Abstract
The formation of G protein-coupled receptor (GPCR) heteromers elicits signaling diversification and holds great promise for improved drug selectivity. Most studies have been conducted in heterologous expression systems; however, in vivo validation is missing from most cases thus questioning the physiological significance of GPCR heteromerization. Melatonin MT1 and MT2 receptors have been shown to exist as homo- and heteromers in vitro. We show here that the effect of melatonin on rod photoreceptor light sensitivity is mediated by melatonin MT1/MT2 receptor heteromers. This effect involves activation of the heteromer-specific PLC/PKC pathway and is abolished in MT1−/− and MT2−/− mice as well as in mice overexpressing a non-functional MT2 receptor mutant that competes with the formation of functional MT1/MT2 heteromers in photoreceptor cells. This study establishes the essential role of melatonin receptor heteromers in retinal function and supports the physiological importance of GPCR heteromerization. Finally, our work may have important therapeutic implications, as the heteromer complex may provide a unique pharmacological target to improve photoreceptor functioning and to extend the viability of photoreceptors during aging.
Introduction
G protein-coupled receptors (GPCRs), also called ‘seven-transmembrane receptors’, form the largest protein family of the human genome with approximately 800 members. GPCRs sense the extracellular environment and are involved in many cellular processes. The structural resolution of several GPCRs confirmed the high degree of conservation of their overall structure despite well-known ligand diversity ranging from photons, metabolites, lipids and peptides to proteins (1). In addition, GPCRs are major drug targets accounting for up to 30% of currently marketed drugs (2). Many reports indicate that GPCRs have the potential to interact with themselves (homomers) and with other GPCRs (heteromers). Structural studies have shown that some GPCRs crystallize as homodimers displaying several putative dimer interfaces, and these homodimers are awaiting confirmation in a physiologically relevant cellular environment (3). Although monomeric GPCRs represent the minimal signaling unit (4, 5), GPCR oligomerization, in particular heteromerization, may provide additional pharmacological and functional properties distinct from those of the individual receptors of which they are comprised (6–8). GPCR heteromers could provide additional pharmaceutical targets leading to improved drug selectivity by acting only on those cells coexpressing both receptors (9). Whereas there is compelling evidence for the existence of a number of GPCR heteromers in transfected cells, in vivo evidence is still lacking in most cases (10, 11) and their physiological relevance remains an intense matter of debate (12). Selected examples, for which strong in vivo evidence for GPCR heteromerization exist, underscore the great potential of GPCR heteromers as future therapeutic targets (13–17).
Two members of the melatonin receptor subfamily in humans, melatonin receptor type 1 (MT1) and melatonin receptor type 2 (MT2), have a high potential to homo- and heteromerize in a constitutive manner when transfected in HEK293T cells at physiological concentration (18). Moreover, the propensity for homo- and heteromer formation does not seem to be identical. Whereas the propensity of human MT1/MT2 heteromer and MT1 homomer formation is similar, that of MT2 homomer formation is 3- to 4-fold lower, suggesting that the MT2 receptor preferentially exists as a heteromeric complex with MT1 (19). MT1 and MT2 receptors bind melatonin with similar affinity, and both inhibit the adenylyl cyclase pathway through Gi proteins (20, 21). The functional consequences of melatonin receptor heteromerization are currently unknown. The formation of MT1/MT2 heteromers has been proposed to occur in the retina and in other tissues where both receptors are detected (22). However, direct evidence is still missing. In humans, both melatonin receptors have been located on rod photoreceptors and on ganglion cells, making these cells likely candidates for MT1/MT2 heteromer formation (23–26).
Previous studies have shown that melatonin is synthesized during the night in the mammalian retina reaching concentrations in the pico to low nanomolar range (27, 28), where it plays an important role in the regulation of retinal physiology and pathophysiology (see (29) for a recent review). Indeed melatonin modulates the visual functions by increasing photoreceptor light sensitivity at night (30–32) and is implicated in the pathogenesis of age-related macular degeneration and glaucoma (33–35).
The electroretinogram (ERG) is a commonly used method to assess retinal functioning and it mainly consists of a-wave and b-wave. In the dark-adapted ERGs, the a-wave represents the response of the photoreceptors to a flash of light whereas the b-wave represents the response of the bipolar cells (36), thus the amplitudes of the a- and b-wave can be used to determine the effects of genetic mutations or pharmacological treatments on specific retinal cell types (36–37). Indeed, work from our laboratories has shown that administration of exogenous melatonin during the day increases the amplitudes of a- and b-waves of the scotopic ERG to values observed at night under control conditions (32); the removal of MT1 receptors abolishes these effects (32). Furthermore, the removal of MT1 receptors abolished the daily and circadian rhythms in the ERG responses (32,38) and decreased photoreceptor and ganglion cell viability during aging (32, 35).
Results
Removal of the MT2 receptor replicates the ERG phenotype of MT1 deletion in mice
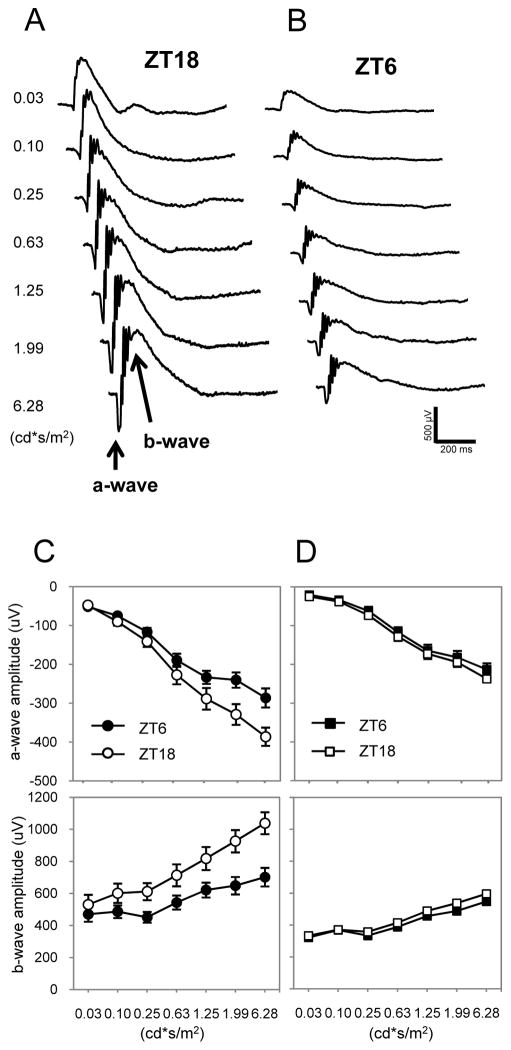
If MT1/MT2 heteromers are the functionally relevant signaling unit in photoreceptor cells, the deletion of either component of the heteromer would be expected to result in the same phenotype. We generated C3H-f+/+ MT2−/− mice and compared the scotopic ERG of these mice with those of previously described C3H f+/+ MT1−/− mice (32). As previously reported (32) in C3H/f+/+ wild-type mice, the amplitudes of the a- and b-wave were significantly greater at midnight (ZT18) than at midday (ZT6, Fig. 1, A to C), whereas in C3H f+/+ MT2−/− mice, no changes in the amplitude of the a- and b-wave were observed between ZT18 and ZT6 (Fig. 1D).
Figure 1. Removal of the MT2 receptor replicates the ERG phenotype of MT1 deletion in mice.
(A, B) Representative ERG traces recorded at ZT 18 and ZT 6 in C3H-f+/+ (WT) mice are shown. (C) Quantification of dark-adapted ERG responses to flashes of light recorded in the middle of the day (ZT 6, ●, ■) and middle of the night (ZT 18, ○,□) in C3H-f+/+ (C) and MT2−/− (D) mice. Mice (3–4 months old) were dark-adapted for at least 30 min before the recordings were performed. A clear daily rhythm in the amplitude of the a- and b-wave were observed in WT mice (two-way ANOVA, P< 0.01 in both cases) but not in MT2−/− (two-way ANOVA, P > 0.1 in both cases). Data are presented as mean ± SEM; n = 6 for each time point and genotype. Luminance is expressed in candela-seconds per meter squared (cd*s/m2).
