Abstract
Background
There is growing evidence to support an important role for vitamin D and related hormones PTH and FGF23 on cardiac remodeling in chronic kidney disease (CKD). Our objective was to determine the relationships between vitamin D and cardiac remodeling in CKD and the effects of PTH and FGF23 on these associations.
Methods and Results
In 1,431 participants from the Chronic Renal Insufficiency Cohort (CRIC) study, we measured 25(OH)D, 1,25(OH)2D, FGF23, and PTH, and performed quantitative echocardiography. Using linear regression methods, we determined significant negative interactions between 25(OH)D and FGF23 on LV mass (p=0.016); end-diastolic ([EDV], p=0.029); and end-systolic volumes ([ESV], p=0.021). In participants with an FGF23 level >the median of 123.5 RU/ml, each doubling of 25(OH)D was associated with a 2.5% (95%CI −4.8,−0.2) lower LV mass. This association was less pronounced with FGF23 levels <the median (0.4%, 95%CI −1.9,2.7). Conversely, in participants with deficient 25(OH)D levels <20 ng/ml, each doubling of FGF23 was associated with a 3.4% (95%CI 1.2,5.6) greater LV mass compared to only a 1.6% (95%CI −0.2,3.5) difference in participants with sufficient 25(OH)D. Similar findings were observed with 25(OH)D and volumes (p<0.05), and 1,25(OH)2D and LV mass and volumes (p<0.005). There was no effect modification by PTH.
Conclusions
We identified significant interactions between 25(OH)D, 1,25(OH)2D, and FGF23 on cardiac remodeling. Increased LV mass and cavity dilatation were observed with low 25(OH)D and high FGF23. Our findings suggest that consideration of both hormones is crucial to understanding the role of either in cardiac remodeling, and may have important therapeutic implications.
Keywords: vitamin D, echocardiography, cardiac remodeling
Vitamin D deficiency and related abnormalities in mineral metabolism are highly prevalent in chronic kidney disease (CKD).1 With advancing renal dysfunction, 25-hydroxyvitamin D deficiency and downregulation of the renal 1-α-hydroxylase result in impaired conversion of vitamin D [25(OH)D] to the active 1,25-dihyroxyvitamin D [1,25(OH)2D]; abnormal mineral metabolism; and increases in parathyroid hormone(PTH). 1,25(OH)2D is further downregulated by FGF23, the latter which is also markedly increased in renal failure.1, 2
There is a growing body of basic and clinical evidence that supports an important role for vitamin D metabolism in the maintenance of cardiovascular homeostasis. Animal studies demonstrate a protective effect of vitamin D against adverse cardiac remodeling.3 Vitamin D is a negative regulator of the renin-angiotensin system, and acts to reduce hypertrophic gene expression.4 Mice lacking VDR have elevated production of renin and angiotensin II, resulting in hypertension and cardiac hypertrophy.5 Studies of a cardiomyocyte-specific genetic deletion of VDR mouse model suggest an increase in myocyte size and left ventricular (LV) hypertrophy with the conditional knockout.6
Epidemiologically, vitamin D deficiency is associated with renal disease, hypertension, heart failure, and adverse cardiovascular outcomes.7–9 Clinical studies demonstrate a relationship between vitamin D, blood pressure (BP), and renin activity.4 Furthermore, low 25(OH)D levels are associated with more advanced New York Heart Association (NYHA) Class heart failure and impaired LV function.10 Most recently, in 115 participants with chronic kidney disease, although paracalcitriol administration was not associated with a significant reduction in LV mass over a 48-week period, treated patients did demonstrate smaller increases in BNP, fewer cardiovascular events, and a reduction in atrial volume compared to controls, suggestive of an effect of vitamin D on cardiac remodeling.11
Furthermore, recent studies have also demonstrated important effects of vitamin D-related markers of mineral metabolism on cardiac remodeling. In 2,312 participants in the Cardiovascular Health Study, PTH levels greater than ≥65 pg/ml were associated with a 30% greater risk for heart failure.12 Faul, et al demonstrated a significant relationship between increased FGF23 levels and LV hypertrophy in CKD that persisted after adjustment for 25(OH)D, and defined a role for FGF23 in the development of pathologic hypertrophy.13
However, the interactions amongst these markers of mineral metabolism and cardiac remodeling, and the potential relationships between 25(OH)D and 1,25(OH)2D, and FGF23 and PTH, have not been comprehensively defined in CKD. We were specifically interested in the interaction by PTH and FGF23 on the association between 25(OH)D and 1,25(OH)2D and LV mass and cardiac remodeling. We thus measured the following biomarkers: 25(OH)D, 1,25(OH)2D, FGF23, and PTH in 1,431 participants from the Chronic Renal Insufficiency Cohort (CRIC) study who had quantitative echocardiography measures of mass, volumes, and ejection fraction. Our objective was to determine the relationships between 25(OH)D and 1,25(OH)2D and echocardiographic parameters of cardiac size and function in a large, diverse chronic kidney disease population, and define the potential effects of PTH and FGF23 on these associations. We hypothesized that FGF23 excess and vitamin D deficiency would exert multiplicative effects on increasing LV mass and hypertrophy.
