, 2007). Understanding how such multicomponent devices act to integrate input signals that regulate the basic function of opening a hole for ions to pass remains a major challenge.
There are many channel families in which the gating mechanisms are still very obscure, including thermosensation by TRP channels (Nilius and Owsianik, 2011; Ramsey et al., 2006a), mechano-sensation by Piezo channels (Coste et al., 2012; Kim et al., 2012), and the gating of CRAC channels via formation of multiprotein complexes that involve both plasma and intracellular membrane components (McNally and Prakriya, 2012). Basic questions of how the hole opens and closes, what drives the transitions, and what regulates gating remain central to understanding these fascinating molecules. Amazingly, even in this genomics era, the molecular identity of some channels and channel-mediated signaling processes in the nervous system remain elusive. Notable among these is the still mysterious molecular identity of the mechanotransduction channel that is responsible for hearing (Kazmierczak and Muller, 2012). Molecular identification of these still missing in action molecules will bring us back to the basic questions of how does the hole open and what goes through it?
It will be essential to obtain multiple structures of different types of channels captured in each of their functional states so that gating transitions measured functionally and molecular motions detected optically can be understood in terms of atomic rearrangements. Only this knowledge will bring us to the point where our understanding of molecular mechanism can be put to the test of recapitulation by realistic molecular dynamics simulations and movies of structures in action. Moreover, information of this kind should make it possible to understand how disease mutations (Ashcroft, 2000; Ashcroft, 2006) affect function. Reaching these goals will require further developments in structural studies, new ways to trap channel states, and additional methods for observing gating in real time in the manner of voltage-clamp fluorometry. Additionally, as computational power continues to increase and simulations approach the timescales of actual gating events (Jensen et al., 2010; Jensen et al., 2012), we also expect that more insights into molecular mechanism will come from a combination of simulation and experiment (Dror et al., 2012; Ostmeyer et al., 2013; Sauguet et al., 2013; Stansfeld and Sansom, 2011).
Pharmacological reagents, channel control, and brain function
Although some of the classically studied channels have well developed pharmacologies (Hille, 2001), most channel types lack selective agents that could be used to manipulate their function or identify them in a native setting. This inability to control function not only hinders studies of basic mechanisms, but prevents understanding of what particular channels do in complex environments such as a brain slice or whole animal. To return to 1988, one of the studies in the Neuron inaugural year used a selective high-affinity compound, saxitoxin, to follow the maturation of NaVs in rat retina (Wollner et al., 1988). Why, twenty-five years later, do we still lack high-affinity and highly selective compounds for most of the cloned channels? Similar to the call placed in 1977 that highlighted the need for the tools of physical chemistry to be marshaled to understand channels better, we make the call for the tools of chemical biology and ligand discovery to be employed to develop small molecules (Bagal et al., 2013; Dunlop et al., 2008; Wulff et al., 2009) and biologics (Baron et al., 2013; Klint et al., 2012; Lewis et al., 2012) that can selectivity affect channel function. One approach would be to use the emerging structural data as a platform for virtual docking to find leads, as is being done using the multitude of new GPCR structures (Shoichet and Kobilka, 2012).
In addition to structure-guided modulator discovery, another promising approach for gaining control of a particular channels is the use of tethered ligands, in which covalent tethering provides specificity and high local concentration to overcome a lack of ligand selectivity and low affinity (Erlanson et al., 2004). A variant of this approach that is particularly suited to the study of the nervous system is the photoswitched-tethered ligand. In this case, the linker connecting the active moiety to the protein can be rapidly and reversibly photoisomerized using two wavelengths of light to alternatively present ligand to its binding site and remove it and thereby activate or antagonize channels or block their pores (Szobota and Isacoff, 2010). Another promising strategy would be to engineer channels to respond to non-native and normally inert ligands (Shapiro et al., 2012), as has been done in the so-called RASSL and DREADD G protein coupled receptors (Alexander et al., 2009; Pei et al., 2008). The attraction of these latter methods is that they can bring the precision of studies that have been carried out in non-neuronal cells in Neuron’s first 25 years to the natural world of supermolecular complexes in neurons and within the intact neural circuits in vivo.
The answer, then, to the question ‘Is there anything left to learn?’ is a resounding ‘Yes!!!’ There remain many critical issues of basic mechanism that need to be sorted out for many channel classes. The exciting thing for the coming quarter century is that channelologists will have an ever increasing ability to move from approaching channels as macromolecules to channels as biological entities. Making such connections should take us closer to the dream of understanding the function of the most complex device of all driven by life’s spark: the human brain.