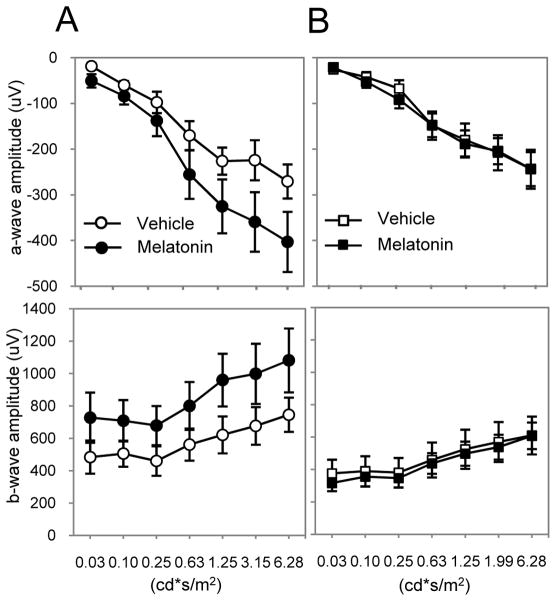
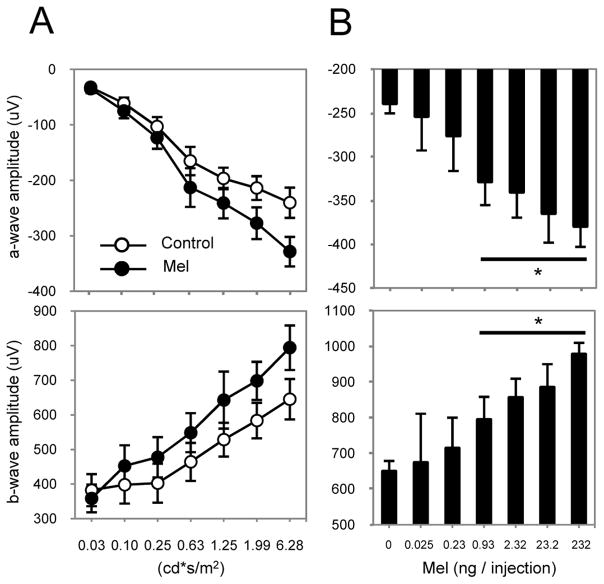
We then tested whether intraperitoneal injection (i.p.) of melatonin (1mg/kg) during the day (ZT6) would affect the dark-adapted ERG. C3H-f+/+ MT2−/− mice were injected with melatonin and dark-adapted for one hour prior to recording the ERG. Contrary to what was observed in C3H-f+/+ mice (Fig. 2A), melatonin injection during the daytime did not produce any changes in the ERG of MT2−/− mice (Fig. 2B). In conclusion, the ERG data obtained with the MT2−/− mice were identical to those previously obtained with the MT1−/− and, thus, are compatible with our hypothesis of the involvement of MT1/MT2 heteromers in photoreceptor function.
Figure 2. Administration of melatonin does not affect ERGs in MT2−/−.
Quantification of the dark-adapted response of the a- (upper panels) and b-wave (lower panels) to flashes of light recorded after 1 h of dark adaptation and intraperitoneal injection of melatonin (1 mg/kg) or vehicle in C3H-f+/+ (A) and MT2−/− (B) mice. Data are presented as mean ± SEM; n = 5–8 for each group. Melatonin injection did not induce any significant changes in the amplitude of the a- and b-wave in MT2−/− mice (two-way ANOVA, P > 0.1). Luminance is expressed in candela-seconds per meter squared (cd*s/m2). ERGs in this experiment were performed at ZT 6.
Murine MT1/MT2 heteromers form in transfected HEK293T cells
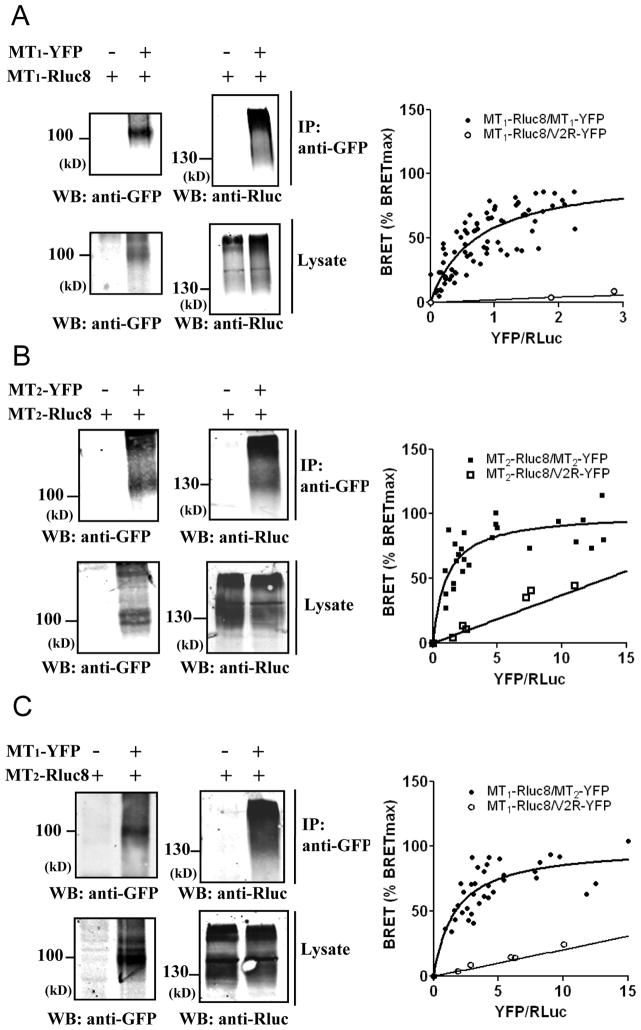
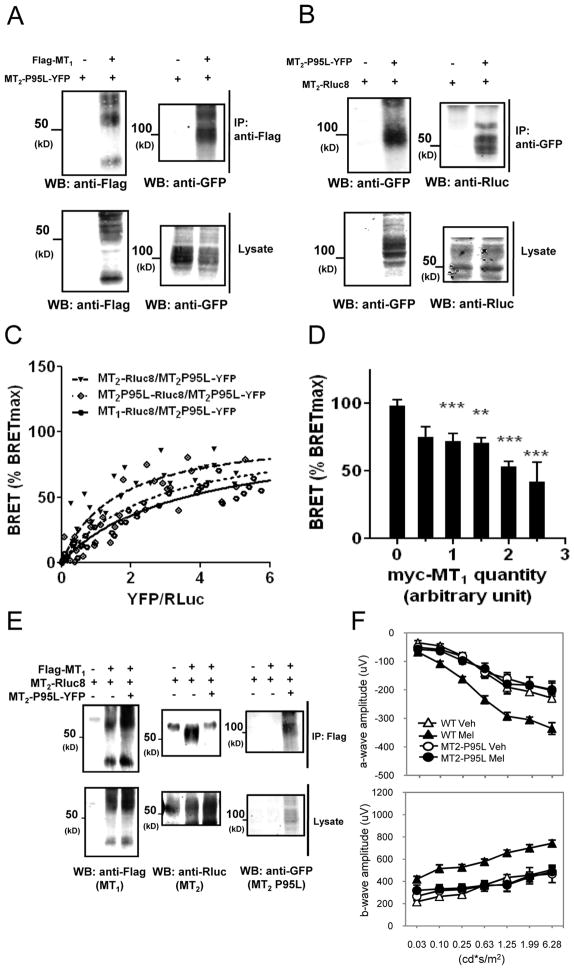
To more directly assess the possible interaction between murine MT1 and MT2 receptors, we carried out coimmunoprecipitation experiments. These experiments revealed that MT1 and MT2 engage into homo- and heteromeric complexes in HEK293T cells (Fig. 3, A to C, left panels). Several diffuse bands typical for glycosylated and hydrophobic seven-transmembrane proteins were observed. To further validate these results in intact cells, we performed bioluminescence resonance energy transfer (BRET) donor saturation experiments. The Renilla luciferase 8 (Rluc8) energy donor and the yellow fluorescent protein (YFP) energy acceptor were fused to the carboxyl terminus of MT1 and MT2 receptors. Co-transfection of a fixed amount of MT1-Rluc8 expression plasmid and increasing amounts of MT1-YFP or MT2-YFP expression plasmids (Fig. 3, A to C, right panel) resulted in the expected hyperbolic saturation curve with increasing YFP/Rluc ratios for all receptor combinations, reflecting a specific interaction between BRET donor and acceptor pairs (BRET50=0.71±0.15 and 1.67±0.42 (n=5) for MT1 homomers and MT1/MT2 heteromers, respectively). Similar results were obtained in cells expressing a fixed amount of MT2-Rluc8 and increasing amounts of MT2-YFP (Fig. 3B, right panel) (BRET50=1.07 ±0.24 (n=5) for MT2 homomers). Expression of the MT1-Rluc8 donor with the negative control vasopressin V2-YFP receptor resulted in a linear, non-saturable BRET increase, characteristic of random interactions. Overall, coimmunoprecipitation and BRET results show that murine MT1 and MT2 receptors can form homo- and heteromers in HEK293T cells as previously shown for their human homologues.
Figure 3. Formation of murine MT1/MT2 heteromers in transfected HEK293T cells.
(A–C, left panels) Coimmunoprecipitation of MT1 and MT2 receptors. Lysates from HEK293T cells expressing the indicated Rluc8 and YFP fusion proteins were immunoprecipitated with antibodies recognizing the GFP and the presence of coprecipitated Rluc fusion proteins detected with antibodies against Rluc (A–C; upper western blot). Expression of fusion proteins in lysates was assessed by immunoblotting with indicated antibodies. (A–C, right panels) BRET donor saturation experiments with HEK293T cells co-expressing a fixed amount of MT1-Rluc8 (A, C) or MT2-Rluc8 (B) in the presence of increasing amounts of MT1-YFP, MT2-YFP or vasopressin receptor V2-YFP (negative control). Western blots were representative of two further experiments. The saturation curves were obtained from five independent experiments. Anti-GFP, anti-Rluc, antibodies recognizing GFP and Rluc, respectively.