Methods
Study Population
The CRIC study is an NIH/NIDDK-sponsored, multi-center prospective cohort study established to study the progression of cardiovascular and renal disease among patients with CKD.14 Participants were recruited based upon age-related entry criteria for estimated glomerular filtration rate (eGFR). Exclusion criteria included life expectancy less than 3 years, NYHA Class III or IV heart failure, known cirrhosis, HIV, prior end stage renal disease, organ or bone marrow transplant, immunosuppressive therapy within the prior 6 months, chemotherapy within the past 2 years, polycystic kidney disease, pregnancy and institutionalized patients.
A total of 3,612 individuals aged 21 to 74 years with an eGFR between 20 and 70 ml/min/1.73 m2 were recruited between June 2003 and March 2007. Participants underwent extensive baseline and follow-up evaluations involving blood sampling and detailed questionnaires. All participants underwent two dimensional (2D) transthoracic echocardiograms at the Year 1 visit.
These analyses used data from an ancillary Vitamin D Study which was conducted at four of the seven clinical centers of the CRIC study: University of Pennsylvania, University of Michigan, Kaiser/University of California at San Francisco, and Johns Hopkins University. In these participants, serum vitamin D and PTH levels were obtained at the Year 1 visit concurrent with echocardiograms from participants representative of the clinical sites. FGF23 levels were obtained at baseline. The protocol was approved by the Institutional Review Board at each site.
Vitamin D, PTH, and FGF-23 Measurements
Serum 25(OH)D was measured using mass spectrometry and 1,25(OH)2D was measured using an established 125I-labeled radioimmunoassay from banked, previously unthawed Year 1 samples stored at −80°C. For 25(OH)D, the limit of quantitation was 1.3 ng/ml. The interassay coefficient of variation (CV) for 25(OH)D was 7.3–10.0% and for 1,25(OH)2D was 7–11%. Year 1 plasma iPTH (pg/ml) was quantified using a radioimmunoassay with 125I-labeled antibody (Scantibodies Clinical Laboratory, Santee, CA, USA). The CV for iPTH was 3–5%. FGF23 was measured in duplicate after a single thaw of stored baseline plasma samples using a second generation C-terminal enzyme-linked immunosorbent assay (Immunotopics) from baseline samples stored at −80°C. The CV was 7.6%.15 Aldosterone was measured after a single thaw of stored baseline plasma samples using a commercially available ELISA (BioVendor). The CV ranged from 6.5 to 8.7%.
Quantitative Echocardiography
Digital 2D transthoracic echocardiograms were analyzed on TomTec computer workstations (TomTec Imaging Systems, Unterschleissheim, Germany). Linear measurements of LV dimensions were performed in the parasternal long axis view using 2D echo and included LV internal dimension at end-diastole (LVIDd) and end-systole (LVIDs). LV length at end-systole and end-diastole were obtained by measuring the distance from the apical endocardium to the center of the mitral valve plane in the 4-chamber view.
LV mass was estimated at end-diastole by digitizing the endocardial and epicardial surfaces of the LV short axis to obtain short axis myocardial areas.16 LV mass (g) was calculated using the area-length method (5/6 short axis myocardial area x LV cavity length x myocardial density (1.055)). LV mass was indexed to height to the 2.7th power. Presence of LV hypertrophy was identified using standard, gender-specific cut points: LVMI ≥50 g/m2.7 in men or ≥ 47 g/m2.7 in women.13 LV end-diastolic (EDV) and end-systolic (ESV) volumes were obtained using Simpson’s method of discs as recommended by the American Society of Echocardiography.16 LV volumes were indexed to height2.7. Ejection fraction (EF) was calculated from 2D LV volumes as (EDV-ESV)/EDV × 100%.
Statistical Methods
The data were examined using summary statistics (percentiles, means, standard deviations(s.d.), medians with interquartile ranges denoting the 25th and 75th percentiles) and markers log-transformed when appropriate. In initial analyses, the univariate cross-sectional associations with clinical correlates at time of vitamin D measure were determined using chi-square test for categorical variables and t-test for continuous variables. These included variables pertaining to demographics (age, sex, and race), social (tobacco and ethanol history), and medical history (cardiovascular disease, hypercholesterolemia, atrial fibrillation, hypertension, and diabetes); clinical measures (body mass index, blood pressure, and eGFR using the CRIC equation17); medical therapy (ace-inhibitor, angiotensin receptor blocker and beta-blocker), and season of blood draw.
Univariable models were then generated using continuous (natural log transformed) and categorical forms of 25(OH)D or 1,25(OH)2D as predictors and echocardiographic parameters as the outcomes. We defined categories according to percentile distributions for both measures; for 25(OH)D we also defined deficient versus not according to the recent Institute of Medicine guideline definitions of < 20 ng/ml versus ≥ 20 ng/ml.18 We selected potential confounders for multivariable adjustment both on the basis of the univariable associations with vitamin D metabolite or cardiac remodeling parameter using a p-value<0.05 as the significance level19 and clinical judgment. Model assumptions were verified using regression diagnostics, and lack of significant collinearity was also confirmed using the vif command following construction of the multivariable regression models.
