Abstract
We evaluated Toll-like receptor (TLR) function in primary human dendritic cells from 104 young (age 21–30) and older (≥ 65 years) individuals. We used multicolor flow cytometry and intracellular cytokine staining of myeloid (mDC) and plasmacytoid (pDC) DCs and found substantial decreases in older, compared to young individuals in TNF-α, IL-6 and/or IL-12 (p40) production in mDCs and in TNF-α and IFN-α production in pDCs in response to TLR1/2, TLR2/6, TLR3, TLR5, and TLR8 engagement in mDCs and TLR7 and TLR9 in pDCs. These differences were highly significant after adjustment for heterogeneity between young and older groups (e.g. gender, race, body mass index [BMI], number of comorbid medical conditions) using mixed effect statistical modeling. Studies of surface and intracellular expression of TLR proteins, and of TLR gene expression in purified mDCs and pDCs revealed potential contributions for both transcriptional and post-transcriptional mechanisms in these age-associated effects. Moreover, intracellular cytokine production in the absence of TLR ligand stimulation was elevated in cells from older, compared to young individuals, suggesting a dysregulation of cytokine production that may limit further activation by TLR engagement. Our results provide evidence for immunosenescence in dendritic cells; notably, defects in cytokine production were strongly associated with poor antibody response to influenza immunization, a functional consequence of impaired TLR function in the aging innate immune response.
Introduction
Aging is associated with a progressive decline in immune function (immunosenescence) resulting in increased susceptibility to viral and bacterial infections and decreased response to vaccines (1–3). Age-associated perturbations in the humoral and cell-mediated arms of the adaptive immune system are well documented (3, 4); however, the impact of aging on the innate immune system is less well defined. Age related deficiencies of the innate immune system are incompletely understood but include reduced recruitment, phagocytosis, granule release and microbial activity by PMN or macrophages, suggesting an age-related dysfunction in signal transduction as a manifestation of immunosenescence (5–9).
Toll-like receptors (TLRs) are pattern recognition receptors that recognize conserved molecular patterns on microbes and are key to triggering antimicrobial host defense responses (10). Recognition of microbial components by TLRs initiates MyD88 or TRIF-dependent signal transduction pathways that culminate in the elaboration of type I interferons and proinflammatory cytokines that facilitate the linkage of innate to adaptive immune responses control innate immune responses (11). Deficiencies in human TLR signaling lead to increased severity of several diseases, including sepsis, immunodeficiencies, atherosclerosis and asthma (12). We previously evaluated TLR function in monocytes and macrophages in the context of human aging, and observed an age-associated deficit in TLR1/2-induced cytokine production associated with reduced surface expression of TLR1 in monocytes and dysregulation of TLR3 expression in macrophages (13, 14). Monocytes from older individuals also demonstrated a generalized age-associated defect in TLR-induced expression of the CD80 costimulatory molecule associated with reduced protective antibody responses to influenza immunization (15). Similar reduced levels of TLRs have been noted in whole blood samples and colonic biopsies from older individuals (16), and in macrophages and pDCs from aged mice (17–19); other studies in different inbred mouse strains have implicated signaling differences in the absence of age-associated alterations in TLR expression (20, 21).
Dendritic cells (DCs) are professional antigen-presenting cells (APC) that play a pivotal role in the linkage between innate and adaptive immunity (22). Human blood DCs, classified as myeloid DC (mDC) or plasmacytoid DC (pDC), have distinct functional activities. mDCs produce large amounts of IL-12 and induce strong T helper type1 (TH1) and cytotoxic T lymphocyte (CTL) responses (23). In contrast, pDCs produce large amounts of type I IFN in response to viral and bacterial stimuli. Stimulation of DCs and production of effector mechanisms involves phagocytosis, the upregulation of costimulatory and MHC molecules, a switch in chemokine receptor expression, the secretion of cytokines and chemokines, and the presentation of antigens by DCs (24). The consequences of aging on TLR function in human mDC and pDC populations remain incompletely understood. Here, we report on age-associated alterations in TLR function in such cells, and the consequences of age-associated alterations in innate immunity for vaccine responsiveness in humans.
Materials and Methods
Study Participants
We recruited participants at vaccination clinics organized by the Yale University Health Services in October and November of 2007. Heparinized blood from healthy volunteers was obtained with informed consent under a protocol approved by the Human Investigations Committee of the Yale University School of Medicine. Older (age≥65) or young (21–30 years) participants with no history of acute illness in the two weeks prior to enrollment were evaluated. Immunocompromised individuals were excluded as described previously (14). After informed consent was obtained, participants were evaluated using a screening questionnaire, and self-reported information was collected which included demographic information, height, weight, medications, and comorbid conditions (with specific inquiries regarding coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, diabetes, hypertension, peptic ulcer disease, kidney disease, and chronic lung disease).
Isolation of human peripheral blood dendritic cells
Human peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (GE Healthcare, NJ) gradient centrifugation. For FACS assays, DC populations were identified in PBMCs by staining with fluorescent antibodies to specific surface markers. Circulating DC numbers were calculated by multiplying the percentage of DCs by the absolute count of PBMCs in the cell populations gated by light scatter in flow cytometry. For PCR assays, DCs were purified by sorting on FACSAria (Becton Dickinson, CA). Purified mDC and pDC were stored in RLT buffer at −80°C for RNA isolation.
Cell stimulation and flow cytometry
For prevaccination samples, PBMCs were suspended in RPMI 1640 medium plus 10% fetal bovine serum (FBS), and adjusted to a concentration of 2 × 106 cells/mL. The following ligands in medium were added to PBMCs: TLR1/2 ligand (Pam3CSK4, 10 µg/mL), TLR2/6 ligand (LTA, 2 µg/mL), TLR3 ligand (Poly I:C, 10 µg/ml), TLR5 ligand (flagellin 5 µg/mL), TLR7/8 ligand (R848 0.5 µg/mL), TLR9 ligand (CpG-ODN2216, 3 µg/ml). RPMI 1640 medium plus FBS alone was used in control cultures. All ligands were purchased from Invivogen. Cells were incubated for 6 h and Brefeldin A (Golgi-Stop, BD Biosciences) was added for the last 4 h of incubation to retain intracellular cytokines. Cell suspensions were labeled with primary antibodies for surface staining as follows: lineage cocktail (CD3 [Clone SK7], CD14 [Clone MphiP9], CD16 [3G8] and CD19 [SJ25C1])-APC-Cy7, HLA-DR-PE-Cy7, CD11c [B-ly6]-APC and CD123 [9F5]-PE-Cy5. For analyses of surface TLR expression in unstimulated samples, antibodies against TLR1 (PE, clone GD2.F4), TLR2 (Alexa700, clone TL2.1) were used; reagents and procedures for the analyses of intracellular TLR expression in unstimulated samples is described below. Anti-CD3, CD14, CD16, CD19, CD11c, CD123, TLR1 and TLR 2 antibodies were purchased from BD Biosciences. Cell surface labeling at 4 °C was followed by washing in PBS containing 2% FBS. Cells were fixed in 4% paraformaldehyde, resuspended in 90% FBS containing 10% DMSO, and stored at −80°C.
Samples obtained 4–6 weeks postvaccination for analysis of TLR responses were incubated for 4 hours in medium alone or with distinct TLR ligands as follows: TLR3 ligand (Poly I:C, 50 µg/ml); TLR7 ligand (Gardiquimod, 3 µM); TLR8 ligand (3M002, 10 µM) and TLR7/8 ligand (R848, 3 µg/ml). Brefeldin A was added for the last 2 hours to retain intracellular cytokines. Cell suspensions were labeled, fixed and frozen as described above.
On the day of analysis, cells were thawed, permeabilized in BD Perm/Wash buffer (BD Pharmingen, CA), pelleted at 500×g for 10 min at 4°C, and resuspended in BD Perm/Wash buffer containing Abs for intracellular staining of cytokines or intracellular TLRs as follows: IL-6-PE (Clone MQ2-6A3), TNF-α Alexa700 (Clone MAb11), IL-12 (p40)-Pacific Blue (Clone C8.6), IFN-α-FITC (Clone225.2C), TLR3-biotin (Clone TLR3.7), TLR 8-biotin (Clone 44C143) and TLR 9-FITC (Clone 26C593.2), with appropriate isotype controls. Streptavidin (SA)-FITC was used as a secondary staining agent for TLR3-biotin and TLR8-biotin, and a biotinylated isotype control plus SA-FITC was used for all experiments employing biotinylated antibodies. IL-6, IL-12 and TNF-α antibodies were purchased from BD Pharmingen. Antibody to IFN-α was purchased from Chromaprobe, Inc. TLR3-biotin was purchased from eBioscience, and TLR 8-biotin and TLR9-FITC were purchased from Imgenex. Approximately 0.5–1 million total events were collected per sample using an LSR II instrument (BD Biosciences, CA), with analysis using FlowJo software (Tree Star, OR). Initial gating on parent DC subsets was further characterized by cytokine positive subpopulations. We used Boolean combinations of gated DC subsets in the three cytokine gates (IL-12, IL-6, and TNF-α) as described to uniquely discriminate responding cells based on their cytokine production (25) .