Acknowledgements
We thank B. Hille and W. A. Catterall for assistance with the figures and K. Brejc for critical comments on the manuscript. This work was supported by grants to D.L.M. from NIH R01-HL080050, R01-DC007664, R01-MH093603, and U54-GM094625 to E.Y.I. from NIH R01 NS35549 and L.Y.J. from the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- Alam A, Jiang Y. Structural studies of ion selectivity in tetrameric cation channels. J Gen Physiol. 2011;137:397–403. doi: 10.1085/jgp.201010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JK, Sagher D, Krivoshein AV, Criado M, Jefford G, Green WN. Ric-3 promotes alpha7 nicotinic receptor assembly and trafficking through the ER subcompartment of dendrites. J Neurosci. 2010;30:10112–10126. doi: 10.1523/JNEUROSCI.6344-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Arrigoni C, Schroeder I, Romani G, Van Etten JL, Thiel G, Moroni A. The voltage-sensing domain of a phosphatase gates the pore of a potassium channel. J Gen Physiol. 2013;141:389–395. doi: 10.1085/jgp.201210940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. Ion Channels and Disease. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science. 1992;257:248–251. doi: 10.1126/science.1321496. [DOI] [PubMed] [Google Scholar]
- Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, et al. Ion channels as therapeutic targets: a drug discovery perspective. Journal of medicinal chemistry. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- Baker OS, Larsson HP, Mannuzzu LM, Isacoff EY. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in shaker K+ channel gating. Neuron. 1998;20:1283–1294. doi: 10.1016/s0896-6273(00)80507-3. [DOI] [PubMed] [Google Scholar]
- Ballivet M, Nef P, Couturier S, Rungger D, Bader CR, Bertrand D, Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988;1:847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Baron A, Diochot S, Salinas M, Deval E, Noel J, Lingueglia E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon: official journal of the International Society on Toxinology. 2013;75C:187–204. doi: 10.1016/j.toxicon.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Eisenberg D. The evolving role of 3D domain swapping in proteins. Structure. 2004;12:1339–1341. doi: 10.1016/j.str.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Blount P, Merlie JP. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J Cell Biol. 1991;113:1125–1132. doi: 10.1083/jcb.113.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- Bokel C, Dass S, Wilsch-Brauninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclaire MF, Swartz KJ. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–208. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Taghibiglou C, Collingridge GL, Wang YT. Mechanisms involved in the reduction of GABAA receptor alpha1-subunit expression caused by the epilepsy mutation A322D in the trafficking-competent receptor. J Biol Chem. 2008;283:22043–22050. doi: 10.1074/jbc.M801708200. [DOI] [PubMed] [Google Scholar]
- Brohawn SG, Campbell EB, MacKinnon R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc Natl Acad Sci U S A. 2013;110:2129–2134. doi: 10.1073/pnas.1218950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwick JA, MacKinnon R. Solution structure and phospholipid interactions of the isolated voltage-sensor domain from KvAP. J Mol Biol. 2010;403:591–606. doi: 10.1016/j.jmb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of voltage-sensitive sodium channels. Annual review of biochemistry. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Raman IM, Robinson HP, Sejnowski TJ, Paulsen O. The Hodgkin-Huxley heritage: from channels to circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14064–14073. doi: 10.1523/JNEUROSCI.3403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, Bezanilla F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 1997;19:1127–1140. doi: 10.1016/s0896-6273(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Sompornpisut P, Intharathep P, Roux B, Perozo E. The activated state of a sodium channel voltage sensor in a membrane environment. Proc Natl Acad Sci U S A. 2010;107:5435–5440. doi: 10.1073/pnas.0914109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem. 2012;287:40207–40215. doi: 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Chanda B. Perspectives on: conformational coupling in ion channels: thermodynamics of electromechanical coupling in voltage-gated ion channels. J Gen Physiol. 2012;140:613–623. doi: 10.1085/jgp.201210840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SF, Mazzo F, Pistillo F, Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Contreras GF, Castillo K, Enrique N, Carrasquel-Ursulaez W, Castillo JP, Milesi V, Neely A, Alvarez O, Ferreira G, Gonzalez C, et al. A BK (Slo1) channel journey from molecule to physiology. Channels. 2013;7 doi: 10.4161/chan.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annual review of pharmacology and toxicology. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Poitevin F, Prevost MS, Sauguet L, Delarue M, Changeux JP. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure. 2012;20:941–956. doi: 10.1016/j.str.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Wei AA, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- Cuello LG, Jogini V, Cortes DM, Pan AC, Gagnon DG, Dalmas O, Cordero-Morales JF, Chakrapani S, Roux B, Perozo E. Structural basis for the coupling between activation and inactivation gates in K(+) channels. Nature. 2010a;466:272–275. doi: 10.1038/nature09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K(+) channels. Nature. 2010b;466:203–208. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Connolly J, Boulter J, Wada E, Wada K, Swanson LW, Patrick J, Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988;1:45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Deutsch C. Potassium channel ontogeny. Annu Rev Physiol. 2002;64:19–46. doi: 10.1146/annurev.physiol.64.081501.155934. [DOI] [PubMed] [Google Scholar]