In individual models, we investigated the possibility of effect modification by PTH and FGF23 on the association between 25(OH)D and echocardiographic measures of cardiac structure and function. We a priori hypothesized that the association between 25(OH)D and LV mass could differ according to PTH and FGF23 given the biologic interactions between these mineral metabolites and 25(OH)D and 1,25(OH)2D.20 We therefore included interaction terms (e.g. 25(OH)D by FGF23) in the models in order to identify statistically significant interactions. To assess the difference in relationships we present the results as the percent change in echocardiographic remodeling parameter for each doubling of biomarker [e.g. 25(OH)D] above and below the median level of the opposing marker(e.g. FGF23). The parameters and biomarkers were natural log transformed, so that the percentage difference in the parameter of interest (with 95% CI) associated with a doubling in the biomarker was estimated by (2β1 − 1) × 100 where β1= the regression coefficient for natural log of the biomarker. The 95% CI were estimated by ((2Lower − 1) × 100 to (2Upper − 1) × 100) where (Lower,Upper) were the lower and upper values of the 95% CI for β1. Models with interaction terms allowed for the identification of significant interactions, while the estimation of the percentage difference in the parameter of interest with 95% CI in stratified analyses illustrated the nature of the potential interactions that supplemented our formal interaction testing. A similar approach was taken for models whereby we examined the effects of doubling FGF23 above and below the median level of 25(OH)D or 1,25(OH)2D. All analyses were performed using STATA 12.0 (Statacorp, TX).
Results
Patient Characteristics
Across the 1,431 participants with vitamin D levels and a 2D transthoracic echocardiogram, the mean age was 60.1±10.5 years, 53% were male, and 39% were African American (Table 1). The median eGFR was 46.4 (interquartile range [IQR] 34.6, 58.7) ml/min/1.73 m2. Eighty-four percent of the cohort had hypertension. Median 25(OH)D levels were 26.9 ng/ml (IQR 15.8, 37.1); 1,25(OH)2D levels were 26.9 pg/ml (IQR 18.2, 36.9); PTH levels were 61 pg/ml (IQR 42, 93.5); and FGF23 levels were 123.5 RU/ml (IQR 83.8, 194.4), with moderate correlations amongst markers (Supplementary Figure 1 and Table 1). As expected, low levels of 25(OH)D and 1,25(OH)2D were both significantly associated with more advanced renal dysfunction, as measured by eGFR (p<0.001 for both). The echocardiographic parameters of cardiac remodeling are shown in Table 2. There was evidence for LV hypertrophy in 41% of the cohort.
Table 1.
Patient Characteristics
| Parameter | Entire cohort (n=1431) | 25 OHD Deficiency (<20 ng/ml) (n=490) | No 25 OHD Deficiency (≥20 ng/ml) (n=941) | 1,25 OHD Less than Median (<26.9 pg/ml) (n=713) | 1,25 OHD Greater than Median (≥26.9 pg/ml) (n=717) | FGF23 Greater than the Median (≥123.5 RU/ml) (n=703) | FGF23 Less than the Median (<123.5 RU/ml) (n=704) | PTH Excess (≥65 pg/ml) (n=633) | No PTH Excess (<65 pg/ml) (n=735) |
|---|---|---|---|---|---|---|---|---|---|
| Demographic Variables | |||||||||
| Age | 60.1±10.5 | 59.5±10.5 | 60.4±10.5 | 61±10 | 59.2±10.9 | 61.0±10 | 59.2±10.9 | 60.7±10.4 | 59.4±10.6 |
| Male Gender | 761(53) | 264(54) | 497(53) | 379(53) | 381(53) | 342(49) | 412(59) | 340(54) | 390(53) |
| African American | 561(39) | 317(65) | 244(26) | 293(41) | 268(37) | 298(42) | 250(36) | 309(49) | 218(30) |
| Medical History and Risk Factors | |||||||||
| Hypertension | 1198(84) | 451(92) | 747(80) | 645(91) | 552(77) | 640(91) | 538(77) | 584(92) | 561(76) |
| Diabetes | 631(44) | 291(59) | 340(36) | 397(56) | 233(33) | 379(54) | 241(34) | 324(51) | 280(38) |
| Current Tobacco Use | 132(9) | 59(12) | 73(8) | 75(11) | 57(8) | 87(12) | 45(6) | 62(10) | 63(9) |