Evaluation of enriched dendritic cell populations
A random sample of 13 members of the original cohort (6 young, 7 older) were recalled for additional blood samples 20–21 months after initial enrollment. PBMCs from these follow-up samples were subjected to DC isolation using magnetic bead sorting (Blood Dendritic Cell Isolation Kit II [Miltenyi Biotec]), first by depleting CD19- and CD14-positive cells, and then using positive selection with anti-BDCA3, anti-BDCA4, and anti-CD1c. Approximately 5 × 105 DCs (a mixture of mDCs and pDCs) were isolated from each participant using this procedure, and these cells were 70–85% pure using a live cell gate. Stimulations with Pam3CSK4, LTA and R848 were carried out as described previously, and mDC and pDC populations were identified and interrogated for intracellular cytokine production by flow cytometry using the protocol described above—with the exception that cells were processed immediately for intracellular cytokine staining without fixation and freezing.
TLR expression analysis by real-time quantitative-PCR (qPCR)
Total RNA was harvested from purified mDC and pDC using the RNeasy mini kit according to the modified manufacturer’s instructions (Qiagen, CA). Carrier RNA (4 ng/µl, Qiagen, CA) was added to optimize the isolation. cDNA was synthesized using AffinityScript Multi Temperature cDNA Synthesis Kit (Stratagene, TX) according to standard protocols (13). Amplification was performed in an iCycler (Bio-Rad, CA) for 60 cycles with an annealing temperature at 60 as described (13). Primers and probes for qPCRs were synthesized according to customized sequences as follows: TLR3 (Forward: 5’-CCT GGT TTG TTA ATT GGA TTA ACG A −3’, Reverse: 5’ TGA GGT GGA GTG TTG CAA AGG −3’, Probe: 5’- 6FAM ACCCATACCAACATCCCTGAGCTGTCAA TAMRA −3’), TLR7 (Forward: 5’- TTA CCT GGA TGG AAA CCA GCT A −3’, Reverse: 5’- TCA AGG CTG AGA AGC TGT AAG CTA −3’, Probe: 5’- 6FAM-AGAGATACCGCAGGGCCTCCCGMGBNFQ −3’ ), TLR8 (Forward: 5’- GGA AAG CAA GTC CCT GGT AGA ATT −3’, Reverse: 5’- ACC TGT TGT CAT CAT CAT TCC ACA A −3’, Probe: 5’- 6FAM-ATTGCCACTGAAAACTMGBNFQ −3’), TLR9 (Forward: 5’- GGA CCT CTG GTA CTG CTT CCA −3’, Reverse: 5’- AAG CTC GTT GTA CAC CCA GTC T −3’, Probe: 5’- 6FAM-ACG ATG CCT TCG TGG TCT TCG ACA AAT MGBNFQ −3’), β-actin (Forward: 5’-ATC CTG GCC TCG CTG TCC AC-3’, Reverse: 5’-GGG CCG GAC TCG TCA TAC −3’, Probe:5’- 6FAMTCCAGCAGATGTGGATCAGCAAGCATAMRA −3’). The expression level of each gene in each sample was determined in duplicate and results were normalized to β-actin as described (13). Significance was assessed by the Mann-Whitney test using Graphpad Prism (GraphPad Software, Inc.).
Assessment of immunogenicity
A serum sample was collected from each subject before vaccination and <35 days (range, 28–42 days) after vaccination. Serum samples were separated into aliquots and stored at a temperature of −20° C or lower until assayed. We performed hemagglutination inhibition (HAI) assays on pre- and postvaccine serum samples as described (26) to determine antibody titers against each of the strains of influenza included in the 2007–2008 influenza vaccine using antigen reagents specific to the vaccine. The composition of the vaccine was: A/Solomon Islands/3/2006; A/Wisconsin/67/2005; B/Malaysia/2506/2004 (Fluzone: Sanofi Pasteur). Serconversion to a strain in the vaccine was defined as a 4-fold or greater increase in antibody titer between pre- and post-vaccine serum samples. We defined seroprotection as a post-vaccine titer of ≥ 1:64. For the analyses of seroprotection, we excluded members of the cohort who had pre-vaccine titers of ≥ 1:64, since these individuals already met criteria for protection prior to vaccination. Consequently, the analysis of seroprotection included n=32 young and n=49 older for the H1N1 vaccine strain; n=47 young and n=30 older for H3N2; and n=44 young and n=34 older for the B strain.
Statistical analysis
Proportions or means, where appropriate, were used to describe the demographic and clinical characteristics of each cohort at enrollment. In order to model both the variation in our sample of young and old adults, as well as the correlation between ligand specific stimulation, we employed a mixed effects model to estimate the effect of age group on IL6, IL12 and TNF-α percentage change after stimulation in mDCs and TNF-α and IFN levels percentage change after stimulation in pDCs in the cohort (27, 28). Specifically, we used an unstructured covariance structure that permitted each participant to have a unique correlation structure for each ligand stimulation; this accounted for the inherent variation of each participant. Tests of normality were applied to IL6, IL12, TNF-α and IFN-α percentage change by cell type. The full models tested for the fixed effect of age group (older versus young), ligand, the interaction between age group and ligand, controlled for potential confounding by including the covariates gender, race, number of comorbid conditions (heart disease, stroke, peripheral vascular disease) and body mass index using restricted maximum likelihood. Least-squared means were estimated for the fixed effects of age group and age group by ligand interaction and the differences were tested.
Next, we estimated strain-specific seroconversion and seroprotection in the cohort using generalized linear models controlling confounding by the same covariates listed above (29). Then, we estimated the effect of age group on the number of strain seroprotected and seroconverted using Poisson regression controlling confounding by covariates listed above (29). For models of seroprotection only those with prevaccination titers < 1:64 were included.
Subsequently, we used mixed effects models to estimate the effect of seroconversion and seroprotection on the IL6, IL12 and TNF-α percentage change after stimulation in mDCs and TNF-α and IFN levels percentage change after stimulation in pDCs, again each of these models were adjusted for correlations between ligand specific stimulation and covariates (29).
We used multivariable linear regression to estimate the effect of age group on the percent-positive cells expressing specific TLR proteins, controlling for confounding by covariates listed above. To test the whether there were differences between the young and older age groups for the enriched dendritic cell populations we used a t-test. Statistical tests were 2-tailed, and p<.05 considered to indicate statistical significance. Analyses used SAS version 9.1 (SAS Institute, Cary, NC).
Results
Age-associated defect in TLR-induced cytokine production in human dendritic cells
We evaluated TLR function in DCs from 50 younger (age 21–30 years) and 54 older (age ≥65) individuals prior to influenza immunization. Clinical and demographic characteristics of participants are shown in Table 1. Notably, the older individuals evaluated represented a generally healthy group, with nearly 75% reporting no comorbid illnesses. Heterogeneity was found in the young vs. older group in terms of gender, race, and number of comorbid illnesses, reflecting the expected consequences of aging and differences in composition of the two age groups; we used mixed effects multivariable statistical modeling to adjust for potential confounding by these covariates as in our previous work (14).
Table 1.
Characteristics of young and older cohort.
| Young (n=50) | Older (n=54) | P valuea | |
|---|---|---|---|
| Age, mean (range) | 24.8 (21–30) | 75.4 (64–87) | <0.0001 |
| Female Gender | 35 (70%) | 22 (40.7%) | 0.006 |
| Race: % White | 35 (70%) | 48 (88%) | <0.0001 |
| % Black | 5(10%) | 2 (3.7%) | 0.49 |
| % Asian | 10(20%) | 2 (3.7%) | 0.003 |
| % Hispanic | 0 | 4 (7.4%) | 0.006 |
| Body Mass Index (BMI) | 24.4 | 26.4 | 0.09 |
| Number of comorbidities |
0.23 | 1.24 | <0.0001 |
| No reported comorbidities |
49 (98%) | 43 (79.6%) | 0.009 |
| Specific comorbidities: | |||
| Stroke | 0 (0%) | 3 (5.5%) | 0.12 |
| Heart Diseaseb | 0 (0%) | 9(16.6%) | <0.0001 |
| PVD | 0 (0%) | 2 (3.7%) | 0.49 |
| Lung Disease | 0 (0%) | 2 (3.7%) | 0.49 |
| Peptic Ulcer Disease | 1 (2%) | 5 (10%) | 0.12 |
Based on t tests for normally distributed characteristics and Fisher’s exact tests for categorical characteristics.