MT1 and MT2 receptors are expressed in the mouse photoreceptor cells where they heteromerize
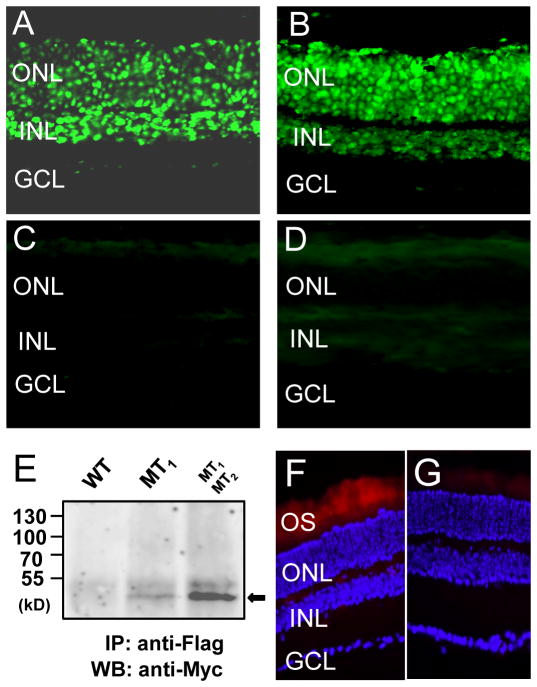
By fluorescent in situ hybridization we detected MTNR1A (MT1) mRNAs in the outer nuclear layer (ONL) and the inner nuclear layer (INL) and in the ganglion cell layer (GCL) of mouse retina (Fig. 4A), whereas MTNR1B2 (MT2) transcripts were detected in the ONL and INL (Fig. 4B). No signal was detected in sections treated with the sense probes (Fig. 4, C and D). In the ONL MT1 and MT2 mRNAs were detected in almost all the nuclei of the photoreceptors.
Figure 4. MT1 and MT2 receptors are expressed in mouse photoreceptor cells where they heteromerize.
MTNR1A and MTNR1B transcripts in the retina were detected by in situ hybridization using a fluorescein-labeled probe. (A) The MT1 antisense probe detected a clear signal in the outer nuclear layer (ONL) and inner retinal layer (INL); a faint signal was also present in some ganglion cells (GCL). (B) The MT2 antisense probe detected a clear signal in the ONL and INL but not in the GCL. No signal was detected using the MT1 (C) or MT2 sense probe (D). (E) Coimmunoprecipitation of MT1 and MT2 receptors from the retina of wild-type C57/bl6 mice or transgenic C57/bl6 mice expressing either Flag-MT1 or Flag-MT1 and Myc-MT2 receptors in photoreceptor cells under the control of the rhodopsin promoter. Representative images and western blots are shown. (F,G) Visualization of MT1 and MT2 receptors heterodimerization by in situ PLA on retinal sections of transgenic C57/bl6 mice expressing Flag-MT1 and Myc-MT2 receptors in photoreceptor cells. MT1/MT2 heterodimers were visualized by staining cells with proximity probes directed against Flag and Myc, followed by ligation and rolling –circle amplification. The hybridization probes were labeled with a fluorophore which can visualized using Texas red filter. The nuclei were stained with DAPI (blue) A strong and specific fluorescent signal was detected in the photoreceptors’ outer segments (OS) (F), no specific signal was detected in control sections (G). Similar results have been obtained in two further experiments. Anti-Flag, anti-Myc, antibodies recognizing Flag or Myc epitopes, respectively.
To test whether MT1 and MT2 receptors form functional heteromers in the mouse photoreceptors, we developed a series of transgenic mice by transducing mouse fertilized eggs with lentiviral vectors containing Flag-MT1 or Myc-MT2 wild-type receptor coding regions under the control of the rhodopsin promoter to drive expression of these receptors in photoreceptor cells. Tagging the receptors was necessary to detect them properly as reliable antibodies, recognizing that wild-type rodent MT2 receptors are currently lacking. Immunofluorescence in these mice was only detected in the photoreceptors using antibodies recognizing the Flag or Myc epitopes to detect the tagged MT1 or MT2 receptors (Fig. S1). We then crossed Flag-MT1 with Myc-MT2 mice to produce Flag-MT1/Myc-MT2 mice and performed coimmunoprecipitation experiments. Retinal cell lysates were prepared from wild-type and transgenic mice expressing either Flag-MT1 or co-expressing Flag-MT1 and Myc-MT2 receptors (Fig. 4E). Flag-MT1 receptors were precipitated, and Myc-MT2 receptors were only co-precipitated in lysates prepared from double transgenic mice consistent with the formation of MT1/MT2 heteromers in photoreceptors. We then performed in situ proximity ligation assay (PLA) (39) on retinal sections obtained from Flag-MT1/Myc-MT2 mice to verify the close proximity of MT1 and MT2 receptors in the murine photoreceptors. A specific fluorescence signal was observed indicating that MT1 and MT2 receptors exclusively interact in the outer segment of photoreceptors as predicted (Fig. 4, F to G).
Pharmacological characterization of retinal MT1/MT2 heteromers by scotopic ERGs
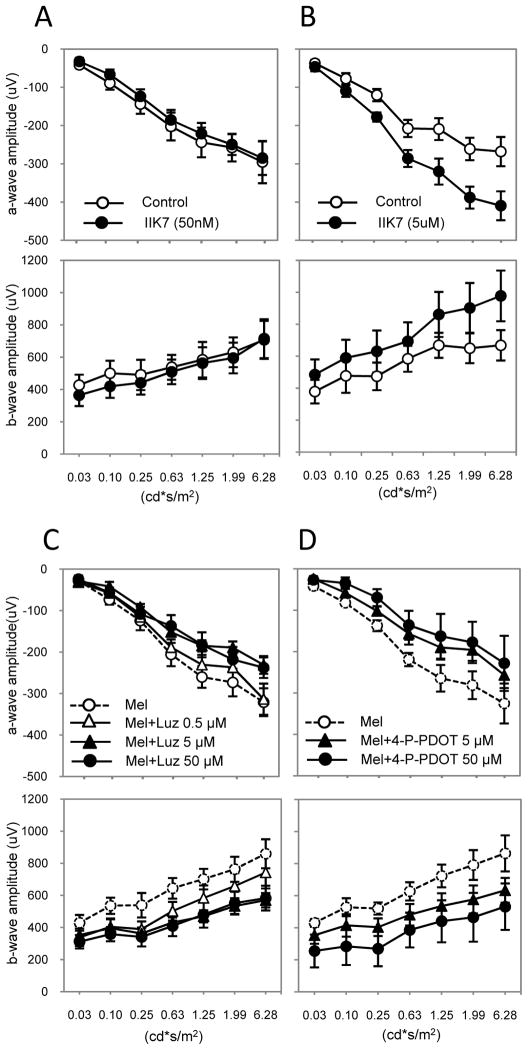
To further decipher the involvement of MT1 and MT2 receptors in the effect of melatonin on scotopic ERGs, we performed intravitreal injections (i.v.) into the eyes of C3H-f+/+ mice. Intravitreal injection of melatonin induced a dose-dependent increase in the amplitude of the a- and b-wave of the scotopic ERGs as expected (Fig. 5, A and B). We then injected the IIK7 compound that has been reported to behave as a selective agonist of the human MT2 receptor (40). Competition of 2-[125I]iodo-melatonin binding at mouse MT1 and MT2 receptors showed also high affinity and selectivity of IIK7 for murine MT2 receptors with Ki values of 73 ± 23 nM and 0.052 ± 0.015 nM, for MT1 and MT2, respectively (Fig. S2, A and D). When injected intravitreally at a concentration that selectively activates MT2 receptors (50 nM), IIK7 did not increase the amplitude of the a- or b-wave (Fig. 6A) whereas a higher concentration (5 μM) that activates MT1 and MT2 receptors, significantly increased the amplitude of the a- and b-wave (Fig. 6B). Conversely, injection of luzindole or cis-4-phenyl-2- propionamidotetralin (4P-PDOT), two melatonin receptor antagonists with high preference for human MT1/MT2 heteromers over MT2 homomers (19) and high affinity for murine MT1 and MT2 receptors (Fig. S2, B to D), prevented the increase in the amplitude of the a- and b-wave that followed administration of melatonin via i.p., which is consistent with the involvement of MT1/MT2 heteromers and validates the experimental protocol (Fig. 6, C and D). Injection of luzindole or 4P-PDOT alone did not produce any significant changes in the amplitude of the a- and b-wave excluding any agonistic and inverse agonistic effects of these compounds in the retina (Fig. S3). Collectively, these data confirm that the effect of melatonin on the amplitude of the a- and b-wave of the scotopic ERGs occurs with the expected pharmacology and depends on the simultaneous activation of both protomers of the MT1/MT2 heteromer.