| Alcohol Use | 939(66) | 271(55) | 668(71) | 442(62) | 496(69) | 421(60) | 507(72) | 364(58) | 537(73) |
| Atrial fibrillation | 235(16) | 91(19) | 144(15) | 133(19) | 101(14) | 139(20) | 92(13) | 122(19) | 107(15) |
| Hypercholesterol. | 1209(85) | 436(89) | 773(82) | 642(90) | 566(79) | 627(89) | 562(80) | 560(89) | 598(81) |
| Clinical Measures | |||||||||
| BMI | 31.5±7.6 | 34.1±8.8 | 30.1±6.6 | 32.7±8.2 | 30.3±6.8 | 32.6±8.2 | 30.3±6.8 | 33±8.3 | 30±6.4 |
| Systolic blood pressure | 124±20 | 129±21 | 121±19 | 125±21 | 123±20 | 127±22 | 121±18 | 129±21 | 120±19 |
| eGFR | 47.1±17.3 | 43.4±18.1 | 49±16.6 | 40.5±15.1 | 53.7±16.8 | 39.3±15.2 | 55±15.7 | 38.5±15.8 | 54.2±15.1 |
| Medication Use | |||||||||
| Beta blocker | 656(46) | 256(53) | 400(43) | 365(52) | 290(41) | 386(55) | 258(37) | 340(54) | 292(40) |
| Ace-I/ARB | 985(69) | 344(71) | 641(68) | 538(76) | 446(62) | 525(75) | 445(64) | 455(72) | 490(67) |
| Any BP Drug | 1272(89) | 461(95) | 811(86) | 669(94) | 602(84) | 674(96) | 576(82) | 599(95) | 619(84) |
| Measures related to Vitamin D | |||||||||
| 25(OH)D(ng/ml) | 27.8±14.7 | 12.7±4.3 | 35.7±11.8 | 23.7±13.7 | 31.9±14.6 | 26.1±15.1 | 29.6±14.3 | 22.9±13.5 | 32.1±14.4 |
| 1,25(OH)2D(pg/ml) | 30.4±20.0 | 23.4±13.1 | 34.1±21.9 | 17.5±6.1 | 43.3±20.7 | 26±17.2 | 34.7±21.5 | 27.3±18.1 | 33.1±21.4 |
| PTH(pg/ml) | 83.6± 98.8 | 115.5±145.5 | 66.9±55 | 95.8±114.7 | 71.5±78.3 | 103.9±116.2 | 61.7±69.4 | 132.1±128.6 | 41.8±13.2 |
| FGF23(RU/ml) | 200.4±467.5 | 276.7±749.5 | 160.3±185.3 | 216.8±284.6 | 183.7±596.8 | 316.8±639.8 | 83.7±23.3 | 263.6±664.5 | 145.5±180.7 |
| Calcium (mg/dl) | 9.3±0.47 | 9.2±0.5 | 9.4±0.5 | 9.3±0.5 | 9.4±0.4 | 9.3±0.5 | 9.4±0.4 | 9.2±0.5 | 9.4±0.4 |
| Phosphate (mg/dl) | 3.6±0.6 | 3.7±0.7 | 3.6±0.6 | 3.7±0.66 | 3.5±0.54 | 3.7±0.64 | 3.5±0.54 | 3.7±0.7 | 3.5±0.5 |
| Aldosterone(pg/ml) | 141.4±176.2 | 128.6±121.7 | 148.1±198.5 | 149.8±207.3 | 133.0±137.9 | 138.2±154.8 | 144.7±195.5 | 159.1±228.5 | 128.1±120.6 |
| Winter Season of Blood Draw | 587(41) | 233(48) | 354(38) | 286(40) | 301(42) | 297(42) | 279(40) | 281(44) | 278(38) |
Mean±sd or No.(%)
Table 2.
Echocardiographic Parameters of Cardiac Remodeling
| Entire Cohort (n=1431) | Males (n=761) | Females (n=670) | |
|---|---|---|---|
| Indexed LV Mass (g/m2.7) | 49.8±13.5 | 50.4±13.2 | 49.1±13.9 |
| Left Ventricular Hypertrophy (LVH) | 589(41) | 302(40) | 287(42) |
| Indexed End-Diastolic LV Volume (ml/m2.7) | 33.0±8.9 | 34.2±9.4 | 31.6±8.2 |
| Indexed End-Systolic LV Volume (ml/m2.7) | 15.2±6.8 | 16.2±7.5 | 14.1±5.7 |
| Ejection Fraction (%) | 54.9±8.1 | 53.8±8.6 | 56.0±7.4 |
Mean±sd or No.(%)
Associations between Vitamin D, FGF23, PTH and LV Mass
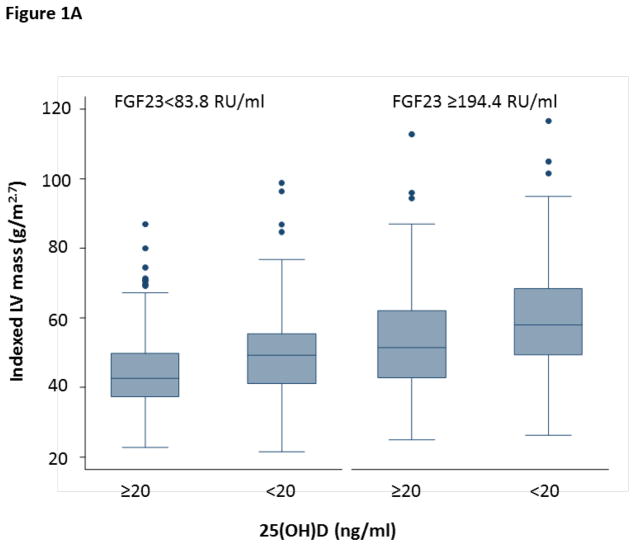
25(OH)D levels were significantly associated with LV mass in unadjusted models, where each doubling in 25(OH)D level was associated with a 7.4% (95% CI −5.9,−8.8, p<0.001) lower LV mass. After adjusting for multiple potential confounders including age, gender, African American race, BMI, eGFR, diabetes, tobacco use, ethanol use, and atrial fibrillation, this effect remained significant (−1.6%, 95% CI −0.1,−3.1, p=0.032). Furthermore, consideration of both PTH and FGF23 in these models revealed a significant interaction between FGF23 and 25(OH)D on LV mass (interaction p=0.016), independent of PTH levels. In participants with FGF23 levels greater than the median, each doubling of 25(OH)D was associated with a 2.5% lower LV mass (95% CI −4.8,−0.2) (Table 3). However, the effect of 25(OH)D on LV mass in the participants with FGF23 levels less than the median was significantly less pronounced (0.4%, 95% CI −1.9,2.7). These findings suggest that the effects of 25(OH)D on LV mass are more evident in the setting of high FGF23 levels (Figure 1A).