Includes coronary artery disease, congestive heart failure, and arrhythmias.
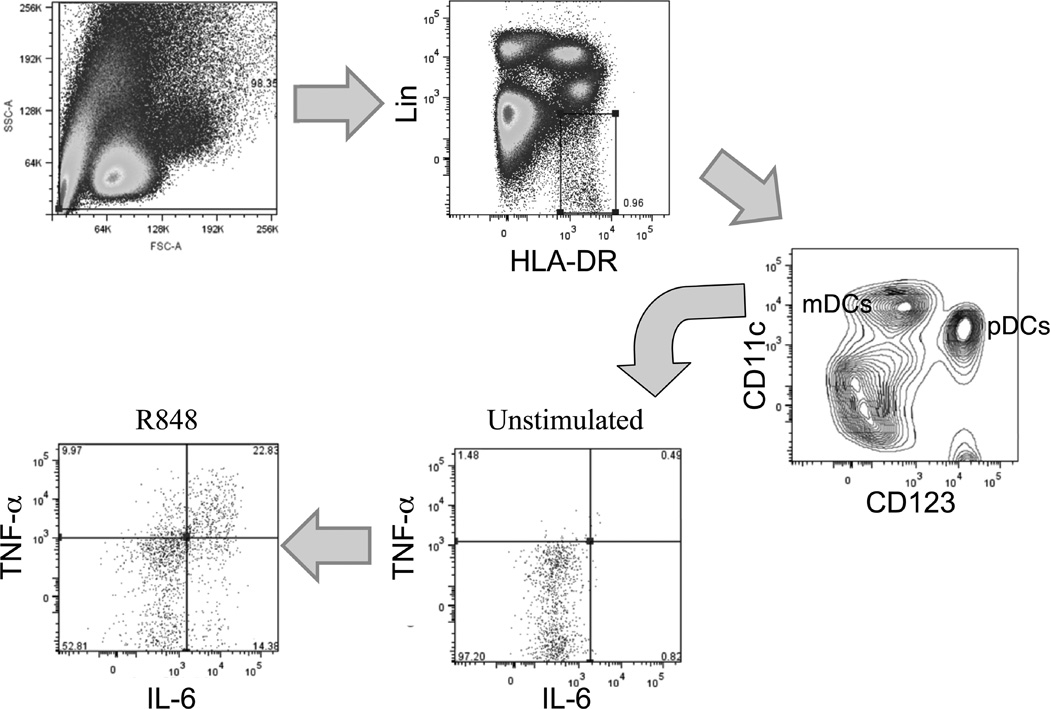
To elucidate the consequences of aging on TLR function in primary human DCs, we employed multicolor flow cytometry and intracellular cytokine staining in peripheral blood mononuclear cell (PBMC) samples from young and older participants. An example of this analysis is shown in Figure 1; we gated on a Lineage (consisting of a cocktail of antibodies against CD3, CD14, CD16, and CD19)-negative, HLA-DR-positive population, with mDCs and pDCs subsequently identified using staining against CD11c and CD123. We found that while the number of mDCs did not change between the young and older groups, the number of circulating pDCs decreased significantly (P<0.01, n = 36 young, n = 34 older) from 3725 + 472 cells/ml to 1975 + 194 cells/ml in young and older groups, respectively, consistent with previous reports of reduced type I IFN production or lower numbers of pDCs in aging (30–32).
Figure 1.
Representative example of identification of mDC and pDC populations from peripheral blood. Lin− HLADR+ cells were further fractionated into CD11c+ (mDC) or CD123+ (pDC) populations. In this example, following treatment with R848, production of TNF-α and IL-6 are evaluated using intracellular cytokine staining, gating in this case on mDCs.
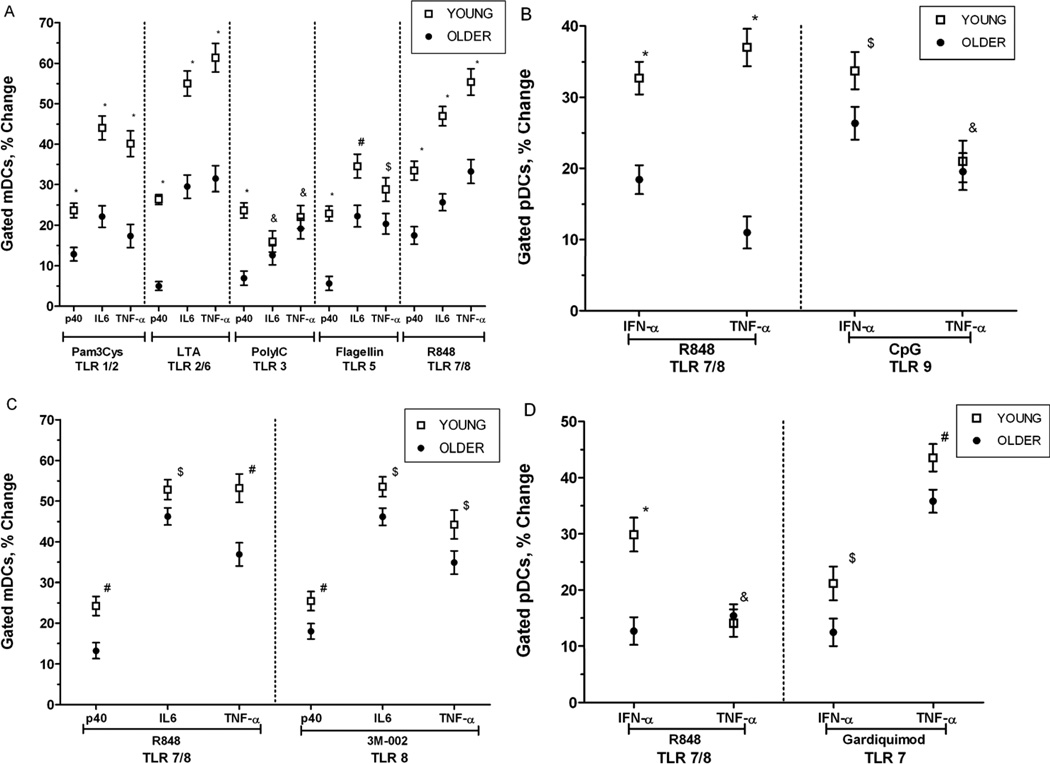
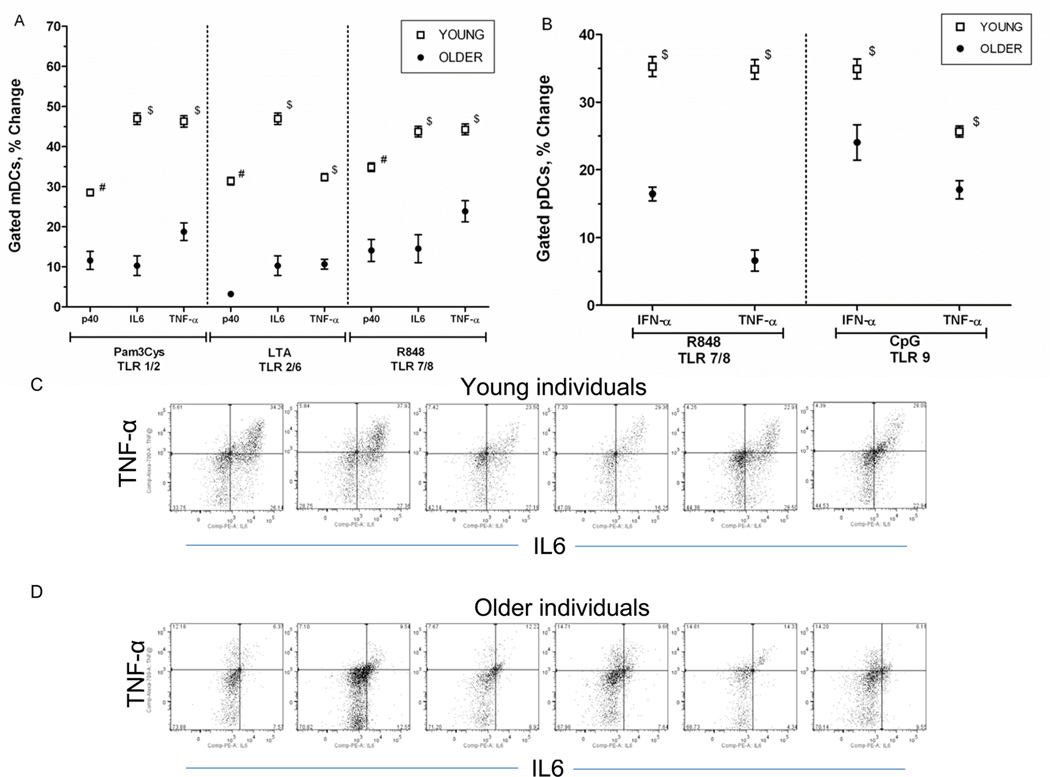
Using intracellular cytokine staining, we evaluated mDCs for the production of TNF-α, IL-6 and the p40 subunit of IL-12/23 (IL-12p40) following treatment with TLR ligands as follows: Pam3CSK4 (TLR1/2), lipoteichoic acid (LTA; TLR2/6), poly (I:C) (TLR3), flagellin (TLR5), R848 (TLR7/8). In general, we observed decreased production of TLR-induced cytokines in mDCs from older compared to young individuals, including lower levels of TNF-α, IL-6 and IL-12/p40 (Figure 2A). In addition, pDCs from older individuals produced decreased levels of TNF-α and IFN-α after treatment with the TLR ligands R848 (TLR7/8) and CpG (TLR9) (Figure 2B). These differences were highly statistically significant after adjustment for covariates including gender, race, BMI, and number of comorbid conditions.