Figure 5. Dose-response to intravitreal injection of melatonin.
(A) Quantification of the dark-adapted response of the a- (upper panel) and b-wave (lower panel) to flashes of light recorded after 1 h of dark adaptation and intravitreal injection of melatonin or vehicle in C3H-f+/+ mice. Intravitreal injection of melatonin (0.93 ng) induced a significant increase of the a- and b-wave amplitude (two-way ANOVA, P < 0.001). (B) Quantification of the dark-adapted response of the a- (upper panel) and b-wave (lower panel) to different doses of melatonin injected intravitreally. Intravitreal injection of melatonin induced an increase in the amplitude of the a- and b-wave in a dose-dependent manner. Data are presented as mean ± SEM; n = 5–8 for each group. Luminance is expressed in candela-seconds per meter squared (cd*s/m2). ERGs in this experiment were performed at ZT 6. * denotes P < 0.05 with respect to vehicle injection (t-tests).
Figure 6. Effect of subtype specific melatonin receptor agonists and antagonists on the ERG.
Intravitreal injection of the MT2-specific agonist IIK7 did not affect the amplitude of the a- and b-wave at lower concentration (50 nM) when it acts as MT2 selective agonist (A, two-way ANOVA, P > 0.1) whereas at higher concentrations (5μM) activating MT1 and MT2 receptors, IIK7 significantly increases the amplitude of the a- and b-wave (B, two-way ANOVA, P < 0.05). Black circles represent IIK7-treated, white circles the control conditions. Intravitreal injections of Luzindole (C) or 4P-PDOT (D) blocked the effect of melatonin injection (i.p.) on the amplitude of the a- and b-wave in a dose-dependent manner. Controls received an intra-vitreal injection of vehicle in the left eye. Data are presented as mean ± SEM; n = 6 for each time point. t-tests, * P < 0.05; ** P < 0.01. Luminance is expressed in candela-seconds per meter squared (cd*s/m2). ERGs in this experiment were performed at ZT 6.
Expression of the non-functional MT2-P95L mutant in the mouse photoreceptors blocks the effects of melatonin injection on the dark-adapted ERGs
In an attempt to interfere with the formation of functional heteromers, we generated transgenic mice expressing a non-functional murine MT2-P95L mutant that has been designed based on the recently described naturally occurring loss-of-function MT2-P95L mutant identified in diabetic patients (41). In vitro experiments performed in HEK293T cells confirmed that the murine mutant expressed properly and was devoid of any 2-(125I)iodo-melatonin binding and signaling capacity as described for the human mutant (Fig. S4, A to C). However, the MT2-P95L mutant maintained its oligomerization capacity as this mutant readily formed homomers and heteromers with MT1 and MT2 wild-type receptors as demonstrated by coimmunoprecipitation (Fig. 7, A and B) and BRET donor saturation experiments (Fig. 7C) with similar BRET50 values, indicating similar propensities to form oligomers (BRET50=2.61±0.71, 3.48±0.73 and 1.52±0.40 (n=3) for MT2-P95L homomers and MT2-P95L/MT1 and MT2-P95L/MT2 heteromers, respectively). Consistently, the MT2-P95L mutant interfered with MT1/MT2 heteromer formation as illustrated in BRET competition and coimmunoprecipitation experiments (Fig. 7D and upper middle western blot of panel E). To examine the consequence of MT2-P95L expression on melatonin receptor function in the retina, we generated transgenic mice expressing the MT2-P95L mutant in photoreceptors and tested the effect of melatonin injection (i.p., 1 mg/Kg) on the dark-adapted ERGs. Control mice responded to melatonin injection with an increase in the amplitude of the a- and b-wave (Fig. 7F), whereas MT2-P95 transgenic mice showed no increase in the amplitude of the a- and b-wave when injected with melatonin (Fig. 7F). These results phenocopy those observed in MT1−/− and MT2−/− mice, suggesting that the effect of melatonin on dark-adapted ERGs depends on the presence of functional MT1/MT2 heteromers in photoreceptor cells.
Figure 7. The non-functional MT2-P95L mutant interferes with MT1/MT2 heteromer formation and blocks the effects of melatonin injection on the dark-adapted ERGs in the mouse photoreceptors.
(A, B) Coimmunoprecipitation of MT2-P95L-YFP with Flag-MT1 (A) or MT2-Rluc8 (B) receptors in HEK293T cells (upper right panel). (C) BRET donor saturation curves were performed by coexpressing equivalent amounts of MT1-Rluc8, MT2-Rluc8 or MT2-P95L-Rluc8 together with increasing quantities of MT2-P95L-YFP in HEK 293T cells. (D) Competition of the BRET between MT1-Rluc8 and MT2-P95L-YFP by increasing concentration of coexpressed Myc-MT1. Expression of the Myc-MT2-P95L mutant was determined by western blot. (E) MT2-P95L-YFP competes for MT1/MT2 heteromer formation as monitored by coimmunoprecipitation (upper middle panel). (F) Quantification of the dark-adapted response of the a- (upper graph) and b-wave (lower graph) to flashes of light recorded in MT2-P95L mutant mice after 1 h of dark adaptation and i.p. injection of melatonin (1 mg/kg) or vehicle in the middle of the day. Data are presented as mean ± SEM; n = 5–8 for each group. Melatonin injection induced no significant changes in the amplitude of the a- and b-wave (two-way ANOVA, P > 0.1). Coimmunoprecipitation data are representative of two further experiments. BRET data are obtained from 3–5 independent experiments, * P < 0.05; ** P < 0.01. Ab, antibody cross-reactivity. Anti-GFP, anti-Rluc, anti-Flag, antibodies recognizing GFP, Rluc or the Flag epitope, respectively. Luminance is expressed in candela-seconds per meter squared (cd*s/m2). ERGs in this experiment were performed at ZT 6.
Melatonin directly affects mouse photoreceptor functioning via the Phospholipase C (PLC)/ Protein kinase C (PKC) signaling pathway
We then decided to investigate the intracellular signaling pathway leading to the effect of melatonin on the dark-adapted photoreceptors. As shown in Figure 8A, the administration of exogenous melatonin directly affected the amplitude of the a-wave 7 ms after the initial flash of light, demonstrating that melatonin can directly affect photoreceptor response. We then performed intravitreal injection of several signaling inhibitors and activators (Fig. 8, B and C and Fig. S5). Whereas the pan-inhibitor of heterotrimeric G proteins 7-[2-amino-1-oxo-3-thio-propyl]-8-cyclohexylmethyl-2-phenyl-5,6,7,8 -tetrahydro-i midazo-[1,2a]-pyrazine dimer, hydrochloride (BIM 46187) inhibited the melatonin-induced response, the Gi protein inhibitor pertussis toxin (PTX) had no effect. The injection of 8-bromoadenosine 3′,5′-cyclic monophosphate (8-br-cAMP) had no effect on the melatonin response, consistently intravitreal injection of the Protein kinase A (PKA) inhibitor H89 did not mimic the photoreceptors response to melatonin. These results exclude the involvement of the cyclic adenosine monophosphate (cAMP)/PKA pathway. In contrast, inhibiting inositol trisphosphate (IP3) receptors with 2-Aminoethoxydiphenyl borate (2-APB), and the inhibition or activation of PKC by Bisindolylmaleimide II (Bisl) or Phorbol 12-myristate 13-acetate (PMA), respectively, abolished or mimicked the effect that administration of exogenous melatonin produces on the photoreceptor response. These data indicate that the effect of melatonin on the photoreceptors is independent of the Gi/cAMP pathway but involves instead activation of the PLC/PKC pathway.
Figure 8. Melatonin directly affects mouse photoreceptor function via the PLC/PKC signaling pathway.