- Deutsch C. The birth of a channel. Neuron. 2003;40:265–276. doi: 10.1016/s0896-6273(03)00506-3. [DOI] [PubMed] [Google Scholar]
- Doerner D, Alger BE. Cyclic GMP depresses hippocampal Ca2+ current through a mechanism independent of cGMP-dependent protein kinase. Neuron. 1988;1:693–699. doi: 10.1016/0896-6273(88)90168-7. [DOI] [PubMed] [Google Scholar]
- Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple arg motif mediates alpha(2B)-adrenergic receptor interaction with Sec24C/D and export. Traffic. 2012;13:857–868. doi: 10.1111/j.1600-0854.2012.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dror RO, Dirks RM, Grossman JP, Xu H, Shaw DE. Biomolecular simulation: a computational microscope for molecular biology. Annual review of biophysics. 2012;41:429–452. doi: 10.1146/annurev-biophys-042910-155245. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nature reviews Drug discovery. 2008;7:358–368. doi: 10.1038/nrd2552. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Yang J, Sather WA, Zhang JF, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Erlanson DA, Wells JA, Braisted AC. Tethering: fragment-based drug discovery. Annual review of biophysics and biomolecular structure. 2004;33:199–223. doi: 10.1146/annurev.biophys.33.110502.140409. [DOI] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R, 2nd, Smith SS, Robinson LC, Leidenheimer NJ. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res. 2010;1346:1–13. doi: 10.1016/j.brainres.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F, Minor DL., Jr Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation. Channels. 2010;4:459–474. doi: 10.4161/chan.4.6.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites JA, Tobias DJ, von Heijne G, White SH. Interface connections of a transmembrane voltage sensor. Proc Natl Acad Sci U S A. 2005;102:15059–15064. doi: 10.1073/pnas.0507618102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski C, Dagcan A, Roux B, Deutsch C. Biogenesis of the pore architecture of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 2011;108:3240–3245. doi: 10.1073/pnas.1017097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi CS, Clark E, Loots E, Pralle A, Isacoff EY. The orientation and molecular movement of a k(+) channel voltage-sensing domain. Neuron. 2003;40:515–525. doi: 10.1016/s0896-6273(03)00646-9. [DOI] [PubMed] [Google Scholar]
- Garrett S, Rosenthal JJ. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science. 2012;335:848–851. doi: 10.1126/science.1212795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman MS, Chang W, Thomas DY, Bergeron JJ, Prives JM. Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J Biol Chem. 1995;270:15085–15092. doi: 10.1074/jbc.270.25.15085. [DOI] [PubMed] [Google Scholar]
- Gill MB, Kato AS, Roberts MF, Yu H, Wang H, Tomita S, Bredt DS. Cornichon-2 modulates AMPA receptor-transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J Neurosci. 2011;31:6928–6938. doi: 10.1523/JNEUROSCI.6271-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauner KS, Mannuzzu LM, Gandhi CS, Isacoff EY. Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature. 1999;402:813–817. doi: 10.1038/45561. [DOI] [PubMed] [Google Scholar]
- Gofman Y, Shats S, Attali B, Haliloglu T, Ben-Tal N. How does KCNE1 regulate the Kv7.1 potassium channel? Model-structure, mutations, and dynamics of the Kv7.1-KCNE1 complex. Structure. 2012;20:1343–1352. doi: 10.1016/j.str.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Goldman D, Brenner HR, Heinemann S. Acetylcholine receptor alpha-, beta-, gamma-, and delta-subunit mRNA levels are regulated by muscle activity. Neuron. 1988;1:329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32:2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy HR, Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci U S A. 1986;83:508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass FA, Jonikas M, Walter P, Weissman JS, Jan YN, Jan LY, Schuldiner M. Identification of yeast proteins necessary for cell-surface function of a potassium channel. Proc Natl Acad Sci U S A. 2007;104:18079–18084. doi: 10.1073/pnas.0708765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitin Y, Attali B. The C-terminus of Kv7 channels: a multifunctional module. J Physiol. 2008;586:1803–1810. doi: 10.1113/jphysiol.2007.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv : European journal of physiology. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Furukawa H, Traynelis SF. Control of assembly and function of glutamate receptors by the amino-terminal domain. Molecular pharmacology. 2010;78:535–549. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GL, Henderson LP, Spitzer NC. Changes in densities and kinetics of delayed rectifier potassium channels during neuronal differentiation. Neuron. 1988;1:739–750. doi: 10.1016/0896-6273(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Man-Son-Hing H. Low- and high-voltage-activated calcium currents: their relationship to the site of neurotransmitter release in an identified neuron of Helisoma. Neuron. 1988;1:919–927. doi: 10.1016/0896-6273(88)90149-3. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Terlau H, Stuhmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Herring BE, Shi Y, Suh YH, Zheng CY, Blankenship SM, Roche KW, Nicoll RA. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron. 2013;77:1083–1096. doi: 10.1016/j.neuron.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J Cell Sci. 2006;119:4353–4363. doi: 10.1242/jcs.03196. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Bertozzi C, Zimmermann I, Reiter A, Trauner D, Dutzler R. Structural basis of open channel block in a prokaryotic pentameric ligand-gated ion channel. Nature structural & molecular biology. 2010;17:1330–1336. doi: 10.1038/nsmb.1933. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels of nerve: questions for theoretical chemists. Bio Systems. 1977a;8:195–199. doi: 10.1016/0303-2647(77)90040-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977b;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual review of neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M, O'Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989;342:643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Hoopengardner B, Bhalla T, Staber C, Reenan R. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–836. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- Horak M, Chang K, Wenthold RJ. Masking of the endoplasmic reticulum retention signals during assembly of the NMDA receptor. J Neurosci. 2008;28:3500–3509. doi: 10.1523/JNEUROSCI.5239-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tan BZ, Shen Y, Tao J, Jiang F, Sung YY, Ng CK, Raida M, Kohr G, Higuchi M, et al. RNA editing of the IQ domain in Ca(v)1.3 channels modulates their Ca(2)(+)-dependent inactivation. Neuron. 2012;73:304–316. doi: 10.1016/j.neuron.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Kan MC, Lin CL, Richter JD. CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J. 2006;25:4865–4876. doi: 10.1038/sj.emboj.7601322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annual review of neuroscience. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- Jensen MO, Borhani DW, Lindorff-Larsen K, Maragakis P, Jogini V, Eastwood MP, Dror RO, Shaw DE. Principles of conduction and hydrophobic gating in K+ channels. Proc Natl Acad Sci U S A. 2010;107:5833–5838. doi: 10.1073/pnas.0911691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MO, Jogini V, Borhani DW, Leffler AE, Dror RO, Shaw DE. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- Ji S, George AL, Jr, Horn R, Barchi RL. Paramyotonia congenita mutations reveal different roles for segments S3 and S4 of domain D4 in hSkM1 sodium channel gating. J Gen Physiol. 1996;107:183–194. doi: 10.1085/jgp.107.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Taly A, Grutter T. Moving through the gate in ATP-activated P2X receptors. Trends in biochemical sciences. 2013;38:20–29. doi: 10.1016/j.tibs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods in enzymology. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Ho MT, Yu H, Tu Y, Siuda ER, Wang H, Qian YW, Nisenbaum ES, Tomita S, et al. Hippocampal AMPA receptor gating controlled by both TARP and cornichon proteins. Neuron. 2010;68:1082–1096. doi: 10.1016/j.neuron.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Muller U. Sensing sound: molecules that orchestrate mechanotransduction by hair cells. Trends in neurosciences. 2012;35:220–229. doi: 10.1016/j.tins.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SH, Lindstrom J, Ellisman M, Taylor P. Adjacent basic amino acid residues recognized by the COP I complex and ubiquitination govern endoplasmic reticulum to cell surface trafficking of the nicotinic acetylcholine receptor alpha-Subunit. J Biol Chem. 2001;276:18384–18391. doi: 10.1074/jbc.M100691200. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klint JK, Senff S, Rupasinghe DB, Er SY, Herzig V, Nicholson GM, King GF. Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads. Toxicon : official journal of the International Society on Toxinology. 2012;60:478–491. doi: 10.1016/j.toxicon.2012.04.337. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci U S A. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi R, Xu H, Ren D, Navarro B, Spiller BW, Shi Q, Clapham DE. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. doi: 10.1074/jbc.M313100200. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Ten commandments: lessons from the enzymology of DNA replication. Journal of bacteriology. 2000;182:3613–3618. doi: 10.1128/jb.182.13.3613-3618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosolapov A, Tu L, Wang J, Deutsch C. Structure acquisition of the T1 domain of Kv1.3 during biogenesis. Neuron. 2004;44:295–307. doi: 10.1016/j.neuron.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kovalevskaya NV, van de Waterbeemd M, Bokhovchuk FM, Bate N, Bindels RJ, Hoenderop JG, Vuister GW. Structural analysis of calmodulin binding to ion channels demonstrates the role of its plasticity in regulation. Pflugers Archiv: European journal of physiology; 2013. [DOI] [PubMed] [Google Scholar]
- Kreusch A, Pfaffinger PJ, Stevens CF, Choe S. Crystal structure of the tetramerization domain of the Shaker potassium channel. Nature. 1998;392:945–948. doi: 10.1038/31978. [DOI] [PubMed] [Google Scholar]
- Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Via L, Bonini D, Russo I, Orlandi C, Barlati S, Barbon A. Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing. Nucleic Acids Res. 2013;41:617–631. doi: 10.1093/nar/gks1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Lechleiter JD, Dartt DA, Brehm P. Vasoactive intestinal peptide activates Ca2(+)-dependent K+ channels through a cAMP pathway in mouse lacrimal cells. Neuron. 1988;1:227–235. doi: 10.1016/0896-6273(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Lee SY, Letts JA, MacKinnon R. Functional reconstitution of purified human Hv1 H+ channels. J Mol Biol. 2009;387:1055–1060. doi: 10.1016/j.jmb.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology. 2011;26:424–437. doi: 10.1152/physiol.00029.2011. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Blair LA, Dionne VE, Barnard EA. Biophysical and pharmacological properties of cloned GABAA receptor subunits expressed in Xenopus oocytes. Neuron. 