Table 3.
Association between Vitamin D Levels and LV Mass
| Marker | Percent Difference in LV Mass(95% CI) | Marker | Percent Difference in LV Mass(95% CI) |
|---|---|---|---|
| Doubling of 25(OH)D | Doubling of FGF23 | ||
| Stratified by FGF23 | Stratified by 25(OH)D | ||
| FGF≥123.5 RU/ml | −2.5(−4.8,−0.2) | 25(OH)D≥20 ng/ml | 1.6(−0.2,3.5) |
| FGF<123.5 RU/ml | +0.4(−1.9,+2.7) | 25(OH)D<20 ng/ml | 3.4(1.2,5.6) |
|
| |||
| Doubling of 1,25(OH)2D | Doubling of FGF23 | ||
| Stratified by FGF23 | Stratified by 1,25(OH)2D | ||
| FGF≥123.5 RU/ml | −2.6(−4.6,−0.5) | 1,25(OH)2D≥27.1pg/ml | 3.4(1.5,5.3) |
| FGF<123.5 RU/ml | 0.5(−1.8,2.8) | 1,25(OH)2D<27.1 pg/ml | 1.9(−0.1,3.9) |
The regression models for log transformed indexed LV Mass includes log transformed Vitamin D(either 25(OH)D or 1,25(OH)2D) or log transformed FGF23. In addition, the models include age, gender, African American race, BMI, eGFR, diabetes, tobacco use, ethanol use, atrial fibrillation, and natural log of PTH. The percentage difference in LV Mass(with 95% CI) associated with a doubling in Vitamin D(either 25(OH)D or 1,25(OH)2D) or FGF was estimated by (2β1 − 1) × 100 where β1= the regression coefficient for(natural log) of Vitamin D or of(natural log) FGF23. The 95% CI were estimated by((2Lower − 1) × 100 to (2Upper − 1) × 100) where (Lower,Upper) were the lower and upper values of the 95% CI for β1.
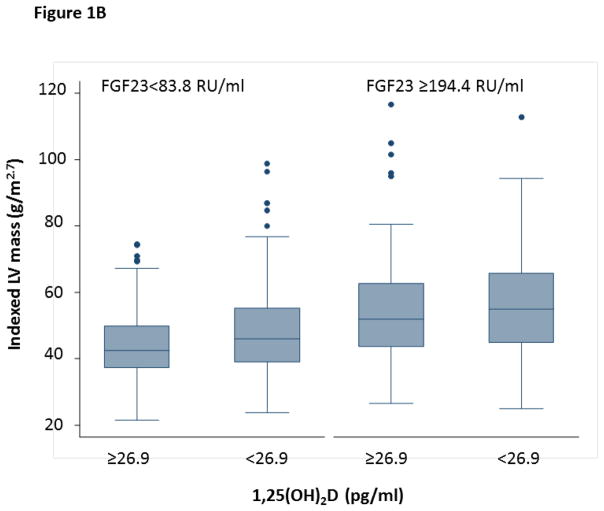
Figure 1.
Figure 1A. The Effects of 25(OH)D Deficiency on LV Mass are More Pronounced with High FGF23
This graph displays the median and interquartile ranges for LV mass according to 25(OH)D Deficiency and the 25th and 75th percentile levels of FGF23
Figure 1B. The Effects of Low 1,25(OH)2D on LV Mass are More Pronounced with High FGF23
This graph displays the median and interquartile ranges for LV mass according to median percentile cutpoints for 1,25(OH)2D and the 25th and 75th percentile levels of FGF23
Furthermore, in the 410 participants with 25(OH)D deficiency, each doubling of FGF23 was associated with a 3.4% higher LV mass (95% CI 1.2,5.6), whereas in the 792 participants with sufficient 25(OH)D levels, each doubling of FGF23 was associated with only a 1.6% difference LV mass (95% CI −0.2,3.5) (Table 3). These findings suggest that the effects of FGF23 levels on LV mass are more evident in the setting of 25(OH)D deficiency.
1,25(OH)2D was also significantly associated with LV mass. In unadjusted models, each doubling in 1,25(OH)2D was associated with a 6.5% lower LV mass (95% CI −7.9,−5.0, p<0.001). Again, in adjusted models including age, gender, African American race, BMI, eGFR, diabetes, tobacco use, ethanol use, and atrial fibrillation this effect was attenuated, with a 1.4% lower LV mass with each doubling of 1,25(OH)2D (95% CI 0.10,−2.9, p=0.067). Consistent with our findings above, there was also a significant interaction between 1,25(OH)2D and FGF23 on LV mass (interaction p=0.003). In participants with FGF23 levels greater than the median, each doubling of 1,25(OH)2D was associated with a 2.6% lower LV mass (95% CI −4.6,−0.5%) (Table 3). Again, this effect differed in participants with low FGF23 levels (0.5%, 95% CI −1.8,2.8%). These findings suggest that the effects of 1,25(OH)2D are also more pronounced in the setting of high FGF23 (Figure 1B).