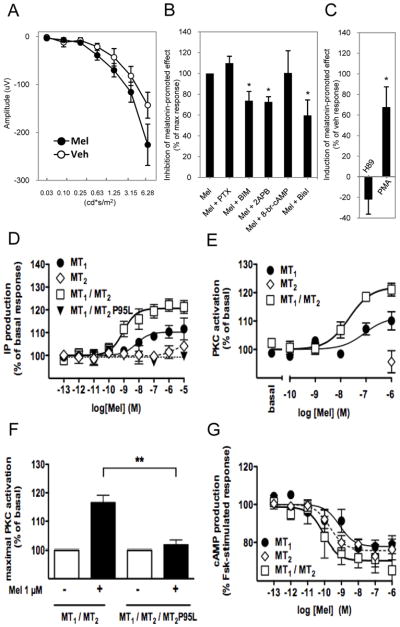
(A) The photoreceptor response (a-wave at 7 ms, see main text for details) is significantly increased after the administration of exogenous melatonin. (B) Pertussis toxin (PTX, Gi protein inhibitor) did not affect the melatonin-induced increase in a-wave amplitude at 7 ms (t-tests, P > 0.1), whereas the pan heterotrimeric G proteins inhibitor BIM 46187 or inhibition of the IP3 receptors by 2-ABP significantly reduced the melatonin-promoted increase of the a-wave amplitude at 7 ms (t-tests, P < 0.05). On the other end, activation of PKA pathway by injecting a cAMP analogue (8-Br-cAMP) did not produce a significant effect on the photoreceptor response (t-test, P > 0.1), whereas inhibition of the PKC pathway by Bisl significantly reduced the melatonin-promoted increase in the a-wave amplitude (t-tests, P < 0.05). (C) Inhibition of PKA activity by H89 did not affect the response of photoreceptors to exogenous melatonin (t-test, P > 0.1) whereas activation of PKC by PMA significantly reduced the effect of melatonin of the a-wave (P< 0.05, t-test). Inositol phosphate production (D) and PKC activity (E,F) following stimulation with increasing concentrations (D,E) or 100 nM (F) melatonin was assessed in HEK 293T cells expressing the indicated receptors. (G) Inhibitory effect of the indicated receptors on forskolin-stimulated cAMP production. Data shown in panels A and B represent the mean ± SEM (n= 6–8), whereas data in panels D-G represent the mean ± SEM of at least 4 experiments performed in triplicate (* P < 0.05; ** P < 0.01). Luminance is expressed in candela-seconds per meter squared (cd*s/m2). ERGs in this experiment were performed at ZT 6.
Consistent with this finding, physiological melatonin concentrations stimulated inositol phosphate production in HEK293T cells coexpressing MT1 and MT2 receptors (EC50= 2.8 nM ±1.1 nM) (Fig. 8D). Interestingly, melatonin was less potent (EC50= 51.0 nM ± 35.2 nM) or inactive in cells expressing MT2 or MT1 alone, respectively (Fig. 8D) suggesting a positive allosteric effect of the MT2 protomer on the MT1 protomer in the heteromer. The allosteric behavior was further confirmed in cells coexpressing the wild-type MT1 receptor with the inactive MT2 -P95L mutant, which completely abolished the effect of melatonin in the heteromer (Fig. 8D). To explore the existence of a potential cross-antagonism in MT1/MT2 heteromers, we compared the effect of the MT2-selective 4P-PDOT antagonist (see Fig. S2C) on melatonin-induced inositol phosphate production in cells expressing MT1 receptors in the absence or presence of MT2 (Fig. S6). Irrespective of the co-expression of MT2, competition curves were mono-phasic with similar IC50 values 5.8 ±7.7 nM and 4.0 ±3.5 nM (n=3) for cells expressing MT1 or MT1 and MT2, respectively) suggesting the absence of cross-antagonism.
We then determined the effect of melatonin on PKC activity in HEK293T cells. Melatonin dose-dependently increased PKC activity in cells expressing MT1/MT2 heteromers and less potently in cells expressing MT1 alone (Fig. 8E). The effect on MT1/MT2 heteromers was potently inhibited in cells coexpressing the dominant negative MT2-P95L mutant coroborating our in vivo observation in transgenic mice overexpressing the MT2-P95L mutant (Fig. 8F). A possible switch between Gi protein towards Gq protein coupling from homomers to heteromers could be excluded as the inhibition of cAMP production was not impaired but rather improved in cells co-expressing MT1 and MT2 receptors (Fig. 8G).
Taken together, MT1/MT2 heteromers are able to signal through the Gi/cAMP and the PLC/PKC pathway. The effect of melatonin on the scotopic ERG involves the activation of the PLC/PKC pathway through MT1/MT2 heteromers, in which the MT2 protomer most likely allosterically regulates the MT1 protomer.
Discussion
Establishing whether GPCRs form physiologically relevant functional homo- and heteromers in vivo has been a major biochemical challenge. Apart from obligate class-C GPCR dimers (13, 41), only a few recent studies support the formation of physiologically relevant heteromers (15–17, 42–44). Here, we provide compelling biochemical and functional in vivo evidence for the formation of MT1/MT2 heteromers in photoreceptors. ERG measurements have been instrumental in establishing the pivotal role of MT1/MT2 heteromers in the regulation of retinal light sensitivity as demonstrated by the complete loss of the modulatory effect of melatonin in MT1−/− and MT2−/− mice as well as in mice overexpressing a non-functional MT2 receptor mutant that competes with the formation of functional MT1/MT2 heteromers. Formation of a novel functional signal transduction unit, composed of MT1 and MT2 receptors allosterically interacting with each other, is indicated by the activation of the PLC/PKC signaling pathway in photoreceptors. The existence of two melatonin receptor subtypes with apparently redundant functional properties (similar affinity for melatonin and signaling properties) and overlapping expression patterns in several tissues remains enigmatic. Based on these observations, we hypothesized that co-expression of MT1 and MT2 receptors might be justified by the formation of MT1/MT2 heteromers with unique functional properties. Based on previous reports, we considered the retina as a promising target tissue to verify this hypothesis. Here, we report that MT2 receptors have indeed a similar distribution to MT1 receptors with dominant co-expression in photoreceptors. The functional expression of melatonin receptors in photoreceptors is further supported by the fact that melatonin affects the early photoreceptor-specific component of ERGs and that this effect is abolished by the photoreceptor-specific expression of a non-functional MT2 mutant.
Previous in vitro studies on human MT1 and MT2 receptors indicated the formation of MT1/MT2 heteromers (18, 19). We now confirm these in vitro findings with murine MT1 and MT2 receptors and provide additional experimental data suggesting that heterodimerization can also occur in vivo. Due to the lack of reliable antibodies for murine melatonin receptors to perform coimmunoprecipitation experiments, we decided to generate mice expressing tagged MT1 and MT2 receptors in photoreceptors and to cross these mice in order to produce a double transgenic mouse line in which both tagged receptors were expressed in photoreceptors (Flag-MT1/Myc-MT2). Coimmunoprecipitation and in situ PLA experiments performed with retinas obtained from these mice indicated that these receptors indeed form a complex in the mouse photoreceptors. It is worth noting that within the retina, melatonin receptor heteromer formation appears to be restricted to photoreceptors since ganglion cell viability is not affected by the removal of MT2 receptors (35) and the circadian rhythms in retinal dopamine are not affected by MT1 removal (38), further underscoring the specificity of our observations on photoreceptor cells.
To examine the functional consequences of MT1/MT2 heteromerization, we either deleted receptor subtype or used selective pharmacological tools. Removal of the MT2 receptor produced on the ERGs an effect identical to that previously reported in the mice lacking MT1; namely, a complete loss of the effect of melatonin on the scotopic ERGs (32). This demonstrates that the effect of melatonin depends strictly on the presence of both receptor subtypes, most likely by targeting MT1/MT2 heteromers expressed in photoreceptors. Consistent with these results, we found that administration of the 4P-PDOT and luzindole, two antagonists with preference for MT1/MT2 heteromers (19), potently inhibited the effect of melatonin on dark-adapted ERGs. In addition, IIK7, a MT2-selective agonist, only reproduced the effect of melatonin at concentrations targeting both protomers of the MT1/MT2 heteromer but not at concentrations specific for the MT2 protomer consistent with the hypothesis that both protomers have to be activated in the heteromer.
We then gathered further evidence for MT1/MT2 heteromer formation in photoreceptors by expressing a melatonin binding-deficient mutant of the MT2 receptor (MT2-P95L) in these cells. We chose this approach because it does not require any prior knowledge of the specific heteromer interaction interface and the signaling pathway activated by the heteromer. The complete inhibition of the effect of melatonin on dark-adapted ERGs in transgenic mice expressing the mutant in photoreceptors demonstrates the feasibility of this approach and provides additional compelling evidence that argues in favor of the formation of functional MT1/MT2 heteromers in mouse photoreceptors.
Finally, we sought to investigate the pathway involved in melatonin-induced ERG regulation. MT1 and MT2 receptors typically inhibit cAMP formation via pertussis toxin-sensitive Gi proteins that are negatively coupled with adenylyl cyclase (20, 21). Surprisingly, manipulating the cAMP signaling pathway by either inhibiting Gi proteins or PKA, or by administrating a cell-permeable cAMP analogue, did not block or mimic the melatonin-induced effects in photoreceptors, respectively. In contrast, the inhibition of PKC by Bisl partially blocked (about 40%) the effect of exogenous melatonin on photoreceptors. Consistently, the PKC activator PMA mimicked the effect of melatonin. The effect of PKC is likely to be mediated by inositol phosphate production since inhibition of IP3 receptors by 2-APB produced a similar effect. Stimulation of inositol phosphate production through MT1/MT2 heteromers is further supported by our in vitro data in transfected HEK293T cells. Indeed, inositol phosphate concentrations were increased at physiological melatonin concentrations (EC50=2 nM) in cells co-expressing MT1 and MT2 receptors, whereas MT2 alone was unable to modulate this pathway and MT1 only at supra-physiological melatonin amount. Similar results were obtained for PKC activation.