Figure 2.
Age-associated alteration in Toll-like receptor (TLR)–induced cytokine production in mDCs and pDCs in older, compared to young adults. A. Age-associated defects (n=50 young, n=54 older) in IL-12p40, IL-6 and TNF-α production in mDCs (as measured by intracellular cytokine staining) following stimulation with indicated TLR ligands B. Age-associated defects in IFN-α and TNF-α production in pDCs (n=50 young, n=54 older) following stimulation with R848 and CpG. Stable defects in TLR8-induced cytokine production in mDCs (C) and in TLR7-induced cytokine production in pDCs (D), assessed both with R848 and TLR7- or TLR8-specific agonists 4–6 weeks after studies in A and B (n=36 young, n=34 older). Each plot depicts the mean ± SEM percent positive difference in intracellular cytokine production between samples stimulated with the indicated TLR ligand and unstimulated samples.; P values after adjustment for covariates are indicated: *< 0.0001, #<0.01, $<0.05, &<NS).
We reevaluated a subset of our cohort six weeks later with new blood samples to assess the stability of responses to selected TLR ligands and to provide a more in depth examination of those receptors recognizing components of nucleic acids, such as in the course of influenza or other viral infections. We repeated stimulation with R848 (TLR 7/8) and in addition used more selective ligands for TLR7 and TLR8 (Gardiquimod and 3M-002, respectively). In keeping with our initial observations, mDCs from older, compared to young individuals produced lower levels of IL-6, TNF-α and IL-12 in response to both R848 and 3M-002. In addition, we found that Gardiquimod or R848 engagement of TLR7 in pDCs resulted in diminished IFN-α production in pDC from older, compared to young individuals (Figure 2C, 2D). These findings indicate that at least for TLR 7 and TLR8, the age-associated alterations in cytokine responses we observed are stable over time. Taken together, these results reveal a generalized defect in TLR-induced cytokine production in primary human mDCs and pDCs.
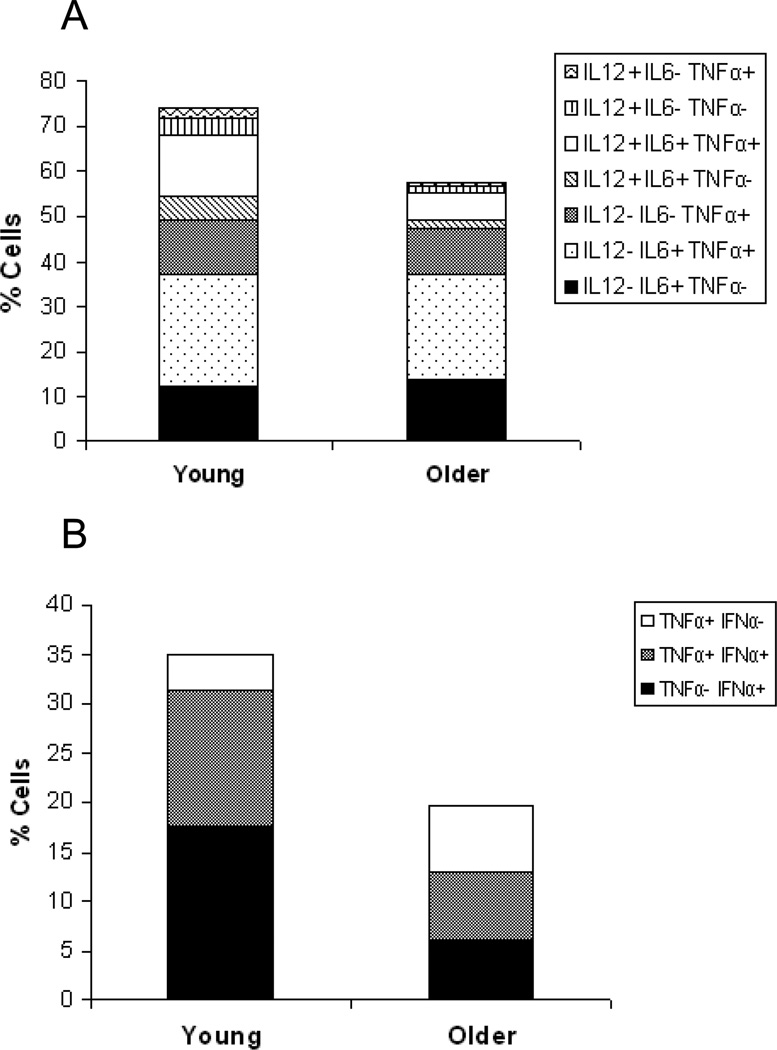
Reduced production of cytokines by DCs in older individuals may be especially relevant for particular sub-populations of cells. Distinct populations of cytokine-producing cells can be delineated at the single-cell level based on the combination of cytokines produced. To reveal age-dependent differences, we have employed Boolean gating strategies that allow rapid comparison of distinct sub-populations of stimulated DCs (25). The response of mDCs and pDCs to R848 stimulation is depicted (Figure 3). It is readily apparent that both age groups have equivalent populations of single positive mDCs that produce IL-6 and/or TNF-α with low expression of IL-12. However, the most significant difference between mDCs of young vs. older individuals is in the multifunctional triple positive cells that simultaneously produce IL-12, IL-6, and TNF-α, which are dramatically lower in DCs from older subjects (Figure 3A). For pDCs, while overall production of cytokines was reduced in pDCs from older individuals, the total subpopulation of IFN-negative cells is actually larger in pDCs from older, compared to young participants (Figure 3B).
Figure 3.
Boolean gating of DC subpopulations. (A) the frequency of mDCs expressing each of the seven possible combinations of IL-6, TNF-α, and IL-12 (p40) after stimulation with the TLR7/8 ligand R848; (B) the frequency of pDCs expressing each of the three possible combinations of IFN-α and TNF-α after stimulation with R848.
