Abstract
Background
Fluorescence confocal mosaicing microscopy is an emerging technology for rapid imaging of nuclear and morphologic detail directly in excised tissue, without the need for frozen or fixed section processing. Basal cell carcinomas (BCCs) can be detected with high sensitivity and specificity in Mohs excisions with this approach. For translation to clinical trials and toward potentially routine implementation, a new and faster approach called strip mosaicing confocal microscopy was recently developed.
Objectives
To perform a preliminary assessment of fluorescence strip mosaicing confocal microscopy for detecting skin cancer margins in Mohs excisions.
Methods
Tissue from 17 Mohs cases was imaged in the form of strip mosaics. Each mosaic was divided into two halves (submosaics) and graded by a Mohs surgeon, who was blinded to the pathology. The 34 submosaics were compared to the corresponding Mohs pathology.
Results
The overall image quality was excellent for resolution, contrast and stitching in the 34 submosaics. Components of normal skin including the epidermis, dermis, dermal appendages and subcutaneous tissue were easily visualized. Preliminary measure of sensitivity and specificity was 94% for detecting skin cancer margins.
Conclusions
The new strip mosaicing approach represents another advance in confocal microscopy for imaging of large areas of excised tissue. Strip mosaicing may enable rapid assessment of BCC margins in fresh excisions during Mohs surgery and may serve as an adjunct for frozen pathology.
Introduction
Mohs surgery is the standard of care with high cure rates for treatment of high risk skin cancers. However, the preparation of frozen pathology during Mohs surgery is labor-intensive, time-consuming and expensive1. Fluorescence confocal mosaicing microscopy is an emerging technology for imaging nuclear and morphologic detail and assessing tumor margins directly in fresh Mohs excisions, without the need for frozen sectioning2. In confocal mosaics, basal cell carcinomas (BCCs) were detected with sensitivity of 96.6% and specificity 89.2% 3,4. More recently, rapid pathology at the bedside to guide surgery was demonstrated in shave biopsies of BCCs5 and nail matrix biopsies of subungual melanoma6. However, translation to clinical trials and eventually routine implementation will necessitate faster and more efficient mosaicing. Toward this goal we developed a faster approach called strip mosaicing confocal microscopy7,8. Here we report the preliminary clinical testing of this approach on Mohs excisions.
Methods
Imaging was performed with our strip mosaicing confocal microscope with laser illumination at 488 nm and ~5 milliwatts of power on the tissue. Nuclear morphology was stained with acridine orange and imaged in fluorescence at 500–700 nm. Long strip images were acquired, each of dimensions 0.5 mm × 10 mm, and stitched together to create a mosaic. Approximately 25 strips are acquired to cover 10 mm × 10 mm of Mohs excised tissue, and the imaging required less than 2 minutes. By comparison, our earlier slower approach required 9–10 minutes2. Improved methods for registration of neighboring strip images, correction for illumination fall-off and blending at the edges result in mosaics appearing seamless with better consistency. This technology has been described in detail elsewhere7,8.
Discarded tissue from Mohs surgery was collected under IRB approval, thawed, rinsed in isotonic saline, placed in 0.6 milliMolar acridine orange solution for 20 seconds, and rinsed again in saline to wash excess unbound dye. This protocol results in optimal staining of nuclear morphology with minimal pooling artifact in the dermis9. The tissue was placed in a specially-engineered mount8 to flatten the edges of the excision for consistent imaging of the epidermis. This simulates the manual procedure of pressing the tissue edges onto the cryostat chuck by Mohs histotechnicians.
Tissue from 17 Mohs cases (16 BCCs and 1 squamous cell carcinoma (SCC)) was imaged. Each strip mosaic was divided in half, resulting in 34 submosaics. Each submosaic was displayed on a large monitor with 2500 × 1900 pixels and graded by a Mohs surgeon (KN) who was blinded to the pathology. The submosaics were graded for overall image quality based on (a) contrast, (b) quality of stitching, (c) resolution and (d) appearance of epidermis and dermis. BCC, if present, was classified as superficial, nodular, micronodular or infiltrative. The submosaics were subsequently compared to the corresponding hematoxylin and eosin (H&E)-stained Mohs frozen pathology.
Results
The overall image quality was excellent for resolution, contrast, stitching, and visibility of the epidermis and dermis (Table 1).