Activation of the PLC/PKC pathway has been previously reported to be of functional importance in rod outer segments (45, 46). Recent data indicate that this pathway may play an important role in the light-dependent translocation of arrestin, the major regulator of rhodopsin function in rod photoreceptors (47). Whether melatonin regulates arrestin translocation has to be established in future studies.
Activation of the PLC/PKC pathway by melatonin has been observed at other occasions. Previous reports showed that MT1, when expressed alone in transfected cells, activates this pathway (48) and that in tissues such as the suprachiasmatic nucleus (SCN) of the hypothalamus, which is known to co-express MT1 and MT2 receptors, PKC is also activated (49). Whether PKC activation or any other effect of melatonin in the SCN is dependent on MT1/MT2 heteromers is currently unknown. Phenotypic characterization of MT1−/− and MT2−/− mice suggests that the action of melatonin in the SCN does not appear to be mediated by MT1/MT2 heteromers at least in the outputs so far investigated (50). Nevertheless, it is worthwhile mentioning that no study so far has investigated in detail the expression of these receptors in the SCN and whether these receptors may be co-expressed in some SCN neurons during the circadian cycle where they may form heterodimers and control a yet to be identified SCN output.
Increased potency and efficacy of melatonin on inositol phosphate production and PKC activity in cells expressing MT1/MT2 heteromers vs. the corresponding homomers indicate an allosteric behavior of the heteromer. No activation of this pathway was observed when the MT2 wild type receptor was replaced by the inactive P95L mutant further confirming the need for a functional MT2 protomer to allosterically activate the MT1 protomer in the heteromer. In contrast, specific activation of the MT2 protomer is not sufficient to activate the PLC/PKC pathway, which requires rather activation of both protomers. The absence of cross-antagonism or negative allosterism in the MT1/MT2 heteromer is in agreement with previous observation on human MT1/MT2 heteromers (19). Collectively, these data suggest a working model where the MT2 protomer allosterically potentiates/facilitates the activation of the PLC/PKC pathway by the MT1 receptor.
In conclusion, our data provide novel evidence that the modulatory effect of melatonin on mouse photoreceptor light sensitivity is mediated by MT1/MT2 heteromers involving the activation of the PLC/PKC pathway. The involvement of MT1/MT2 heteromers may have important therapeutic implications, as the heteromer complex may provide a unique pharmacological target to improve photoreceptor functioning and to extend the viability of photoreceptors during aging.
Materials and Methods
Animals
C3H- MT2−/− knockout mice homozygous for the rd1 mutation, generously donated by Drs. Reppert and Weaver (University of Massachusetts Medical School), were backcrossed with C3H/f+/+ mice in which the rd1 mutation had been removed to produce C3H/f+/+MT2−/−. The genotypes were determined according to the protocols previously described (32, 50). All the experimental procedures were carried out in accordance with Association for Assessment of Laboratory Animal Care policies and approved by the Morehouse School of Medicine Animal Care and Use Committee.
In situ hybridization
Mouse were killed by CO2 asphyxiation, the eyeballs were immediately removed, punctured and then fixed with 4% paraformaldehyde in phosphate-buffered saline (pH 7.0) for 6 h at 4°C. The eyes were transferred to a 30% sucrose solution for 12–14 h, embedded in Tissue-Tek OCT compound (Miles), and cut into 20-μm-thick cryosections. The template for transcription was a cDNA fragment of mouse MT2 subcloned into a pZLI vector (GenBank NM145712). The correct orientation of the construct was verified by sequence analysis and restriction enzyme digestion. Antisense and sense cRNA probes were generated by using Fluorescein-12-UTP (Perkin Elmer Life Science) by in vitro transcription (MT2, F: 5′-acactcacatagggcgattg-3′; R: 5′-agtgtgctggaattcggttc-3′, 512 bp). The templates for transcribing RNA probes were made by linearizing recombinant plasmids. Details about the MT1 probes are reported in Baba et al. (32). Sections were immersed in pre-hybridization buffer containing 50% formamide, 5×Denhardt s solution, and 5×SSC (1×SSC=150 mM NaCl, 15 mM sodium citrate, pH 7.0) for 2 h at room temperature. The sections were then hybridized with 75 μl hybridization buffer, covered with a coverslip, and incubated overnight in a humidified chamber at 67°C. The best labeling was obtained at a probe concentration of 1:100. Slides were then washed in 5×SSC/50% formamide at 68°C for 1 h and in 2×SSC for 1 h at 68°C, and then incubated in 20 mg/ml RNase A at 37°C for 30 min followed by 2×SSC for 1 h and 0.2×SSC for 30 min (twice) at room temperature. Slides were mounted and then viewed with a Zeiss Axioskop microscope equipped with epifluorescence.
In situ proximity ligation assay (PLA)
Eyeballs obtained from MT1-Flag/MT2-Myc were fix 4% PFA overnight, transferred to a 20% sucrose solution for 12–14 h and embedded in Tissue-Tek OCT compound (Miles) and then sectioned (10 μm). PLA was conducted using Duolink In Situ-Fluorescence kit (Sigma) (39). After blocking (Duolink Blocking solution) the sections were incubated with the mouse anti-c-Myc (1:250, Santa Cruz Biotechnology, Inc) and anti-FLAG (1:500, Sigma-Aldrich) antibodies overnight. Anti-mouse Minus PLA probe (1:5, Sigma), anti-rabbit Plus PLA probe (1:5, Sigma) and Duolink® In Situ Detection Reagents Red kit were used to detect protein interactions. Sections were washed with buffer A (8.8g/L NaCl, 1.2g/L Tris base, 0.5ml/L Tween 20, pH 7.4) after the 1st and 2nd incubation and the wash buffer B (5.84g/L NaCl, 4.24g/L Tris base and 26g/L Tris-HCL, pH 7.5) was used after amplification process. After drying at room temperature, the slides were mounted with cover slip using Duolink In Situ Mounting Medium with DAPI (Sigma). Slides were mounted and then viewed with a Zeiss Axioskop microscope equipped with epifluorescence. Primary antibodies or the proxity probe were omitted in control sections.
Scotopic electroretinogram (ERG)
Mice were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg). The pupils were dilated with 1% atropine and 2.5% phenylephrine (Sigma, St. Louis, MO, USA), and mice were placed on an heating pad set at 37°C with feedback from the rectal temperature probe. The eye was lubricated with saline solution, and a contact lens type electrode (LKC Technologies model: N1530NNC) was topically applied on the cornea. A needle reference was inserted in other side of cheek, and the ground needle was inserted into the base of tail. All preparation of ERG recordings was conducted under red dim light (<3 lux, 15 W Kodak safe lamp filter 1A, Eastman Kodak, Rochester, NY, USA).
All electrodes were connected to a Universal DC Amplifier (LKC Technologies model UBA-4200), and bands were filtered from 0.3 to 500 Hz. Data were recorded and analyzed by EM for Windows (ver. 8.2.1, LKC Technologies). Core body temperature was maintained in 37°C by a feedback temperature control system (FHC inc., Bowdoin, ME) throughout the entire ERG recording. In the dark-adapted ERG protocol, seven series of flash intensities between from 0.03 to 6.28 cd*s/m2 were presented to the mouse eye. Flashes were generated by 530-nm green LEDs in a Ganzfeld illuminator (LKC Technologies), and the interval of the flashes increased from 0.612 to 30 s as the intensity of the flashes increased. Responses of 3–10 flashes were averaged to generate a waveform for each step of light intensity, and the a- and b-wave of ERG measurement were analyzed from the trace of waveform. To directly determine the photoreceptors’ response to melatonin we decided to use an early time-point in the a-wave (i.e., 7 ms after the initial flash of light) (see (36) for details) since at this early time the amplitude of the a-wave is not affected by the activity of the bipolar cells (37).