1988;1:773–781. doi: 10.1016/0896-6273(88)90125-0. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Dutertre S, Vetter I, Christie MJ. Conus venom peptide pharmacology. Pharmacological reviews. 2012;64:259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- Li M, Jan YN, Jan LY. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257:1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Li Q, Jogini V, Wanderling S, Cortes DM, Perozo E. Expression, purification, and reconstitution of the voltage-sensing domain from Ci-VSP. Biochemistry. 2012;51:8132–8142. doi: 10.1021/bi300980q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Su YY, Wang H, Li L, Wang Q, Bao L. Transmembrane segments prevent surface expression of sodium channel Nav1.8 and promote calnexin-dependent channel degradation. J Biol Chem. 2010;285:32977–32987. doi: 10.1074/jbc.M110.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Hess P, Weaver F, Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991;353:752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien RW, Tsien RY. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988;1:355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Liu TI, Lebaric ZN, Rosenthal JJ, Gilly WF. Natural substitutions at highly conserved T1-domain residues perturb processing and functional expression of squid Kv1 channels. J Neurophysiol. 2001;85:61–71. doi: 10.1152/jn.2001.85.1.61. [DOI] [PubMed] [Google Scholar]
- Liu Y, Eisenberg D. 3D domain swapping: as domains continue to swap. Protein science : a publication of the Protein Society. 2002;11:1285–1299. doi: 10.1110/ps.0201402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Lopez GA, Jan YN, Jan LY. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K+ channels. Neuron. 1991;7:327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- Lord C, Ferro-Novick S, Miller EA. The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I, Volterra A, Dash P, Siegelbaum SA, Goelet P. Blockade of ion channel expression in Xenopus oocytes with complementary DNA probes to Na+ and K+ channel mRNAs. Neuron. 1988;1:963–971. doi: 10.1016/0896-6273(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Lu J, Robinson JM, Edwards D, Deutsch C. T1-T1 interactions occur in ER membranes while nascent Kv peptides are still attached to ribosomes. Biochemistry. 2001a;40:10934–10946. doi: 10.1021/bi010763e. [DOI] [PubMed] [Google Scholar]
- Lu Z, Klem AM, Ramu Y. Ion conduction pore is conserved among potassium channels. Nature. 2001b;413:809–813. doi: 10.1038/35101535. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291:316–319. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- MacKinnon R, Reinhart PH, White MM. Charybdotoxin block of Shaker K+ channels suggests that different types of K+ channels share common structural features. Neuron. 1988;1:997–1001. doi: 10.1016/0896-6273(88)90156-0. [DOI] [PubMed] [Google Scholar]
- Maghsoodi B, Poon MM, Nam CI, Aoto J, Ting P, Chen L. Retinoic acid regulates RARalpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–515. doi: 10.1016/0896-6273(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzo F, Pistillo F, Grazioso G, Clementi F, Borgese N, Gotti C, Colombo SF. Nicotine-Modulated Subunit Stoichiometry Affects Stability and Trafficking of alpha3beta4 Nicotinic Receptor. J Neurosci. 2013;33:12316–12328. doi: 10.1523/JNEUROSCI.2393-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker EC, Bagneris C, Naylor CE, Cole AR, D'Avanzo N, Nichols CG, Wallace BA. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nature communications. 2012;3:1102. doi: 10.1038/ncomms2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker EC, D'Avanzo N, Nichols CG, Wallace BA. A simplified bacterial “Pore” provides insight into the assembly, stability and structure of sodium channels. J Biol Chem. 2011;286:16386–16391. doi: 10.1074/jbc.C111.228122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally BA, Prakriya M. Permeation, selectivity and gating in store-operated CRAC channels. J Physiol. 2012;590:4179–4191. doi: 10.1113/jphysiol.2012.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikosch M, Homann U. How do ER export motifs work on ion channel trafficking? Curr Opin Plant Biol. 2009;12:685–689. doi: 10.1016/j.pbi.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Mikosch M, Hurst AC, Hertel B, Homann U. Diacidic motif is required for efficient transport of the K+ channel KAT1 to the plasma membrane. Plant Physiol. 2006;142:923–930. doi: 10.1104/pp.106.087064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikosch M, Kaberich K, Homann U. ER export of KAT1 is correlated to the number of acidic residues within a triacidic motif. Traffic. 2009;10:1481–1487. doi: 10.1111/j.1600-0854.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- Miller C. Competition for block of a Ca2(+)-activated K+ channel by charybdotoxin and tetraethylammonium. Neuron. 1988;1:1003–1006. doi: 10.1016/0896-6273(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Miller C, Stahl N, Barrol M. A thermodynamic analysis of monovalent cation permeation through a K(+)-selective ion channel. Neuron. 1988;1:159–164. doi: 10.1016/0896-6273(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Miller EA, Schekman R. COPII - a flexible vesicle formation system. Curr Opin Cell Biol. 2013;25:420–427. doi: 10.1016/j.ceb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Minor DL., Jr A sensitive channel family replete with sense and motion. Nature structural & molecular biology. 2006;13:388–390. doi: 10.1038/nsmb0506-388. [DOI] [PubMed] [Google Scholar]