Table 1.
Evaluation of strip mosaics by a Mohs surgeon (KN) for image quality.
| Acceptable | Not Acceptable | |
|---|---|---|
| Overall image quality | 14 | 3 |
| Contrast | 15 | 2 |
| Quality of stitching | 16 | 1 |
| Resolution | 14 | 3 |
| Epidermis | 12 | 5 |
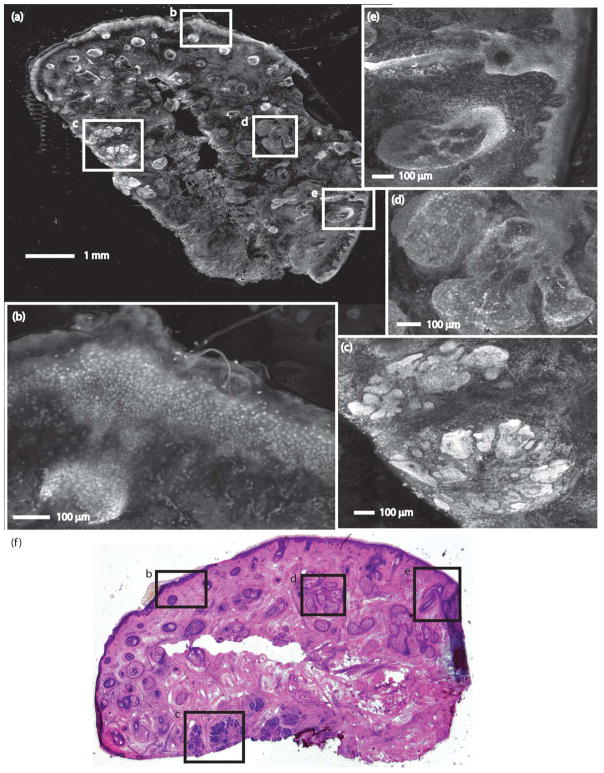
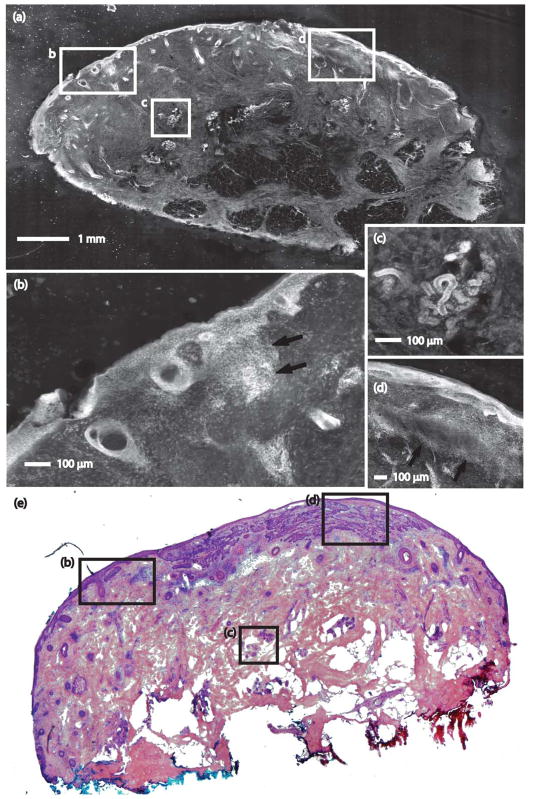
Figures 1 and 2 show strip mosaics with their corresponding Mohs frozen pathology, both shown at 2X magnification. Within the complete mosaics, the viewer may digitally zoom to view tissue at higher magnifications, as seen in the insets. Nuclear and morphologic details of normal skin structures such as the epidermis, dermis, collagen, solar elastosis, hair follicles, sebaceous glands, eccrine glands, and adipose tissue are easily visible.
Figure 1.
A strip mosaic of a Mohs excision and the corresponding Mohs pathology. (a) The strip mosaic shows a large field of view, with insets indicating regions showing: (b) epidermis, (c) nodular BCC, (d) sebaceous gland, and (e) hair follicle and sebaceous gland. (f) The corresponding Mohs pathology with matching features is shown (H&E, 2X).
Figure 2.