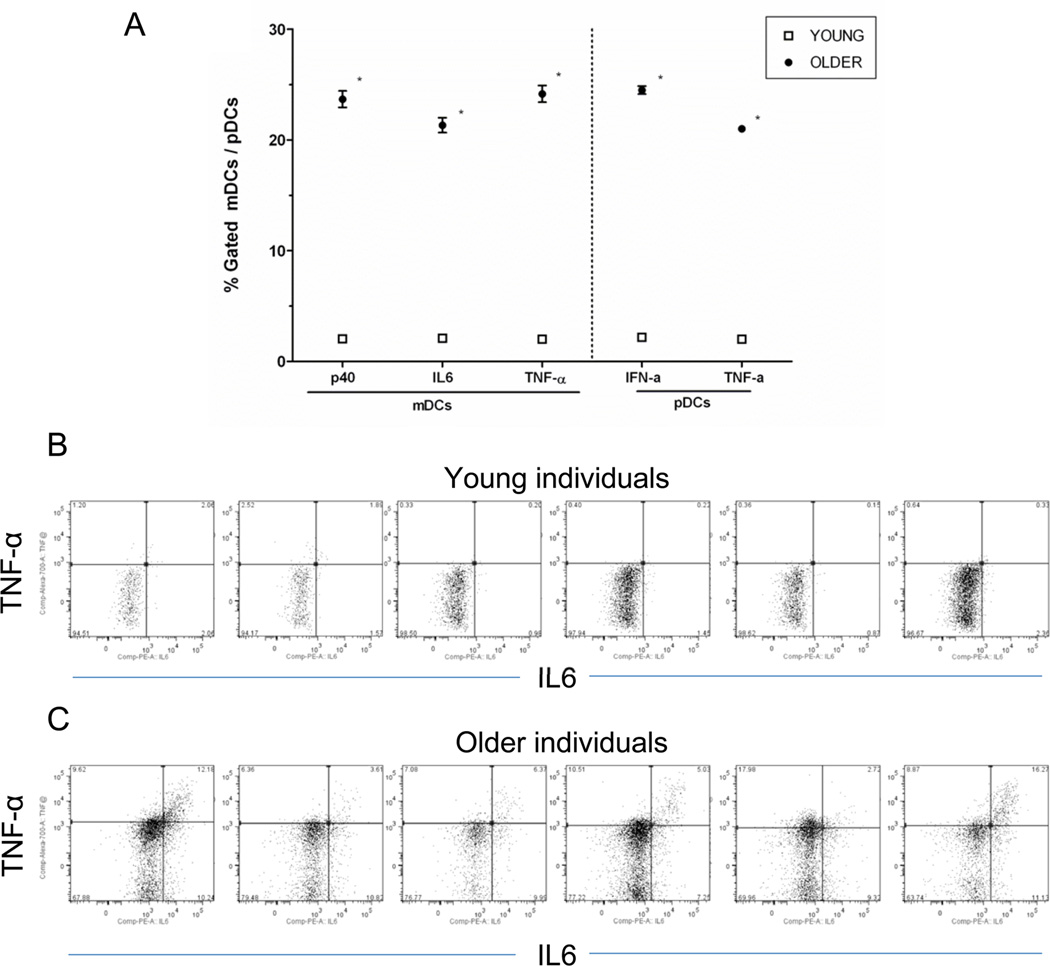
Our observation of decreased TLR-induced cytokine production contrasts with the pro-inflammatory milieu associated with elevated levels of IL-6, TNF-α and other cytokines in older individuals—so called “inflamm-aging” (33). Because of this, we compared levels of cytokine production as measured by intracellular cytokine staining in DC populations that were not subjected to TLR ligand stimulation. Notably, we observed markedly elevated levels of p40, TNF-α, and IL-6 in mDCs, and of TNF-α and IFN-α in pDCs, in older, compared to young individuals (Figure 4); these differences were highly statistically significant. Taken together, these data indicate that basal levels of cytokine production are elevated in DCs from older individuals, and that TLR ligand stimulation of such cells results in a smaller increase in cytokine production than in DCs from young participants.
Figure 4.
Basal cytokine levels in older, compared to young adults. A. Baseline IL-12 (p40,) IL-6 and TNF-α production in mDCs (as measured by intracellular cytokine staining) and IFN-α and TNF-α production in pDCs (n=50 young, n=54 older). The mean ± SEM percent positive mDCs/pDCs for cyokine production in the absence of TLR stimulation are depicted (in some cases because of low variability in the samples, error bars are too narrow to be distinguished). P values after adjustment for covariates are indicated: *< 0.0001. B and C. Representative examples of baseline cytokine production, depicted here baseline TNF-α and IL-6 production in mDCs from 6 young and 6 older adults following R848 treatment.
We chose to evaluate cytokine production in DC populations using flow cytometry-based analyses of PBMCs, rather than study purified DCs, to minimize manipulations that might result in partial activation of DCs, and because purification procedures would substantially decrease the yield of cells for analysis. However, the low percentage of DCs present in the PBMC population raises the possibility that other cell types could contribute to the observed alterations in TLR function in mDCs and pDCs from older, compared to young individuals. To evaluate this possibility, we used a combination of negative and positive magnetic bead sorting to isolate DCs that were 70–85% pure in 6 young and 7 older, randomly chosen members of the original cohorts, evaluated 20–21 months after initial enrollment. The lower yield of cells from this procedure allowed us to test only activation by Pam3CSK4 (TLR1/2), LTA (TLR2/6), and R848 (TLR8) in mDCs, and R848 (TLR7) and CpG (TLR9) in pDCs. Nonetheless, our evaluation of these enriched DC populations yielded results that were consistent with our observations in PBMCs. We observed statistically significant decreases in p40, IL-6, and TNF-α production in mDCs, and IFN-α and TNF-α production in pDCs, in response to these ligands in cells from older, compared to young individuals (note that these estimates of significance were not adjusted for covariates given the lower number of samples) (Figure 5). Indeed, for TNF-α production in response to CpG stimulation in pDCs, a significant difference was observed in the recalled subset while no significant difference was observed in the larger cohort when initially evaluated; the reasons for this may reflect [HGA1]the fact that the older cohort was nearly two years older when recalled. We employed magnetic bead enrichment, instead of cell sorting, since the purity of cell-sorted populations is achieved at the expense of sufficient yield of cells for in vitro studies. While we cannot exclude the possibility that other cell types could still contribute to the alterations in DC function we observed, even in these highly enriched populations, these results nonetheless indicate that age-associated alterations in mDCs and pDCs contribute to the decreased TLR-induced cytokine production found in cells from older individuals.
Figure 5.
Age-associated alteration in Toll-like receptor (TLR)–induced cytokine production in purified DCs isolated using magnetic bead sorting in 6 older, compared to 7 young adults two years after the original study. A. Age-associated decrease (n=13) in IL-12 (p40,) IL-6 and TNF-α production in mDCs (as measured by intracellular cytokine staining) following stimulation with indicated TLR ligands. B. Age-associated decrease in IFN-α and TNF-α production in pDCs (n=13) following stimulation with R848 and CpG. The mean ± SEM percent positive difference in intracellular cytokine production between samples stimulated with the indicated TLR ligand and unstimulated samples are depicted; P values after adjustment for covariates are indicated: #<0.01, $<0.05. C and D. Representative examples of TLR ligand induced cytokine production, depicted here TNF-α and IL-6 production in 6 young (Panel A) and 6 older adults (Panel B) after stimulation with TLR7/8 ligand R848.
Age-associated alterations in TLR expression in human DCs
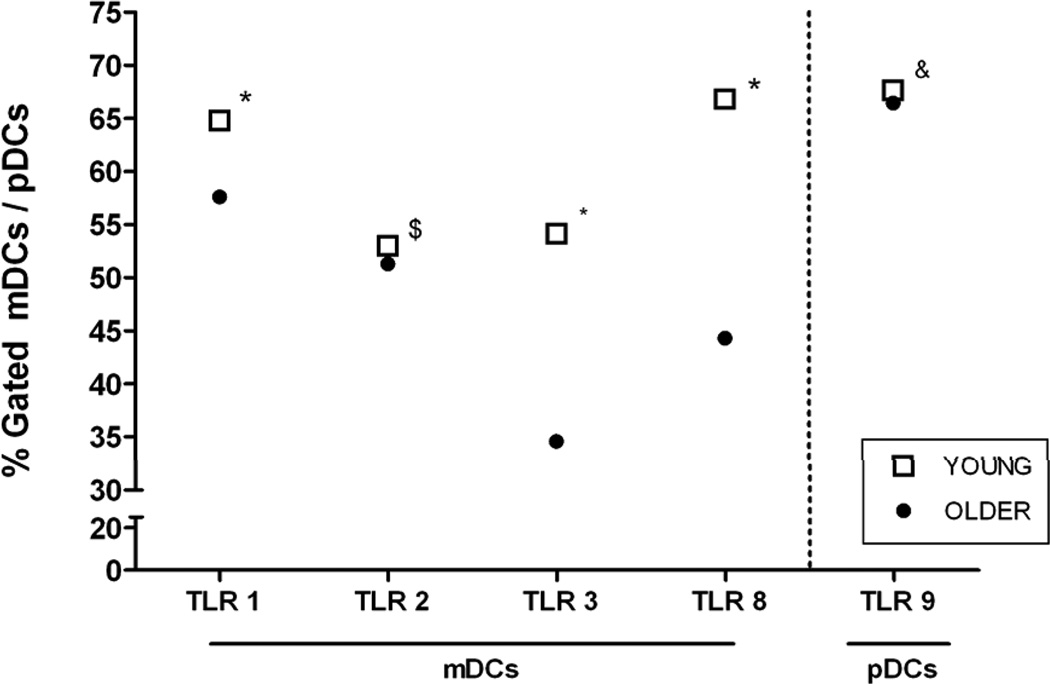
To determine the basis for our observed age-associated defects in TLR-mediated function in human DCs, we employed flow cytometry to examine the expression of TLRs from DCs of young vs. older individuals. We evaluated the surface expression of TLR1 and TLR2, and the intracellular expression of TLR3 and TLR8 in mDCs from young and older individuals; in addition, we determined the intracellular expression of TLR9 in pDCs. We detected decreased expression of TLR1, and essentially unchanged TLR2 expression in mDCs from older individuals, similar to our previous observations in human monocytes (14). In addition, intracellular expression of TLR3 and TLR8 were also decreased in mDCs from older, compared to young individuals, while TLR9 expression in pDCs appeared unchanged (Figure 6). Thus, some of the age-associations alterations we observed may result from decreased expression of TLRs in cell surface and intracellular compartments, particularly in mDCs.