Administration of exogenous melatonin, melatonin agonists, antagonists and inhibitors
Melatonin (Sigma, St Luis, MO) was dissolved in ethanol (8mg/mL) and then diluted with sterilized PBS (0.1mg/mL, 1.25% ethanol). Melatonin was administered by intraperitoneal (1 mg/kg i.p.) or intravitreal (i.v.) injection in different dosages according to the experimental designs. We used melatonin receptor agonists or antagonists, and various activators or inhibitors in the experiments as follows: IIK7 (Sigma, 30mg/mL DMSO stock solution, 0.005% final DMSO concentration), Luzindole (Tocris, Bristol UK, 29mg/mL DMSO stock solution, 0.05% final DMSO concentration), cis-4-Phenyl-2-propionamidotetralin (4P-PDOT, Sigma, 28mg/mL DMSO stock solution, 0.05% final DMSO concentration), 2-Aminoethyl diphenylborinate (2-APB, Sigma, 20mg/mL DMSO stock solution, 0.006% final DMSO concentration), BIM-46187 (51) (6.7mg/mL DMSO stock solution, 0.23% final DMSO concentration), Bisindolylmaleimide I (Sigma, 10mg/mL DMSO stock solution, 0.1% final DMSO concentration), and Phorbol 12-myristate 13-acetate (PMA, Tocris, 61mg/mL DMSO stock solution, 0.0003% final DMSO concentration) were dissolved in DMSO and diluted with sterilized PBS. Identical DMSO concentrations sterilized PBS were used for vehicle control for each particular drug treatment. Pertussis toxin from Bordetella pertussis (PTX, Sigma, 0.1mg/mL stock solution), 8-bromoadenosine 3′,5′-cyclic monophosphate (8-br-cAMP, Sigma, 25mg/mL stock solution), and H-89 dihydrochloride hydrate (H-89, Sigma, 10mg/mL) were dissolved in sterilized double distilled water and sterilized PBS. After mice were anesthetized with the isoflurane vaporizer machine (VetEquip, Pleasanton, CA), the drugs and vehicle control were intravitreally injected for a volume of 1ul using a 10ul Hamilton syringe (Hamilton Company, Reno Nevada) with 30″ gauge needle attached (Becton Dickinson & Co. Franklin Lakes, NJ). A fresh needle was used for each experiment. The dosages of the drugs were calculated according to an estimated vitreal volume of 20 μl in mouse eye (52). All drugs or vehicles, except PTX, were injected 1 h before the ERG recording, and all animals were placed in a dark isolated chamber just after the drug was administered. PTX was administered 4 hours prior to ERG measurement. For some experiments, melatonin was administered via i.p. injection after intravitreal administration of the drug at the dosage of 1mg/kg. Unless differently specified in the text all the ERGs were performed at ZT 6.
Plasmid Constructions
The 3.8 kb fragment of the mouse rhodopsin promoter was generated by chemical synthesis (DNA2.0, Inc.) and flanked with a MluI (5′) and a BamHI site (3′) for insertion into the pJ cloning plasmid from DNA2.0 Inc. The coding regions of the mouse MT1 (NM_008639.2) or the mouse MT2 (NM_145712.2) receptors that were preceded by a Flag or Myc-tag sequence, respectively, were generated by chemical synthesis (DNA2.0, Inc) and flanked with attL1 and attL2 sites and cloned into the pJ cloning plasmid. Flag-MT1 and Myc-MT2 fragments were cloned behind the rhodopsin promoter into the lentiviral pTrip IZI vector to generate the pTrip-rho-Flag-MT1 and pTrip-rho-Myc-MT2 vectors. Flag-MT1 and Myc-MT2 fragments were inserted into the pcDNA3 expression vector behind the CMV promoter to generate pcDNA3-CMV-Flag-MT1 and pcDNA3-Myc-MT2 vectors. The Myc-MT2-P95L mutant was generated by site-directed mutagenesis from the corresponding MT2 wild-type vector. BRET fusion proteins were generated by inserting the coding regions without the STOP codon of Flag-, HA- or Myc-tagged mouse MT1, MT2 or MT2-P95L receptors, respectively, in frame with the coding region of Renilla luciférase variant (Rluc8) or yellow fluorescent protein (YFP): Flag-mMT1-Rluc8, Flag-mMT1-YFP, HA-mMT2-Rluc8, HA-mMT2-YFP, Myc-MT2-P95L-Rluc8, and Myc-MT2-P95L-YFP. All constructs were verified by sequencing.
Coimmunoprecipitation
Transfected cells were solubilized in RIPA buffer (20 mM HEPES pH7.4, 120 mM NaCl, 5 mM EDTA, 10% glycerol), supplemented with protease inhibitors (leupeptine 1 μg/mL, pepstatine 1 μg/ml, benzamidine 2 μg/ml, AEBSF 1 μg/ml) and 1 % Triton X-100 for at least 3 h at 4°C. The soluble fraction was recovered by 1 h of centrifugation at 13,000 g and subjected to immunoprecipitation with 2 μg/mL of antibodies recognizing the Flag (Sigma F7425) or the GFP epitopes (AbCam ab-290). Denatured complexes were heated, separated by SDS-PAGE, transferred to nitrocellulose and immunoblotted either with antibodies against the Flag epitope (Sigma F3165, 1/1000 dilution), Rluc (Millipore MAB4400, 1/500) or GFP (Roche 11814460, 1/500). Immunoreactivity was revealed using secondary antibodies coupled to 680 or 800 nm fluorophores using the Odyssey LI-COR infrared fluorescent scanner (ScienceTec). For co-immunoprecipitation experiments from retinal samples, 3 retinas were solubilized overnight in 1 mL TEM (75 mM Tris pH7.5, 2 mM EDTA, 12 mM MgCl2) supplemented with protease inhibitors and 1% Triton X-100 at 4°C. Immunoprecipitation was performed with mouse antibodies against the Flag epitope (F3165, Sigma) (2 μg) and immunoblots with rabbit antibodies against the Myc epitope (sc-789, A14, 1/500).
BRET Assay
For bioluminescence resonance energy transfer (BRET) donor saturation curves, HEK 293T cells seeded in 6-well plates were transiently transfected with 0.5 ng of MT1-Rluc, 2 ng of MT2-Rluc, or 1 ng of MT2P95L-Rluc and 10–3,000 ng of yellow fluorescent protein (YFP) plasmids. Twenty-four hours after transfection, cells were transferred into a 96-well white Optiplate (Perkin Elmer Life Sciences) precoated with 10 mg/mL poly-L-lysine (Sigma-Aldrich) and incubated for another 24 h before BRET measurements. BRET measurements were performed as described previously (53) with the Mithras lumino/fluorometer (Berthold Technologies). Results are expressed in milliBRET units (mBU), with 1 mBU corresponding to the BRET ratio values multiplied by 1,000.
Radioligand binding assay
Competition binding experiments were performed as previously described (54) using 200 pM [2-125I]iodomelatonin (PerkinElmer Life Sciences) and a range of different concentrations of the indicated melatonin receptor ligands. Ki values were determined according to Cheng–Prussof formula: Ki = IC50/1 + L + Kd.
cAMP assay
Cylic AMP concentrations were determined in cell suspensions stimulated with 2 μM forskolin for 30 min at room temperature in the absence or presence of different concentrations of melatonin (0.1 fM to 1 μM) by HTRF using the Cisbio ‘cAMP-femto-Tb’ kit according to the manufacturer’s instructions.
Inositol Phosphate assay
Inositol phosphate (IP) concentrations were determined in cell suspensions stimulated for 90 min at 37°C in the absence or presence of different concentrations of melatonin (1 fM to 10 μM) by HTRF using the Cisbio ‘IPoneTb’ kit according to the manufacturer’s instructions.
PKC activity assay
PKC activity was measured by a method adapted from Kent et al. (55). Briefly, cells seeded on polylysine-coated 24-well plates were starved over night and stimulated with 100 nM melatonin for 15 min. Cells were then put on ice, washed with ice-cold PBS containing 1 mM Na3VO4 and incubated for 20 min in 25 μL of a buffer (137 mM NaCl, 20 mM HEPES, 10 mM MgCl2, 25 mM β-glycerophosphate, 1 mM DTT, 1 mM Na3VO4, and protease inhibitors) containing 0.05% digitonin. Activation was then performed using the Upstate PKC Assay Kit (Millipore) according to the manufacturer’s instructions.
Lentivirus Production
The viral particles were produced by transient transfection of HEK 293T cells by using the previously described p8.9 and pMD-G plasmids and either pTrip-rho-Flag-MT1, pTrip-rho-Myc-MT2 or pTrip-rho-Myc-MT2-P95L vectors. Supernatants were collected 48 h after transfection, and high titer stocks were prepared as described (56). The stocks were titrated and normalized for the p24 antigen assayed by ELISA.
Generation of transgenic mice
The lentiviral pTrip-rho-Flag-MT1, pTrip-rho-Myc-MT2 or pTrip-rho-Myc-MT2-P95L vectors expressing MT1, MT2 or MT2-P95L receptors under the control of the Rhodopsin promoter were injected into the perivitelline space of mouse fertilized (C57/bl6/N) eggs as described previously (57). The injected eggs were next re-implanted into the oviduct of pseudopregnant females 0.5 DPC. Flag-MT1/Myc-MT2 double transgenic mice were generated by crossing Flag-MT1 and Myc-MT2 transgenic mice.
Supplementary Material
Fig. S1. Flag and Myc immunoreactivity in the photoreceptors Flag-MT1 and Myc-MT2 mice.
Fig. S2. Competition binding of 2-(125I)iodomelatonin on membranes of HEK293 cells mouse MT1 and MT2 receptors.
Fig. S3. Luzindole and 4P-PDOT do not affect the ERG.
Fig. S4. Functional characterization of the MT2-P95L mutant in transfected HEK293T cells.
Fig. S5. Effec of different signaling pathway stimulators and inhibitors on the amplitude of a-wave at 7 ms.
Fig. S6. Inhibition of melatonin-induced inositol phosphate production by 4P-PDOT.
Acknowledgments
We thank Héloïse Pilet, Dr. Chamsy Sarkis and Dr. Marie-José Lecomte (Newvectys Inc., France) for help and advice in the design, construction and production of lentiviral expression vectors. We thank Drs. Julie Dam and Mark Scott (Institut Cochin, France) for their expert comments on the manuscript; and Morehouse School of Medicine Center for Laboratory Animal Resources for housekeeping the transgenic mouse models.