There was no significant effect modification by PTH on the association between 25(OH)D or 1,25(OH)2D and LV mass, although PTH was independently associated with LV mass in these adjusted models. There was a 1.8% (95% CI 0.22,3.4, p=0.026) higher LV mass with each doubling of PTH when considering 25(OH)D and FGF23, and a 2.2% (95% CI 0.65,3.7%, p=0.005) higher LV mass when considering 1,25(OH)2D and FGF23. There was no significant effect modification by race, eGFR, or ACE-inhibitor or ARB therapy. Consideration for potential confounders including phosphate, albuminuria, cholesterol, cardiac disease history, systolic blood pressure, season of blood draw, use of vitamin D supplements, baseline aldosterone levels, and hemoglobin did not have any significant effect on our findings (data not shown).
Associations between Vitamin D Measures and LV Volumes and Ejection Fraction
Given our findings above, subsequent analyses were performed in the context of a potential interaction between 25(OH)D and 1,25(OH)2D and FGF23. In models adjusted for age, gender, African American race, BMI, eGFR, atrial fibrillation, and PTH, the relationships between 25(OH)D and LV EDV and ESV differed according to FGF23 (interaction p= 0.029 and p=0.021, respectively). Although the association between 25(OH)D and LV volumes were not statistically significant within each FGF23 stratum (Table 4), with each doubling of 25(OH)D, lower LV volumes were observed with FGF23 levels greater than the median and higher LV volumes were observed with FGF23 levels less than the median. Furthermore, 25(OH)D deficiency potentiated the adverse cardiac remodeling observed with increased FGF23 levels. As shown in Table 5, in participants with 25(OH)D deficiency, each doubling of FGF23 was associated with higher LV EDV compared to those with sufficient 25(OH)D [3.6% (95% CI 1.4,5.8) versus 2.0% (95% CI 0.1,3.8)]. Similarly, in participants with 25(OH)D deficiency, each doubling of FGF23 was associated with higher LV ESV compared to those with sufficient 25(OH)D levels [5.8% (95% CI 2.7,9.0) versus 2.8% (95% CI 0.1,5.6)].
Table 4.
Association between Vitamin D Levels and LV Volumes
| Percentage Change in LV End Diastolic Volume (95% CI)* | Percentage Change LV End Systolic Volume(95% CI)* | |
|---|---|---|
| Doubling of 25(OH)D | ||
| Stratified by FGF23 | ||
| FGF≥123.5 RU/ml | −1.0(−3.3,1.4) | −2.3(−5.6,1.2) |
| FGF<123.5 RU/ml | 1.4(−0.9,3.8) | 2.3(−1,5.6) |
|
| ||
| Doubling of 1,25(OH)2D | ||
| Stratified by FGF23 | ||
| FGF≥123.5 RU/ml | −1.2(−3.3,0.9) | −1.9(−4.9,1.2) |
| FGF<123.5 RU/ml | 1.3(−1,3.5) | 2.7(−0.5,6.0) |
The regression models for log transformed LV EDV or ESV include log transformed Vitamin D (either 25(OH)D or 1,25(OH)2D). In addition, the models include age, gender, African American race, BMI, eGFR, atrial fibrillation, and natural log of PTH. The percentage differences in LV EDV or ESV (with 95% CI) associated with a doubling in Vitamin D are estimated as in Table 3.
Our findings with 1,25(OH)2D were consistent with a significant interaction observed between 1,25(OH)2D and LV EDV and ESV (p=0.004 and p=0.001, respectively). Again, there were significant differences in the direction of the relationship between 1,25(OH)2D and volumes according to FGF23 levels(Table 4). Furthermore, the effects of FGF23 on LV ESV were more pronounced in the setting of 1,25(OH)2D levels below the median compared to above the median [5.4 (95% CI 2.4,8.5) versus 2.9%(0.2, 5.7)] (Table 5).
Table 5.
Association between FGF23 Levels and LV Volumes
| Percentage Change in LV End Diastolic Volume (95% CI)* | Percentage Change LV End Systolic Volume(95% CI)* | |
|---|---|---|
| Doubling of FGF23 | ||
| Stratified by 25(OH)D | ||
| 25(OH)D≥20 ng/ml | 2.0(0.1,3.8) | 2.8(0.1, 5.6) |
| 25(OH)D<20 ng/ml | 3.6(1.4,5.8) | 5.8(2.7, 9.0) |
|
| ||
| Doubling of FGF23 | ||
| Stratified by 1,25(OH)2D | ||
| 1,25(OH)2D≥26.9 pg/ml | 2.7(0.8, 4.6) | 2.9(0.2, 5.7) |
| 1,25(OH)2D<26.9 pg/ml | 2.7(0.7, 4.8) | 5.4(2.4, 8.5) |
The regression models for log transformed LV EDV or ESV include FGF23. In addition, the models include age, gender, African American race, BMI, eGFR, atrial fibrillation, and natural log of PTH. The percentage differences in LV EDV or ESV (with 95% CI) associated with a doubling in FGF23 are estimated as in Table 3.
Furthermore, there was no significant effect modification by PTH on the association between 25(OH)D or 1,25(OH)2D and LV volumes, although there was an independent association between PTH and EDV (1.9%, 95% CI 0.28,3.5, p=0.021 with each doubling of PTH) when considering FGF23 and 25(OH)D, and when considering FGF23 and 1,25(OH)2D (1.8%, 95% CI 0.28,3.3, p=0.020). There was no independent relationship between PTH and ESV (p=0.11 and p=0.09 for 25(OH)D and 1,25(OH)2D models). There were no significant associations between 25(OH)D, FGF23, and ejection fraction in multivariable adjusted models (all p>0.05), but there was a significant interaction between 1,25(OH)2D and FGF23 on the association with ejection fraction (p=0.011).