Figure 6.
Age-associated decrease in TLR protein expression. Shown are baseline percent positive mDCs for TLR 1, TLR 2, TLR3 and TLR8 expression and pDCs for TLR9 expression (compared with isotype control). Values indicate the mean ± SEM (because of the low amount of variability in these samples, in some cases error bars are too narrow to be distinguished) of the young adults (n = 24) and older adults (n = 24). P values after adjustment for covariates: *< 0.0001, $<0.05, &<NS.
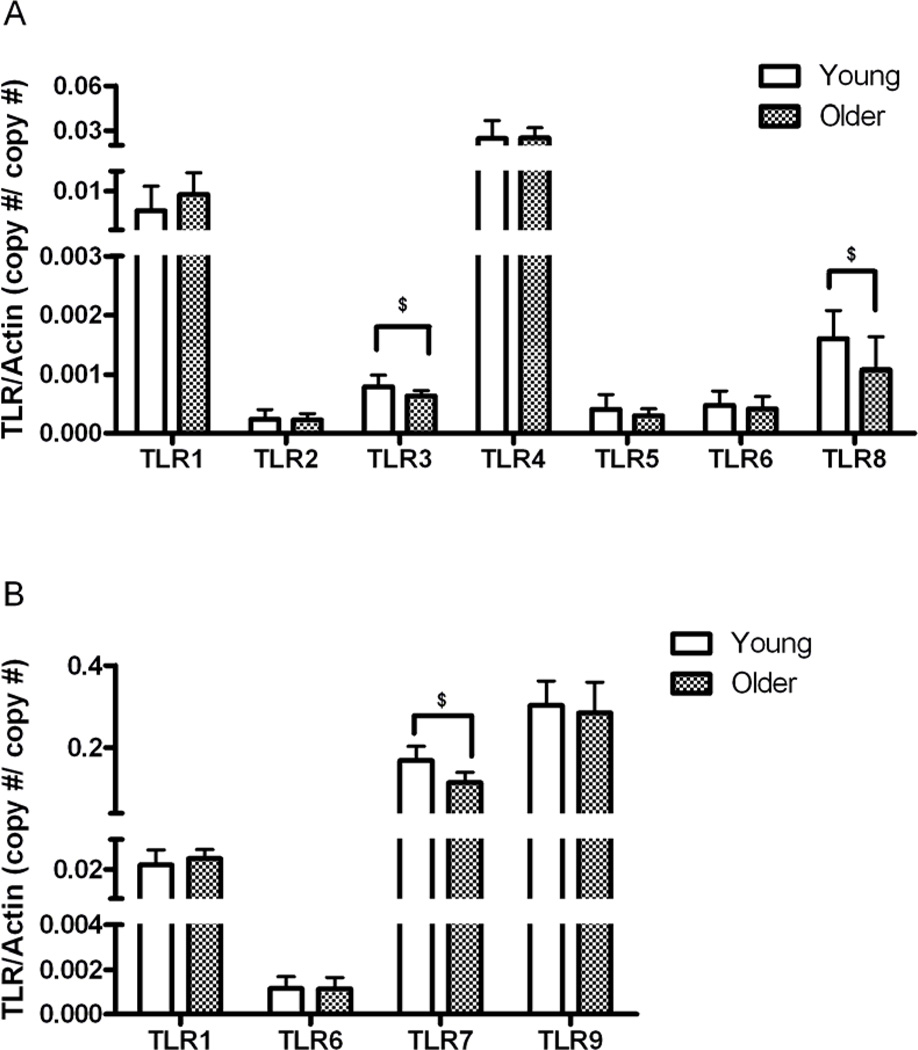
To determine whether transcriptional mechanisms contribute to the observed age-associated differences in TLR expression, we determined TLR mRNA expression levels using qPCR of highly purified mDC and pDC populations from the young and older groups (n = 20/group). We detected cell-type appropriate TLR expression, i.e. mDCs expressed mRNA for TLRs 1, 2, 3, 4, 5, 6, 8, while pDCs expressed mRNA only for TLRs 1, 6, 7, 9 consistent with earlier reports (22, 34). When comparing TLR expression from DCs of older vs. young individuals, we detected significantly lower levels of TLR3 and TLR8 in mDCs from older, as compared to young adults (Figure 7); similarly, the expression of TLR7 was also significantly lower in pDCs from older, compared to young participants (Figure 7). While these data indicate that the expression of TLR3, TLR7 and TLR8 in DCs declines with aging, we observed defects in TLR1/2, TLR2/6 and TLR9-induced cytokine production and protein expression despite unperturbed expression of TLR1, TLR2, TLR6, and TLR9 RNA. Taken together, these findings indicate that both transcriptional and post-transcriptional mechanisms contribute to the observed age-associated defects in TLR function.
Figure 7.
Effect of aging on expression of TLRs in human mDC and pDC. The data display mRNA expression levels of indicated TLRs in purified mDCs (A), and pDCs (B) from 40 individuals (Young, N=20; Older, N=20). mRNA levels were quantified by Q-PCR and normalized to β-actin. The median values and interquartile range are indicated . $ indicates statistical significance between young and older cohort (Mann Whitney, p < 0.05).
Association of TLR stimulated cytokine production with influenza vaccine antibody response
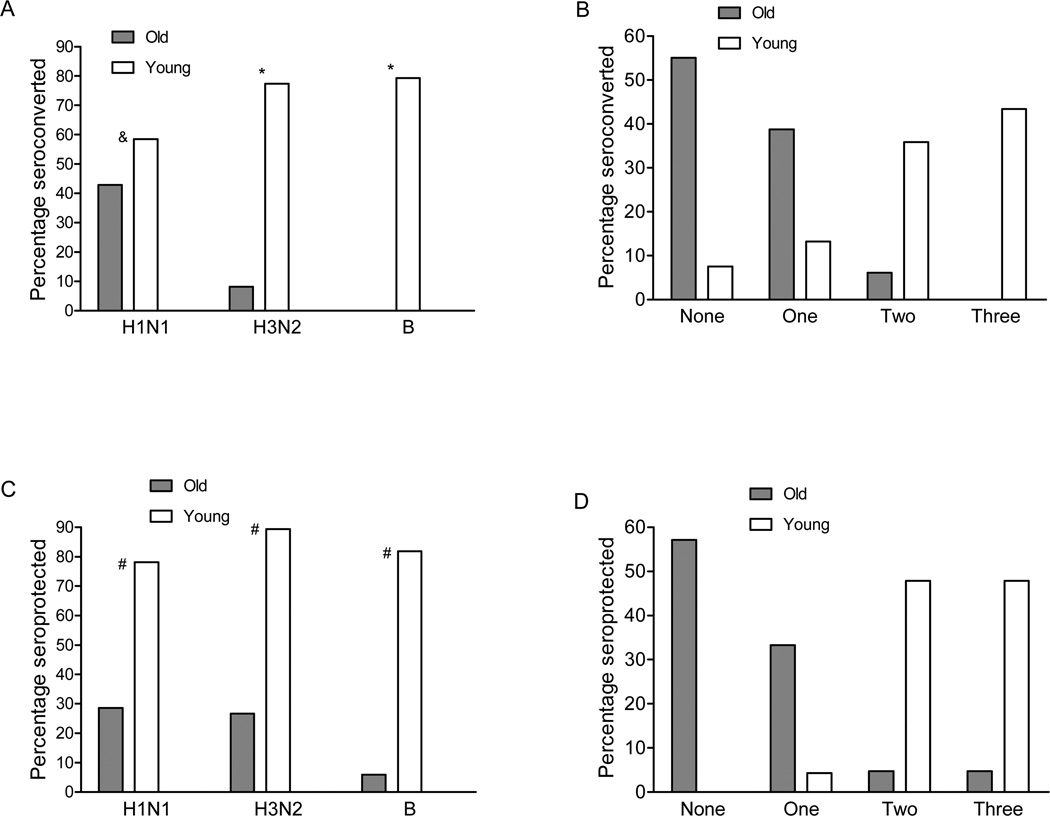
We have shown that both mDCs and pDCs from older individuals have reduced TLR expression and function. To assess the effect of these deficits, we assessed the antibody response to immunization with the trivalent 2007–2008 influenza vaccine (A/Solomon Islands/3/2006; A/Wisconsin/67/2005; B/Malaysia/2506/2004). Serum samples were obtained from the 104 young and older participants prior to and 4–6 weeks following immunization, and influenza serologies were determined using a standard hemagglutination inhibition assay. As expected, rates of seroconversion (defined as a four-fold increase between pre- and post-vaccine serologies) were substantially lower for older individuals, reflecting the known decreased efficacy of the trivalent inactivated vaccine in this population (Figure 8). For example, nearly 80% of young individuals seroconverted to the H3N2 and B vaccine strains, compared to 8% and 0% of older individuals, respectively; notably, 55% of older individuals failed to seroconvert to any of the three vaccine strains. In addition, we also evaluated whether vaccine recipients generated antibody titers post-vaccine that are associated with protection against infection regardless of fold-increase between pre- and post-vaccine titers. Defining seroprotection as a post-vaccine titer of ≥ 1:64, we found that older individuals were much less likely than young participants to show seroprotection against strains in the vaccine (Figure 8).