- Minor DL., Jr The neurobiologist's guide to structural biology: a primer on why macromolecular structure matters and how to evaluate structural data. Neuron. 2007;54:511–533. doi: 10.1016/j.neuron.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor DL, Lin YF, Mobley BC, Avelar A, Jan YN, Jan LY, Berger JM. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell. 2000;102:657–670. doi: 10.1016/s0092-8674(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Mishina M, Kurosaki T, Tobimatsu T, Morimoto Y, Noda M, Yamamoto T, Terao M, Lindstrom J, Takahashi T, Kuno M, et al. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984;307:604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70:20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JV, Olesen C, Winther AM, Nissen P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Quarterly reviews of biophysics. 2010;43:501–566. doi: 10.1017/S003358351000017X. [DOI] [PubMed] [Google Scholar]
- Monod J. Chance and necessity; an essay on the natural philosophy of modern biology, 1st American edn. New York: Knopf; 1971. [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Nakajo K, Kubo Y. Nano-environmental changes by KCNE proteins modify KCNQ channel function. Channels. 2011;5:397–401. doi: 10.4161/chan.5.5.16468. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Suda Y, Neiman AM. Erv14 family cargo receptors are necessary for ER exit during sporulation in Saccharomyces cerevisiae. J Cell Sci. 2007;120:908–916. doi: 10.1242/jcs.03405. [DOI] [PubMed] [Google Scholar]
- Nasu-Nishimura Y, Hurtado D, Braud S, Tang TT, Isaac JT, Roche KW. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J Neurosci. 2006;26:7014–7021. doi: 10.1523/JNEUROSCI.0573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome biology. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean CM, Allen TW. Origins of ion selectivity in potassium channels from the perspective of channel block. J Gen Physiol. 2011;137:405–413. doi: 10.1085/jgp.201010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S. Expression of functional sodium channels from cloned cDNA. Nature. 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, et al. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, Toyosato M, Furutani Y, Hirose T, Asai M, Inayama S, Miyata T, Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982;299:793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Furutani Y, Hirose T, Takashima H, Inayama S, Miyata T, et al. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983;302:528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Murata Y, Iwasaki H. Voltage-sensing phosphatase: actions and potentials. J Physiol. 2009;587:513–520. doi: 10.1113/jphysiol.2008.163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostmeyer J, Chakrapani S, Pan AC, Perozo E, Roux B. Recovery from slow inactivation in K+ channels is controlled by water molecules. Nature. 2013;501:121–124. doi: 10.1038/nature12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature reviews Neuroscience. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Shao XM, Seoh SA, Mock AF, Huang Y, Wainstock DH. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Paulson HL, Ross AF, Green WN, Claudio T. Analysis of early events in acetylcholine receptor assembly. J Cell Biol. 1991;113:1371–1384. doi: 10.1083/jcb.113.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlopoulos E, Trifilieff P, Chevaleyre V, Fioriti L, Zairis S, Pagano A, Malleret G, Kandel ER. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell. 2011;147:1369–1383. doi: 10.1016/j.cell.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Rogan SC, Yan F, Roth BL. Engineered GPCRs as tools to modulate signal transduction. Physiology. 2008;23:313–321. doi: 10.1152/physiol.00025.2008. [DOI] [PubMed] [Google Scholar]
- Penn AC, Greger IH. Sculpting AMPA receptor formation and function by alternative RNA processing. RNA Biol. 2009;6:517–521. doi: 10.4161/rna.6.5.9552. [DOI] [PubMed] [Google Scholar]
- Pfaffinger PJ, Leibowitz MD, Subers EM, Nathanson NM, Almers W, Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988;1:477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- Powers J, Barlowe C. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol Biol Cell. 2002;13:880–891. doi: 10.1091/mbc.01-10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Ashcroft FM. Modeling K(ATP) channel gating and its regulation. Progress in biophysics and molecular biology. 2009;99:7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Rajamani S, Anderson CL, Anson BD, January CT. Pharmacological rescue of human K(+) channel long-QT2 mutations: human ether-a-go-go-related gene rescue without block. Circulation. 2002;105:2830–2835. doi: 10.1161/01.cir.0000019513.50928.74. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006a;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006b;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Xu H, Yue L, Shi Q, Clapham DE. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Garcia EP, Sanders JM, Swanson GT, Marshall J. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci. 2003;23:6608–6616. doi: 10.1523/JNEUROSCI.23-16-06608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Boulin T, Robert VJ, Richmond JE, Bessereau JL. Biosynthesis of ionotropic acetylcholine receptors requires the evolutionarily conserved ER membrane complex. Proc Natl Acad Sci U S A. 2013;110:E1055–E1063. doi: 10.1073/pnas.1216154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal JJ, Seeburg PH. A-to-I RNA editing: effects on proteins key to neural excitability. Neuron. 2012;74:432–439. doi: 10.1016/j.neuron.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Schymkowitz J, Itzhaki LS. Implications of 3D domain swapping for protein folding, misfolding and function. Advances in experimental medicine and biology. 2012;747:137–152. doi: 10.1007/978-1-4614-3229-6_9. [DOI] [PubMed] [Google Scholar]