Discussion
Chronic kidney disease results in significant disturbances in bone and mineral metabolism. Vitamin D deficiency, secondary hyperparathyroidism, and marked elevations in FGF23 concentration are highly prevalent and are believed to contribute directly to cardiac hypertrophy and adverse cardiac remodeling. However, the interactions between these measures are poorly defined. In our cross-sectional analyses of 1,431 patients with chronic renal insufficiency, we identified a significant interaction 25(OH)D and 1,25(OH)2D and FGF23 on LV mass and volumes. Not only were the effects of 25(OH)D deficiency on increased LV mass more pronounced in the setting of elevated FGF23 levels, but 25(OH)D deficiency potentiated the risk of increased cardiac growth (LV mass) and cavity dilatation (LV EDV and ESV) with elevated FGF23 levels. Similarly, the association between 1,25(OH)2D and LV mass was also more pronounced in the setting of elevated FGF23 levels, and 1,25(OH)2D deficiency potentiated the risk of increased cardiac dilatation with elevated FGF23 levels. These effects were independent of PTH, suggesting that there are important effects of vitamin D on cardiac growth and hypertrophy even after consideration of the secondary hyperparathyroidism that occurs with vitamin D deficiency.
Multiple population-based studies have demonstrated an increased risk of cardiovascular events with LV hypertrophy, increased LV mass, and LV cavity dilatation. The Framingham Heart Study established the relationship between LV mass and incident cardiovascular disease in adults free of cardiovascular disease aged 40 years or older.21 Increases in LV mass were associated with an increased risk of cardiovascular disease beyond traditional risk factors. More recently, in the Multi-Ethnic Study of Atherosclerosis, a 10% increase in LV mass was associated with a 40% increase in heart failure over 5 years of followup. Similarly, a 10% increase in LV volumes was associated with a 30% increased risk of heart failure.22 Given the clinical impact of adverse cardiac remodeling, it is critical to improve our understanding of the factors that may modify cardiac hypertrophy and cavity dilatation.
Vitamin D, obtained from dietary supplements in the form of ergocalciferol or from the skin as cholecalciferol is metabolized from the inactive form 25(OH)D to the active form of 1,25(OH)2D by the liver and kidney by 1-α-hydroxylase.2 The renal enzyme 1-α-hydroxylase is stimulated by PTH and inhibited by calcium, phosphate, and 1,25(OH)2D. FGF23 is a peptide hormone that serves to decrease phosphate levels by increasing urinary excretion, decreasing intestinal absorption, and decreasing 1,25(OH)2D(calcitriol) levels through inhibition of the renal and extra-renal 1-α-hydroxylase and increases in the 24-α-hydroxylase.23 While FGF23 also acts to prevent excess calcitriol, 1,25(OH)2D also acts to stimulate FGF23 production via a feedback loop.20
Prior human studies have demonstrated individual associations between 25(OH)D and hypertension.8 The largest study of 12,644 participants from the third National Health and Nutrition Examination Survey (NHANES-III) demonstrated an inverse correlation between systolic blood pressure and pulse pressure and 25(OH)D levels.10 Basic studies have demonstrated individual associations between vitamin D deficiency and hypertrophy4,6 and FGF23 and hypertrophy,13 although the precise mechanisms remain to be more fully elucidated. In animal models, VDR deficient mice demonstrate increased renin angiotensin system activation, hypertension, and ventricular hypertrophy.6,24,25 The effects may also be related to effects on vascular smooth muscle cells or calcineurin activity.26,27 Others have hypothesized that secondary hyperparathyroidism may mediates the association between 25(OH)D and hypertrophy. PTH increases calcium levels and conversion of 25(OH)D to 1,25(OH)2D but has also been shown in observational studies to be associated with increased blood pressure and is believed to have effects on vascular smooth muscle cells.12, 26, 27 PTH did have an independent association with LV mass and EDV in our adjusted models, corroborating the hypothesis that PTH may have some cardiac effects as well. However, the relationships we observed of 25(OH)D and FGF23 on cardiac remodeling were independent of PTH, suggesting that these 25(OH)D/FGF23 effects may occur through additional mechanisms.
FGF23 is associated with cardiac hypertrophy in human andanimal studies.13 Klotho-deficient mice also developed significant LVH, and FGF23 administration results in pathologic hypertrophy. Our findings provide additional insight into the complex interplay within the 25(OH)D, 1,25(OH)2D and FGF23 axes by examining the interactions between these hormones. We found that vitamin D deficiency or FGF23 excess intensifies the adverse cardiac remodeling effects observed with either factor. These findings have important implications in determining which participants might derive the maximum benefit from pharmacologic therapy to either modify vitamin D deficiency or FGF23 excess, and suggest that consideration of both metabolites are critical in establishing the effects of these measures on LV growth and remodeling.