Figure 8.
Responses to influenza vaccination in older adults and young adults. A. Pre-and postvaccine titers showing a ≥ 4-fold increase to each of the influenza strains in the 2007–2008 vaccine (A/Solomon Islands/3/2006-like [H1N1], A/Wisconsin/67/2005-like [H3N2], and B/Malaysia/2506/2004-like [B]) are shown. B. The proportion in each age group with a ≥ 4-fold increase in titer to none, 1, 2, or all of the vaccine strains. C. Pre-and postvaccine hemagglutination inhibition (HI) titers ≥ 1:64. D. The proportion in each age group with a postvaccination titer ≥ 1:64 to none, 1, 2, or all of the vaccine strains. For seroprotection, we excluded members of the cohort who had pre-vaccine titers of ≥ 1:64, who therefore already met criteria for protection prior to vaccination. Consequently, the analysis of seroprotection included n=32 young and n=49 older for the H1N1 vaccine strain; n=47 young and n=30 older for H3N2; and n=44 young and n=34 older for the B strain. P values after adjustment for covariates: *< 0.0001, #<0.01 for difference in distribution in panels A and C.
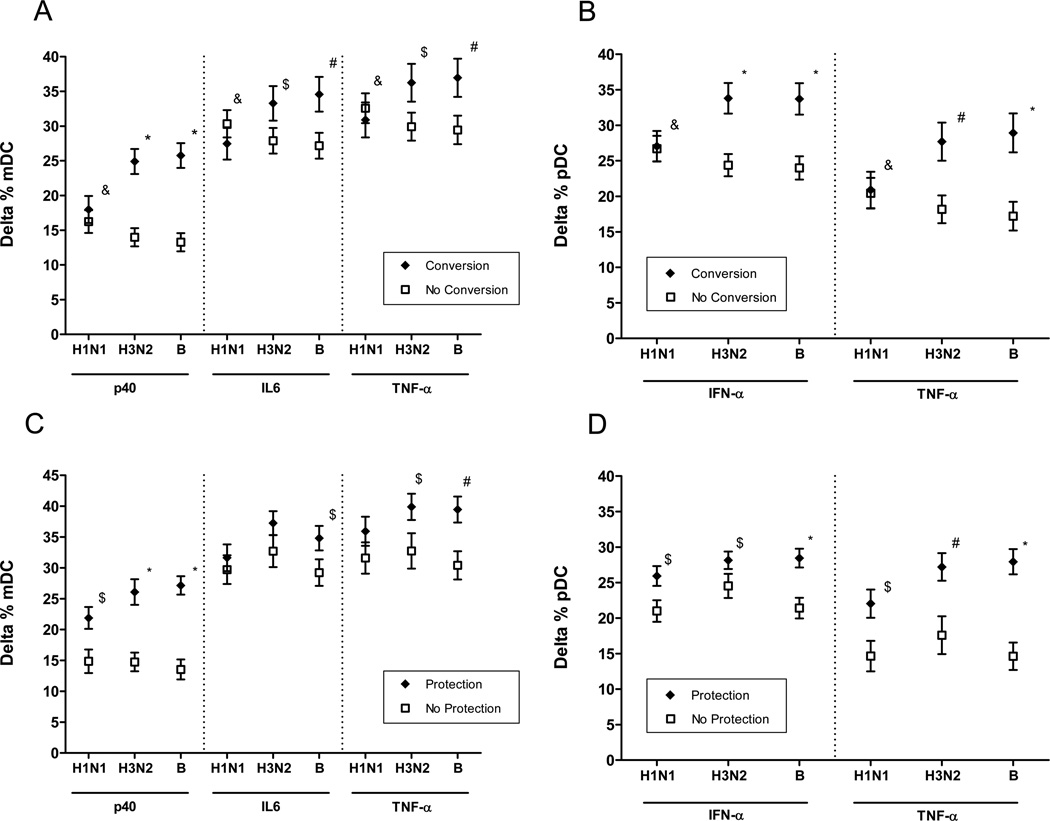
We found that TLR-induced production of TNF-α, IL-6 and IL-12/p40 in mDCs and IFN-α and TNF-α by pDCs was significantly associated with seroconversion to the H3N2 and B strains in the 2007–2008 influenza vaccine; moreover, TLR-induced production of IL-12/p40 in mDCs and of TNF-α and IFN-α in pDCs was significantly associated with seroprotection to all three strains in the vaccine (Figure 9). Taken together, these findings indicate that the defects in TLR-induced cytokine production we have observed have consequences for the generation of a protective antibody response to influenza immunization.
Figure 9.
Prediction of antibody responses to the 2007–2008 influenza vaccine by TLR-induced cytokine production in mDCs and pDCs. Data depict the least squares mean of TLR–induced cytokine production. A. Association of seroconversion to vaccine strains indicated with TLR-induced production of p40 subunit of IL12, IL6, and TNF-α in mDCs. P values after adjustment for covariates. B. Relation of seroconversion to indicated vaccine strains with TLR-induced IFN-α and TNF-α production in pDCs. P values after adjustment for covariates: C. Relation of seroprotection to indicated vaccine strains TLR-induced production of p40 subunit of IL12, IL6, and TNF-α in mDCs. D. Relation of seroprotection to indicated vaccine strains with TLR-induced IFN-α and TNF-α production in pDCs. *< 0.0001, #<0.001, &<NS.
Discussion
Using intracellular staining to detect cytokine production, we evaluated TNF-α, IL-6 and IL-12p40 production following stimulation with a wide range of TLR ligands. We observed a broad ranging, statistically significant decrease in production of these cytokines in response to all TLR ligands tested in both mDCs and pDCs from older, compared to young individuals. This age-associated decrease in TLR function was stable for TLR7 and TLR8 over a 6 week period and was also evident in for TLR1/2, TLR2/6 and TLR7/8 evaluated in magnetic bead-enriched mDCs and pDCs evaluated in a subset of this cohort nearly two years later. These alterations in TLR function are more extensive than the limited defect in TLR1/2-induced production of TNF-α and IL-6 we described previously in monocytes from older individuals (14). Taken together, these results indicate a substantial decrease in TLR function that provides evidence for immunosenescence in human DCs.
The older individuals in our cohort represented a largely healthy aged group, with over 70% reporting no major comorbid conditions; notably, we did not employ limiting enrollment criteria such as SENIEUR, a protocol selecting for successfully aged individuals that excludes perhaps 80% of older individuals (35). Our aim was to obtain a generalizable cohort of community-dwelling older individuals, and to adjust for the heterogeneity in the cohort by using mixed effect multivariable analysis. Such control for covariates among young and older groups strengthens the validity of our findings.