- Roux B, Berneche S, Egwolf B, Lev B, Noskov SY, Rowley CN, Yu H. Ion selectivity in channels and transporters. J Gen Physiol. 2011;137:415–426. doi: 10.1085/jgp.201010577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Hoger JH, Lester HA, Davidson N. At least two mRNA species contribute to the properties of rat brain A-type potassium channels expressed in Xenopus oocytes. Neuron. 1988;1:649–658. doi: 10.1016/0896-6273(88)90164-x. [DOI] [PubMed] [Google Scholar]
- Saheki Y, Bargmann CI. Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat Neurosci. 2009;12:1257–1265. doi: 10.1038/nn.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Santos JS, Asmar-Rovira GA, Han GW, Liu W, Syeda R, Cherezov V, Baker KA, Stevens RC, Montal M. Crystal structure of a voltage-gated K+ channel pore module in a closed state in lipid membranes. J Biol Chem. 2012;287:43063–43070. doi: 10.1074/jbc.M112.415091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JS, Grigoriev SM, Montal M. Molecular template for a voltage sensor in a novel K+ channel. III. Functional reconstitution of a sensorless pore module from a prokaryotic Kv channel. J Gen Physiol. 2008;132:651–666. doi: 10.1085/jgp.200810077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JS, Lundby A, Zazueta C, Montal M. Molecular template for a voltage sensor in a novel K+ channel. I. Identification and functional characterization of KvLm, a voltage-gated K+ channel from Listeria monocytogenes. J Gen Physiol. 2006;128:283–292. doi: 10.1085/jgp.200609572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes. Mol Biol Cell. 2003;14:3605–3616. doi: 10.1091/mbc.E02-12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. Endoplasmic reticulum quality control of unassembled iron transporter depends on Rer1p-mediated retrieval from the golgi. Mol Biol Cell. 2004;15:1417–1424. doi: 10.1091/mbc.E03-10-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Sakaguchi M, Goshima S, Nakamura T, Uozumi N. Integration of Shaker-type K+ channel, KAT1, into the endoplasmic reticulum membrane: synergistic insertion of voltage-sensing segments, S3-S4, and independent insertion of pore-forming segments, S5-P-S6. Proc Natl Acad Sci U S A. 2002;99:60–65. doi: 10.1073/pnas.012399799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Poitevin F, Murail S, Van Renterghem C, Moraga-Cid G, Malherbe L, Thompson AW, Koehl P, Corringer PJ, Baaden M, et al. Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. The EMBO journal. 2013;32:728–741. doi: 10.1038/emboj.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Schrempf H, Schmidt O, Kummerlen R, Hinnah S, Muller D, Betzler M, Steinkamp T, Wagner R. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. The EMBO journal. 1995;14:5170–5178. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwappach B. An overview of trafficking and assembly of neurotransmitter receptors and ion channels (Review) Molecular membrane biology. 2008;25:270–278. doi: 10.1080/09687680801960998. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Shapiro MG, Frazier SJ, Lester HA. Unparalleled control of neural activity using orthogonal pharmacogenetics. ACS chemical neuroscience. 2012;3:619–629. doi: 10.1021/cn300053q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaya D, Findeisen F, Abderemane-Ali F, Arrigoni C, Wong S, Reddy Nurva S, Loussouarn G, Minor DL., Jr Structure of a prokaryotic sodium channel pore reveals essential gating elements and an outer ion binding site common to eukaryotic channels. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.10.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaya D, Kreir M, Robbins RA, Wong S, Hammon J, Bruggemann A, Minor DL., Jr Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteins. Proc Natl Acad Sci U S A. 2011;108:12313–12318. doi: 10.1073/pnas.1106811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichet BK, Kobilka BK. Structure-based drug screening for G-protein-coupled receptors. Trends in pharmacological sciences. 2012;33:268–272. doi: 10.1016/j.tips.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben C, Mikosch M, Brandizzi F, Homann U. Interaction of the K(+)-channel KAT1 with the coat protein complex II coat component Sec24 depends on a di-acidic endoplasmic reticulum export motif. Plant J. 2008;56:997–1006. doi: 10.1111/j.1365-313X.2008.03658.x. [DOI] [PubMed] [Google Scholar]
- Siegel RE. The mRNAs encoding GABAA/benzodiazepine receptor subunits are localized in different cell populations of the bovine cerebellum. Neuron. 1988;1:579–584. doi: 10.1016/0896-6273(88)90107-9. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology. 2011;26:365–376. doi: 10.1152/physiol.00009.2011. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld PJ, Sansom MS. Molecular simulation approaches to membrane proteins. Structure. 2011;19:1562–1572. doi: 10.1016/j.str.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. doi: 10.1146/annurev-physiol-030212-183702. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol. 2007;130:11–20. doi: 10.1085/jgp.200709755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Sukumaran M, Penn AC, Greger IH. AMPA receptor assembly: atomic determinants and built-in modulators. Advances in experimental medicine and biology. 2012;970:241–264. doi: 10.1007/978-3-7091-0932-8_11. [DOI] [PubMed] [Google Scholar]