A strip mosaic of a Mohs excision and corresponding Mohs pathology. (a) The strip mosaic shows a large field of view, with insets indicating regions showing (b) epidermis with superficial BCC (arrow), (c) eccrine gland, and (d) epidermis and superficial BCC (arrow). (e) The corresponding Mohs pathology with matching features is shown (H&E, 2X).
Nuclear and morphologic detail of BCC and SCCs were easily detected and differentiated from normal structures. The bright nuclear staining from acridine orange readily allows detection of BCCs and SCCs with high contrast relative to the darker dermis. Furthermore, nuclear details such as crowding (increased density), pleomorphism, peripheral palisading, and clefting are seen at closer inspection. Overall, normal skin structures and skin cancers in the mosaics correlated well to Mohs frozen pathology.
Of the 34 submosaics, 15 were true positives for BCC, 16 true negatives, 1 was false positive and 1 false negative for BCC, and 1 was true positive for SCC. Thus, a preliminary measure of sensitivity is 94% and specificity 94% for detecting cancer margins. There were 11 submosaics that contained nodular, 5 that contained micronodular, 4 that contained infiltrative and 3 that contained superficial BCCs that were correctly identified. There was one SCC that was correctly identified.
Conclusion and discussion
The new strip mosaicing approach, with preliminary assessment of sensitivity and specificity, represents another advance in confocal microscopy for imaging skin cancer margins directly in excised Mohs tissue without the need for frozen section processing. This approach is being further developed toward implementation at the bedside in a clinical trial. One remaining requirement is to refine our tissue mount to produce more consistent flattening of the epidermis at the edges of the tissue. This is necessary for the beveled-type of Mohs excisions that are commonly performed in the USA. Such flattening, however, may be less critical for Tuebinger torte-, muffin- and other types of excisions10. In the long-term, strip mosaicing may enable rapid assessment of margins in fresh tissue in a Mohs surgery setting, and may serve as an adjunct for frozen pathology.
The high resolution of optical imaging and spectroscopy offers exciting possibilities for microscopic-level detection of margins directly in fresh tissue. Not surprisingly, there is a rich field of technologies being developed for Mohs surgery, which were highlighted in our earlier paper3. Since then, more advances have been reported in brightfield microscopy with Anti-BerEP4-antibody immunostaining11, optical coherence tomography12, near-infrared confocal microscopy with 1300 nm illumination13, polarized optical and terahertz imaging14 and multiphoton multispectral fluorescence lifetime tomography15. Furthermore, some of these are being translated for applications in breast, brain and head-and-neck tissues. In the future, such technologies, either alone or in combination, may produce entirely new tools to serve surgeons in Mohs and other settings.
What’s already known about this topic?
Fluorescence confocal mosaicing microscopy is an emerging technology for imaging of nuclear and morphologic detail and assessing tumor margins directly in fresh Mohs excisions, without the need for frozen section processing. BCC margins were detected with sensitivity of 96.6% and specificity 89.2%.
What does this study add?
Toward potentially routine implementation at the bedside, a new and faster approach called strip mosaicing confocal microscopy has been developed. Preliminary assessment demonstrates sensitivity and specificity of 94% for detecting skin cancer margins.
Acknowledgments
Funding
The National Institutes of Health provided funding under grant R01EB012466.
We thank the NIH for funding (grant R01EB012466), Dr. Yongbiao Li and Dr. Ricardo Toledo-Crow for technical support, and Mr. William Phillips for Mohs pathology.
Footnotes
Disclosures
Milind Rajadhyaksha is a former employee of Lucid Inc., the company that makes and sells the VivaScope confocal microscope. He owns equity in Lucid. The VivaScope is the commercial version of an original laboratory prototype of a confocal scanning laser microscope that was developed by Rajadhyaksha when he was in the Department of Dermatology at Massachusetts General Hospital, Harvard Medical School. The strip mosaicing microscope is based on Lucid’s Vivascope technology platform.