To understand the basis for the defects in TLR-induced cytokine production we observed, we evaluated surface and intracellular expression of specific TLRs expressed in mDCs and pDCs. We found decreased protein expression for all TLRs in mDCs for which antibody reagent availability allowed analysis: TLR1, TLR2, TLR3, TLR5 and TLR8. By contrast, TLR9 protein expression in pDCs appeared unperturbed. To determine whether transcriptional mechanisms contributed to these age-associated decreases in protein expression, we carried out qPCR analysis of the expression of genes encoding TLRs using highly purified samples of mDCs and pDCs isolated using cell sorting. We demonstrated a statistically significant, age-associated decrease in the expression of TLR3 and TLR8 in mDCs and of TLR7 in pDCs; gene expression of other TLRs appeared equivalent in DCs from young and older individuals. Thus, for TLR3 and TLR8 in mDCs and TLR7 in pDCs (although the lack of an appropriate anti-TLR7 antibody precluded evaluation of protein expression), transcriptional mechanisms could contribute to the observed age-associated alterations in cytokine production. However, it is also possible that the magnitude of these differences in transcription may not be sufficient to account for the changes in TLR protein expression we observed. Of note, we observed a statistically significant age-associated defect in TLR3-induced IL-12 (p40) production, but not TNF-α or IL-6; whether TLR3-dependent TNF-α or IL-6 production may be altered in aging individuals under other conditions (e.g. differing or combination ligand concentrations), or whether for these cytokines a threshold for TLR3 signaling is maintained despite decreased TLR3 gene and protein expression remains to be determined in future studies. By contrast, mRNA for TLR9 expression in pDCs was unchanged between young and older individuals despite functional deficits in TLR9-induced cytokine responses (TNF-α and IFN-α) in pDCs from older participants. This finding suggests a potential role for post-transcriptional mechanisms—such as those we reported in the TLR1 defect associated with aging in human monocytes (14). For example, it is notable that the TLR chaperone PRAT4a is critical for the proper surface expression of several TLRs, including TLR1 but not TLR2, and for the proper localization of intracellular TLRs such as TLR9 (36). It is attractive to speculate that age-associated defects in chaperone function represent one potential mechanism contributing to post-translational defects in TLR-induced cytokine production.
Previous studies in humans using a limited range of TLR ligands have reported age-associated defects in LPS-induced IL-12 production in mDCs (also assessed via intracellular cytokine staining) (37), and decreased IFN-α production as measured by ELISA following stimulation of PBMCs with Herpes Simplex virus (32)—results consistent with our findings. In contrast, other recent studies have demonstrated increased production of TNF-α, IL-6 and IFN-α from monocyte-derived dendritic cells from older, compared to young participants in response to TLR ligands (38, 39). It is likely that the use of disparate experimental systems account for differences with our findings, e.g. derivation of DCs from GM-CSF and IL-4 treatment of monocytes in vitro versus our study of primary mDCs and pDCs.
The functional significance of our data is emphasized by the finding that the decreases in TLR-induced cytokine production we observed are strongly associated with the inability to mount a protective antibody response to the trivalent inactivated influenza vaccine currently recommended for all individuals over the age of 65. We observed significant associations between seroprotection and IFN-α and TNF-α production in pDCs or p40 production in mDCs to all three strains in the 2007–2008 vaccine; in addition, strong associations between seroconversion and cytokine production were observed for the H3N2 and B strains in the vaccine. Trends suggesting a relationship between seroprotection or seroconversion and cytokine production were observed that were not significant in some cases (e.g. the H1N1 strain and seroconversion). Whether this reflects differences in immunogenicity among strains in the vaccine is unclear; nonetheless, these findings contrast with our previous studies in monocytes which did not demonstrate an association between TLR-induced cytokine production and influenza vaccine antibody response (14). We observed a strong relationship between aging and defects in TLR-induced cytokine production in DCs, but it is notable that the relationship between cytokine production and seroconversion to influenza immunization was significant regardless of age (in this context, because age and seroconversion were strongly associated, they cannot be independent variables in the same statistical model). Consequently, our results are applicable to the general population receiving influenza vaccine, and provide further evidence linking TLR function to adaptive immune responses.
While the specific TLR ligands present in the influenza vaccine are uncharacterized, the strength of the association we observed with vaccine antibody response suggests that TLR-dependent cytokine production is an indicator for the integrity of the aging innate immune system. Indeed, the decreases we observed in TLR-induced cytokine production in mDCs and pDCs are likely to have a substantial impact on innate immune responses in older individuals, ranging from altered proinflammatory responses mediated by TNF-α and IL-6, to CD4 T cell polarization mediated by IL-12, to type I interferon-dependent antiviral responses (in which pDCs play a crucial role) (40, 41). Consequently, it is attractive to speculate that these alterations in TLR expression and signaling in DCs, combined with other age-associated findings in monocytes and macrophages from aging individuals such as decreased costimulatory protein expression (13–15), could further diminish B and T cell function (42, 43) in contributing to the impaired host responses to infection and immunization characteristic of the aging immune system. Our findings do not exclude the possibility that aberrant elevations in baseline cytokine production renders DCs less responsive to TLR stimulation; such a scenario may contribute to reports of a heightened proinflammatory environment that is particularly associated with disability in aging individuals (33); indeed, our observation that basal levels of intracellular cytokine staining are markedly elevated in mDCs and pDCs from older, compared to young individuals that were not stimulated with TLR ligands is consistent with the hypothesis that proinflammatory cytokine production in DCs is dysregulated in older individuals. Future research is needed as to whether the inability to further increase cytokine production in response to stimulation by pathogen-associated molecular patterns (PAMPs) via TLRs will adversely affect the ability of the innate immune system to facilitate the development of the adaptive immune response. Additional studies of TLR-dependent responses, particularly in older human cohorts with increased functional disability, to address this question.
Acknowledgements
The authors are grateful to Donna Carrano, Sandra Ginter, MaryLou Breitenstein, and Linda Leo-Summers for valuable assistance, Tobi Kollman (U BC) and Chris Wilson (University of Washington) for assistance with FACS analysis, and members of the Yale Program on Aging, and the Yale IMAGIN team for insightful discussions.
Footnotes
This work was supported in part by the NIH (N01-AI-50031, AI 070343), by the Center of Excellence in Aging at Yale University, funded by the John A. Hartford Foundation, and by the Claude D. Pepper Older Americans Independence Center at Yale University (P30 AG021342). E.F. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 3.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 4.McGlauchlen KS, Vogel LA. Ineffective humoral immunity in the elderly. Microbes Infect. 2003;5:1279–1284. doi: 10.1016/j.micinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 6.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 8.Davila DR, Edwards CK, 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. Faseb J. 1990;4:2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 9.Corsini E, Lucchi L, Meroni M, Racchi M, Solerte B, Fioravanti M, Viviani B, Marinovich M, Govoni S, Galli CL. In vivo dehydroepiandrosterone restores age-associated defects in the protein kinase C signal transduction pathway and related functional responses. J Immunol. 2002;168:1753–1758. doi: 10.4049/jimmunol.168.4.1753. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 12.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 13.Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 15.van Duin D, Allore HG, Mohanty S, Ginter S, Newman FK, Belshe RB, Medzhitov R, Shaw AC. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstiel P, Derer S, Till A, Hasler R, Eberstein H, Bewig B, Nikolaus S, Nebel A, Schreiber S. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes Immun. 2008;9:103–114. doi: 10.1038/sj.gene.6364454. [DOI] [PubMed] [Google Scholar]
- 17.Murciano C, Yanez A, O'Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 18.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 19.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 21.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 26.Kendal AP, Pereira MS, Skehel JJ. Hemagglutination inhibition. In: Kendal AP, Skehel JJ, editors. Concepts and procedures for laboratory-based influenza surveillance. Washington DC: US Department of Health and Human Services and Pan-American Health Organization; 1982. pp. B17–B35. [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 28.Verbeke G, Molenberghs G. Linear mixed models in practice. In: Verbeke G, Molenberghs G, editors. A SAS-Oriented Approach. New York: Springer; 1997. [Google Scholar]
- 29.McCullagh P, Nelder JA. Generalized LInear Models. London: Chapman and Hall; 1989. [Google Scholar]
- 30.Abb J, Abb H, Deinhardt F. Age-related decline of human interferon alpha and interferon gamma production. Blut. 1984;48:285–289. doi: 10.1007/BF00320399. [DOI] [PubMed] [Google Scholar]
- 31.Teig N, Moses D, Gieseler S, Schauer U. Age-related changes in human blood dendritic cell subpopulations. Scand J Immunol. 2002;55:453–457. doi: 10.1046/j.1365-3083.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 32.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, Muller-Hermelink HK, Steinmann GG. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, Nagai Y, Takatsu K, Saitoh S, Miyake K. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, Liu YJ, Kadowaki N. Functional diversity and plasticity of human dendritic cell subsets. Int J Hematol. 2005;81:188–196. doi: 10.1532/IJH97.05012. [DOI] [PubMed] [Google Scholar]
- 42.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]