- Szobota S, Isacoff EY. Optical control of neuronal activity. Annual review of biophysics. 2010;39:329–348. doi: 10.1146/annurev.biophys.093008.131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasneem A, Iyer LM, Jakobsson E, Aravind L. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome biology. 2005;6:R4. doi: 10.1186/gb-2004-6-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Timpe LC, Jan YN, Jan LY. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988;1:659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Gorostiza P, Isacoff EY. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. doi: 10.1038/nature05396. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annual review of cell and developmental biology. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetlin V, Kuzmin D, Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. Journal of neurochemistry. 2011;116:734–741. doi: 10.1111/j.1471-4159.2010.07060.x. [DOI] [PubMed] [Google Scholar]
- Tu L, Deutsch C. Evidence for dimerization of dimers in K+ channel assembly. Biophys J. 1999;76:2004–2017. doi: 10.1016/S0006-3495(99)77358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Wang J, Helm A, Skach WR, Deutsch C. Transmembrane biogenesis of Kv1.3. Biochemistry. 2000;39:824–836. doi: 10.1021/bi991740r. [DOI] [PubMed] [Google Scholar]
- Tu LW, Deutsch C. A folding zone in the ribosomal exit tunnel for Kv1.3 helix formation. J Mol Biol. 2010;396:1346–1360. doi: 10.1016/j.jmb.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes. Quarterly reviews of biophysics. 2013:1–40. doi: 10.1017/S0033583513000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkova C, Albrizio M, Roder IV, Schwake M, Betto R, Rudolf R, Kaether C. Sorting receptor Rer1 controls surface expression of muscle acetylcholine receptors by ER retention of unassembled alpha-subunits. Proc Natl Acad Sci U S A. 2011;108:621–625. doi: 10.1073/pnas.1001624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles AS, Barrantes FJ. Chaperoning alpha7 neuronal nicotinic acetylcholine receptors. Biochim Biophys Acta. 2012;1818:718–729. doi: 10.1016/j.bbamem.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Lobo PA, Ahern CA. Seeing the forest through the trees: towards a unified view on physiological calcium regulation of voltage-gated sodium channels. Biophys J. 2012;103:2243–2251. doi: 10.1016/j.bpj.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas E, Yarov-Yarovoy V, Khalili-Araghi F, Catterall WA, Klein ML, Tarek M, Lindahl E, Schulten K, Perozo E, Bezanilla F, et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J Gen Physiol. 2012;140:587–594. doi: 10.1085/jgp.201210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Yan S, Swanson GT. Intracellular trafficking of KA2 kainate receptors mediated by interactions with coatomer protein complex I (COPI) and 14-3-3 chaperone systems. J Biol Chem. 2006;281:15475–15484. doi: 10.1074/jbc.M512098200. [DOI] [PubMed] [Google Scholar]
- Wanamaker CP, Green WN. Endoplasmic reticulum chaperones stabilize nicotinic receptor subunits and regulate receptor assembly. J Biol Chem. 2007;282:31113–31123. doi: 10.1074/jbc.M705369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu HP, Wang XM, Ballivet M, Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988;1:527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Wang YH, Wei KY, Smolke CD. Synthetic biology: advancing the design of diverse genetic systems. Annual review of chemical and biomolecular engineering. 2013;4:69–102. doi: 10.1146/annurev-chembioeng-061312-103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nature reviews Neuroscience. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Morris BJ, Darlison MG, Hunt SP, Barnard EA. Distinct GABAA receptor alpha subunit mRNAs show differential patterns of expression in bovine brain. Neuron. 1988;1:937–947. doi: 10.1016/0896-6273(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Wollner DA, Scheinman R, Catterall WA. Sodium channel expression and assembly during development of retinal ganglion cells. Neuron. 1988;1:727–737. doi: 10.1016/0896-6273(88)90171-7. [DOI] [PubMed] [Google Scholar]
- Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nature reviews Drug discovery. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41:714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Jackson MB. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988;1:615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yan F, Lin CW, Weisiger E, Cartier EA, Taschenberger G, Shyng SL. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem. 2004;279:11096–11105. doi: 10.1074/jbc.M312810200. [DOI] [PubMed] [Google Scholar]
- Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yang N, Horn R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Ye Y, Godzik A. Comparative analysis of protein domain organization. Genome research. 2004;14:343–353. doi: 10.1101/gr.1610504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi BA, Minor DL, Jr, Lin YF, Jan YN, Jan LY. Controlling potassium channel activities: Interplay between the membrane and intracellular factors. Proc Natl Acad Sci U S A. 2001;98:11016–11023. doi: 10.1073/pnas.191351798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004 doi: 10.1126/stke.2532004re15. re15. [DOI] [PubMed] [Google Scholar]
- Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Jan YN, Jan LY. An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1. Proc Natl Acad Sci U S A. 2000;97:3591–3595. doi: 10.1073/pnas.060016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasaka T, He J, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZN, Li Q, Liu C, Wang HB, Wang Q, Bao L. The voltage-gated Na+ channel Nav1.8 contains an ER-retention/retrieval signal antagonized by the beta3 subunit. J Cell Sci. 2008;121:3243–3252. doi: 10.1242/jcs.026856. [DOI] [PubMed] [Google Scholar]
- Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16:1024–1031. doi: 10.1038/nn.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig-Muller R. A di-acidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic. 2007;8:1093–1100. doi: 10.1111/j.1600-0854.2007.00593.x. [DOI] [PubMed] [Google Scholar]