References
- 1.Rajadhyaksha M, Menaker G, Flotte T, Dwyer PJ, González S. Confocal examination of nonmelanoma cancers in thick skin excisions to potentially guide mohs micrographic surgery without frozen histopathology. The Journal of investigative dermatology. 2001;117(5):1137–43. doi: 10.1046/j.0022-202x.2001.01524.x. [DOI] [PubMed] [Google Scholar]
- 2.Gareau DS, Li Y, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. Journal of biomedical optics. 2008;13(5):054001. doi: 10.1117/1.2981828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karen JK, Gareau DS, Dusza SW, Tudisco M, Rajadhyaksha M, Nehal KS. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. The British journal of dermatology. 2009;160(6):1242–50. doi: 10.1111/j.1365-2133.2009.09141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gareau DS, Karen JK, Dusza SW, Tudisco M, Nehal KS, Rajadhyaksha M. Sensitivity and specificity for detecting basal cell carcinomas in Mohs excisions with confocal fluorescence mosaicing microscopy. Journal of biomedical optics. 2009;14(3):034012. doi: 10.1117/1.3130331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennàssar A, Vilalta A, Carrera C, Puig S, Malvehy J. Rapid diagnosis of two facial papules using ex vivo fluorescence confocal microscopy: toward a rapid bedside pathology. Dermatologic surgery: official publication for American Society for Dermatologic Surgery. 2012;38(9):1548–51. doi: 10.1111/j.1524-4725.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- 6.Debarbieux S, Hospod V, Depaepe L, Balme B, Poulalhon N, Thomas L. Perioperative confocal microscopy of the nail matrix in the management of in situ or minimally invasive subungual melanomas. The British journal of dermatology. 2012;167(4):828–36. doi: 10.1111/j.1365-2133.2012.11013.x. [DOI] [PubMed] [Google Scholar]
- 7.Abeytunge S, Li Y, Larson B, Toledo-Crow R, Rajadhyaksha M. Rapid confocal imaging of large areas of excised tissue with strip mosaicing. Journal of biomedical optics. 2011;16(5):050504. doi: 10.1117/1.3582335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abeytunge S, Li Y, Larson B, Peterson G, Seltzer E, Toledo-Crow R, et al. Confocal microscopy with strip mosaicing for rapid imaging over large areas of excised tissue. Journal of biomedical optics. 2013;18(6):61227. doi: 10.1117/1.JBO.18.6.061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bini J, Spain J, Nehal K, Hazelwood V, DiMarzio C, Rajadhyaksha M. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. Journal of biomedical optics. 2011;16(7):076008. doi: 10.1117/1.3596742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schüle D, Breuninger H, Schippert W, Dietz K, Moehrle M. Confocal laser scanning microscopy in micrographic surgery (three-dimensional histology) of basal cell carcinomas. The British journal of dermatology. 2009;161(3):698–700. doi: 10.1111/j.1365-2133.2009.09354.x. [DOI] [PubMed] [Google Scholar]
- 11.Moehrle M, Käflein L, Ziefle S, Metzler G. Rapid lump examination (RLE) - a new tool for Mohs surgery? Journal der Deutschen Dermatologischen Gesellschaft: JDDG. 2011;9(7):534–8. doi: 10.1111/j.1610-0387.2011.07692.x. [DOI] [PubMed] [Google Scholar]
- 12.Cunha D, Richardson T, Sheth N, Orchard G, Coleman A, Mallipeddi R. Comparison of ex vivo optical coherence tomography with conventional frozen-section histology for visualizing basal cell carcinoma during Mohs micrographic surgery. The British journal of dermatology. 2011;165(3):576–80. doi: 10.1111/j.1365-2133.2011.10461.x. [DOI] [PubMed] [Google Scholar]
- 13.Yaroslavsky AN, Patel R, Salomatina E, Li C, Lin C, Al-Arashi M, et al. High-contrast mapping of basal cell carcinomas. Optics letters. 2012;37(4):644–6. doi: 10.1364/OL.37.000644. [DOI] [PubMed] [Google Scholar]
- 14.Joseph CS, Patel R, Neel VA, Giles RH, Yaroslavsky AN. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions Optical and Terahertz skin cancers imaging. Journal of biophotonics. 2012 doi: 10.1002/jbio.201200111. [DOI] [PubMed] [Google Scholar]
- 15.Patalay R, Talbot C, Alexandrov Y, Lenz MO, Kumar S, Warren S, et al. Multiphoton multispectral fluorescence lifetime tomography for the evaluation of basal cell carcinomas. PloS one. 2012;7(9):e43460. doi: 10.1371/journal.pone.0043460. [DOI] [PMC free article] [PubMed] [Google Scholar]