Funding: This work was supported by grants from the National Institutes of Health Grants NS43459, EY028821, EY022216 and by 5U54NS060659, S21MD000101, G12-RR03034, U54RR026137, by the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS) and the “Who am I?” laboratory of excellence No. ANR-11-LABX-0071 funded by the French Gouvernement through its “Investments for the Future” program operated by The French National Research Agency (ANR) under grant No. ANR-11-IDEX-0005-01. A.B.C. was supported by a doctoral fellowship from the CODDIM 2009 (Région Ile-de-France). Authors contributions: K.B. participate in the project design, performed experiments with electroretinography and PLA, interpreted the data and contributed to the writing of the paper; A.B.-C. designed and performed western blots and co-immunoprecipitation experiments, participated in inositol phosphate detection and interpreted the data; A-S.J. Designed and performed all BRET experiments; M.K. generated the MT2-P95L mutant, performed coimmunoprecipitatino from the retina and constructed lentiviral vectors; J-L.G. performed inositol phosphate and cAMP detection and PKC activity; S.D. generated transgenic mice; F.G. constructed BRET fusion potein vectors and supervisd BRET experiments; K.Y. performed initial BRET and coimmunoprecipitation experiments; C.L. performed in situ hybridization; S.A-C. bred and genotyped the varios transgenic mice, R.J. was responsible for the project supervision, experiment design, data interpretation, and manuscript writing; and provided funding support; G.T. was responsible for the project supervision, experiment design, data interpretation, and manuscript writing; and provided funding support.
Footnotes
Competing Interest: The authors declare no competing financial interests.
References and Notes
- 1.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rask-Andersen M, Almen MS, Schioth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10:579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- 3.Audet M, Bouvier M. Restructuring G-protein- coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 6.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre S, Franco R. Oligomerization of G-protein-coupled receptors: a reality. Curr Opin Pharmacol. 2010;10:1–5. doi: 10.1016/j.coph.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferre S, Navarro G, Casado V, Cortes A, Mallol J, Canela EI, Lluis C, Franco R. G protein-coupled receptor heteromers as new targets for drug development. Prog Mol Biol Transl Sci. 2010;91:41–52. doi: 10.1016/S1877-1173(10)91002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohse MJ. Dimerization in GPCR mobility and signaling. Curr Opin Pharmacol. 2010;10:53–58. doi: 10.1016/j.coph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Kamal M, Jockers R. Biological significance of GPCR heteromerization in the neuro-endocrine system. Front Endocrin. 2011;2:2. doi: 10.3389/fendo.2011.00002. 10.3389/ fendo.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvier M, Heveker N, Jockers R, Marullo S, Milligan G. BRET analysis of GPCR oligomerization: newer does not mean better. Nat Methods. 2007;4:3–4. doi: 10.1038/nmeth0107-3. author reply 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KA, Borowsky B, Tamm JA, Gerald C. GABAb receptors function as a heterotrimeric assembly of the subunits GABAbR1 and GAGbR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 14.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, Wang Q, Lu YJ, Bao L, Zhang X. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez S, Moreno-Delgado D, Moreno E, Perez-Capote K, Franco R, Mallol J, Cortes A, Casado V, Lluis C, Ortiz J, Ferre S, Canela E, McCormick PJ. Circadian-related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012;10:e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 19.Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- 20.Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levoye A, Jockers R, Ayoub MA, Delagrange P, Savaskan E, Guillaume JL. Are G protein-coupled receptor heterodimers of physiological relevance?--Focus on melatonin receptors. Chronobiol Int. 2006;23:419–426. doi: 10.1080/07420520500521863. [DOI] [PubMed] [Google Scholar]
- 23.Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Krauchi K, Brydon L, Jockers R, Muller-Spahn F, Meyer P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–526. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- 24.Scher J, Wankiewicz E, Brown GM, Fujieda H. MT1 melatonin receptor in the human retina: Expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- 25.Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, Wirz-Justice A, Savaskan E. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J, Eckert A, Muller-Spahn F, Meyer P. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- 27.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 28.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiechmann AF, Vrieze MJ, Dighe R, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest Ophthalmol Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- 31.Ping Y, Huang H, Zhang XJXL. Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J Physiol. 2008;586:2683–2694. doi: 10.1113/jphysiol.2008.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba K, Pozdeyev NV, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi C, Pan X, Yan H, Guo M, Pierpaoli W. Effects of melatonin in age-related macular degeneration. Ann NY Acad Sci. 2005;1057:384–392. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- 34.Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, Tone P, McCormick SA, Walsh J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–1679. [PMC free article] [PubMed] [Google Scholar]
- 35.Contreras-Alcantara S, Baba K, Tosini G. Removal of Melatonin Receptor Type 1 Increases Intraocular Pressure and Retinal Ganglion Cells Death in the Mouse. Neurosci Lett. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robson JG, Maeda H, Saszik SM, Frishman LJ. In vivo studies of signaling pathways of the mouse using the electroretinogram. Vision Res. 2004;44:3253–3268. doi: 10.1016/j.visres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev NV, Iuvone PM, Bojang P, Jr, Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AF, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AA, Kamermans M, Gregg RG. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90:331–339. doi: 10.1016/j.ajhg.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM, Tosini G. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch JA. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques. 2011;51:111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugden D, Yeh LK, The MT. Design of subtype selective melatonin receptor agonists and antagonists. Reprod Nutr Dev. 1999;39:335–344. doi: 10.1051/rnd:19990306. [DOI] [PubMed] [Google Scholar]
- 41.Bonnefond A, Clement N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N, Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;16:1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- 43.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghalayini AJ, Anderson RE. Activation of bovine rod outer segment phospholipase C by arrestin. J Biol Chem. 1992;267:17977–17982. [PubMed] [Google Scholar]
- 46.Newton AC, Williams DS. Does Protein Kinase-C Play a Role in Rhodopsin Desensitization. Trends in Biochemical Sciences. 1993;18:275–277. doi: 10.1016/0968-0004(93)90032-i. [DOI] [PubMed] [Google Scholar]
- 47.Orisme W, Li J, Goldmann T, Bolch S, Wolfrum U, Smith WC. Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell Signal. 2010;22:447–456. doi: 10.1016/j.cellsig.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brydon L, Roka F, Petit L, deCoppet P, Tissot M, Barrett P, Morgan PJ, Nanoff C, Strosberg AD, Jockers R. Dual signaling of human Mel1a melatonin receptors via G(I2), G(I3), and G(Q/11) proteins. Mol Endocrinol. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- 49.Mc Arthur A, Hunt A, Gillette MU. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: Activation of protein kinase C at dusk and dawn. Endocrinology. 1997;138:627–634. doi: 10.1210/endo.138.2.4925. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 51.Ayoub MA, Damian M, Gespach C, Ferrandis E, Lavergne O, De Wever O, Baneres JL, Pin JP, Prevost GP. Inhibition of heterotrimeric G protein signaling by a small molecule acting on Galpha subunit. J Biol Chem. 2009;284:29136–29145. doi: 10.1074/jbc.M109.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol. 2002;543:899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tadagaki K, Tudor D, Gbahou F, Tschische P, Waldhoer M, Bomsel M, Jockers R, Kamal M. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood. 2012;119:4908–4918. doi: 10.1182/blood-2011-08-372516. [DOI] [PubMed] [Google Scholar]
- 54.Guillaume JL, Daulat AM, Maurice P, Levoye A, Migaud M, Brydon L, Malpaux B, Borg-Capra C, Jockers R. The PDZ protein MUPP1 promotes G(i) coupling and signaling of Mt(1) melatonin receptor. J Biol Chem. 2008;283:16762.16771. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- 55.Kent KC, Mii S, Harrington EO, Chang JD, Mallette S, Ware JA. Requirement for protein kinase C activation in basic fibroblast growth factor-induced human endothelial cell proliferation. Circ Res. 1995;77:231–238. doi: 10.1161/01.res.77.2.231. [DOI] [PubMed] [Google Scholar]
- 56.Couturier C, Sarkis C, Seron K, Belouzard S, Chen P, Lenain A, Corset L, Dam J, Vauthier V, Dubart A, Mallet J, Froguel P, Rouille Y, Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proc Natl Acad Sci U S A. 2007;104:19476–19481. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flag and Myc immunoreactivity in the photoreceptors Flag-MT1 and Myc-MT2 mice.
Fig. S2. Competition binding of 2-(125I)iodomelatonin on membranes of HEK293 cells mouse MT1 and MT2 receptors.
Fig. S3. Luzindole and 4P-PDOT do not affect the ERG.
Fig. S4. Functional characterization of the MT2-P95L mutant in transfected HEK293T cells.
Fig. S5. Effec of different signaling pathway stimulators and inhibitors on the amplitude of a-wave at 7 ms.
Fig. S6. Inhibition of melatonin-induced inositol phosphate production by 4P-PDOT.