Limitations of the study include the possibility for unmeasured confounding, although our models were comprehensive and included multiple potential confounders. While we adjusted for BMI, we cannot completely exclude the possibility that unmeasured confounding between measures of adiposity, vitamin D, and LV mass contributed to the observed association between low vitamin D and increased LV mass. Furthermore, given the observational nature of our study, we cannot determine if adiposity potentially lies in the causal pathway between the relationship between vitamin D and LV mass. Furthermore, our FGF23 levels were obtained from a baseline blood sample, which was approximately one year prior to the assessment of all other variables. Our study design was cross-sectional in nature, limiting the causal inferences of our findings, which can determined more definitively in longitudinal or in intervention studies. Nevertheless, this is the first study to demonstrate a significant interaction between 25(OH)D and 1,25(OH)2D and FGF23 in humans and provide potential insight into future clinical trial design.
In summary, our findings demonstrate that in a large chronic kidney disease cohort, 25(OH)D and 1,25(OH)2D deficiency are independently associated with increased LV mass. There was a significant interaction by FGF23 on these relationships, whereby patients with elevated FGF23 levels had greater effects on LV mass and adverse cardiac remodeling. Similarly, deficiency in vitamin D potentiated the risk of adverse cardiac remodeling observed with elevated FGF23. These findings have important therapeutic implications in determining the effects of vitamin D repletion or FGF23 antagonists on cardiac remodeling.
Supplementary Material
Clinical Perspective.
There is growing evidence to support an important role for vitamin D and related hormones PTH and FGF23 on cardiac remodeling in chronic kidney disease (CKD). In 1,431 participants from the Chronic Renal Insufficiency Cohort (CRIC) study, we measured 25(OH)D, 1,25(OH)2D, FGF23, and PTH, and performed quantitative echocardiography measures of cardiac remodeling. Using linear regression methods, we determined significant negative interactions between 25(OH)D and FGF23 on cardiac size and remodeling, as measured by left ventricular mass, and end-diastolic and end-systolic volumes. Increased LV mass and cavity dilatation were observed with low 25(OH)D and high FGF23 levels. In other words, the effects of 25(OH)D deficiency on increased LV mass more pronounced in the setting of elevated FGF23 levels, and 25(OH)D deficiency potentiated the risk of increased cardiac growth and cavity dilatation with elevated FGF23 levels. Our findings suggest that consideration of both hormones is crucial to understanding the role of either in cardiac remodeling, and may have important therapeutic implications.
Acknowledgments
Sources of Funding
The CRIC ancillary study was supported by R01DK077128 (Dr. Leonard ), K24 DK076808 (Dr. Leonard), and R01DK081374 (Dr Wolf). Dr. Ky was supported by an NHLBI K23HL095661. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the University of Pennsylvania CTRC CTSA UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente Northern California NIH/NCRR UCSF-CTSI UL1 RR-024131.
Appendix
*CRIC Study Investigators include: Lawrence J. Appel, MD, MPH; Harold I. Feldman, MD, MSCE; Alan S. Go, MD; Jiang He, MD, PhD; John W. Kusek, PhD; James P. Lash, MD; Akinlolu Ojo, MD, PhD; Mahboob Rahman, MD; Raymond R. Townsend, MD
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cozzolino M, Ketteler M, Zehnder D. The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail. 2010;12:1031–1041. doi: 10.1093/eurjhf/hfq112. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso P, Rahman A, Hershey SD, Dandu L, Nibbelink KA, Simpson RU. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51:559–564. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 4.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC. Therapeutic Effects of Vitamin D Analogs on Cardiac Hypertrophy in Spontaneously Hypertensive Rats. Am J Pathol. 2010;177:622–631. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameri P, Ronco D, Casu M, Denegri A, Bovio M, Menoni S, Ferone D, Murialdo G. High prevalence of vitamin D deficiency and its association with left ventricular dilation: An echocardiography study in elderly patients with chronic heart failure. Nutr Metab Cardiovasc Dis. 2010;20:633–40. doi: 10.1016/j.numecd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 9.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 10.Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 11.Thadhani R, Appelbaum E, Chang Y, Pritchett Y, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson T, Audhya P, Andress D, Zhang W, Ye J, Packham D, Singh B, Zehnder D, Manning WJ, Pachika A, Solomon SD. Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am J Nephrol. 2011;33:139–149. doi: 10.1159/000323551. [DOI] [PubMed] [Google Scholar]
- 12.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58:1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M Chronic Renal Insufficiency Cohort (CRIC) Study Group. . Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing G, American Society of Echocardiography’s Guidelines and Standards Committee European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. Journal of the American Society of Echocardiography. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI CRIC Study Investigators. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2010. [PubMed] [Google Scholar]
- 19.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 20.Haussler MR, Whitfield GK, Kaneko I, Forster R, Saini R, Hsieh JC, Haussler CA, Jurutka PW. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev Endocr Metab Disord. 2012;13:57–69. doi: 10.1007/s11154-011-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 22.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M. FGF23 inhibits extra-renal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28:46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;88:E125–32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 25.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schierbeck LL, Jensen TS, Bang U, Jensen G, Kober L, Jensen JE. Parathyroid hormone and vitamin D--markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail. 2011;13:626–632. doi: 10.1093/eurjhf/hfr016. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Vanwoerkom RC, Horne BD, Bair TL, May HT, Lappe DL, Muhlestein JB. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J. 2011;162:331–339.e2. doi: 10.1016/j.ahj.2011.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.