Abstract
The Similan Islands (Thailand) in the Andaman Sea are exposed to large amplitude internal waves (LAIW), as evidenced by i.a. abrupt fluctuations in temperature of up to 10°C at supertidal frequencies. Although LAIW have been shown to affect coral composition and framework development in shallow waters, the role of LAIW on coral growth is so far unknown. We carried out a long-term transplant experiment with live nubbins and skeleton slabs of the dominating coral Porites lutea to assess the net growth and bioerosion in LAIW-exposed and LAIW-protected waters. Depth-related, seasonal and interannual differences in LAIW-intensities on the exposed western sides of the islands allowed us to separate the effect of LAIW from other possible factors (e.g. monsoon) affecting the corals. Coral growth and bioerosion were inversely related to LAIW intensity, and positively related to coral framework development. Accretion rates of calcareous fouling organisms on the slabs were negligible compared to bioerosion, reflecting the lack of a true carbonate framework on the exposed W faces of the Similan Islands. Our findings show that LAIW may play an important, yet so far overlooked, role in controlling coral growth in tropical waters.
Introduction
The waxing and waning of coral reefs is determined by two antagonistic processes: the accretion and erosion of calcium carbonate (CaCO3). Scleractinian corals are the most important contributors to the reef framework [1]. Their fast-growing and large CaCO3 skeletons provide the building blocks for the reef, which are reinforced and kept in place by other calcifying organisms, notably calcareous algae, long after the coral colony has died [1], [2]. The largely biogenic accretion of carbonate is kept at bay by a number of physical, chemical and biological processes contributing to the dissolution and erosion of the carbonate rock. Bioeroding organisms, notably boring sponges, excavating fish, and invertebrate grazers, play a dominating role in the destruction of the carbonate [2]–[5]. The balance of accreting and erosive processes determines the net growth of the corals and coral reef framework [6], [7]. In warm, clear, aragonite-rich and nutrient-poor coral reef waters, carbonate accretion exceeds erosion and the reef framework grows [2]–[4], [6], [8]. Less aragonite supersaturated, turbid and nutrient-enriched waters directly or indirectly constrain carbonate accretion [9]–[11] and favour carbonate dissolution [12], [13] and bioerosion [14], [15], tipping the carbonate balance. Low pH conditions coinciding with a shift in the carbonate chemistry and reduced aragonite saturation can exceed the energetic demands of corals for the maintenance of calcification [9], [16] resulting in reduced coral growth or even dissolution [12], [13]. High nutrient concentrations favour the growth of phytoplankton and filter-feeders, many of which are internal bioeroders [2], [14], [15], [17]. They also foster the growth of benthic macroalgae which are an important food source for excavating grazers [2], [18]. Hence, nutrient-enhanced bioerosion is predominantly found in areas influenced by river runoff [8], [17] and upwelling [19], [20].
Recent research mainly focuses on anthropogenic factors threatening the health of corals and coral reefs. The effect of decreasing pH and aragonite saturation [9], [12], [13], [21], increasing temperature [22] and dissolved nutrient loads [23], [24] endanger coral reefs worldwide [25]–[27]. In a rapidly changing environment, corals will be exposed to stronger environmental changes within shorter periods of time (years to decades) with lower chances to acclimatize or adapt [25], [27]. Naturally fluctuating environments provide an opportunity to study corals under conditions similar to future projections of global warming and/or ocean acidification [3], [4], [11]–[14] offering insight into metabolic tolerances and acclimation potentials of corals to extreme conditions. There is mounting evidence that corals exposed to fluctuations in water chemistry (i.e. pH, nutrient concentrations) and/or temperature are more resistant to heat stress ([28], [29] and references therein), and it is possible that corals subjected to natural variations in their physico-chemical environmental may show pre-adaptation potential to cope with climate change.
In this regard, a particular phenomenon occurring in many tropical areas are non-linear large amplitude internal waves (LAIW) occurring at supertidal frequencies (several waves per tidal cycle) [30]–[32]. LAIW may entrain sub-pycnocline waters into shallow reef areas [31], [32] and cause dramatic changes in environmental conditions which exceed by far the daily and seasonal fluctuations in temperature, aragonite saturation and nutrient concentrations in coral reefs [33]. While low magnitude variations at tidal frequencies have shown mixed effects on coral calcification [34], the influence of LAIW on coral growth, calcification and bioerosion has not been studied yet, in spite of their widespread occurrence [35].
The Similan Islands, an archipelago off the west coast of Thailand in the Andaman Sea, are exposed to LAIW. The sub-pycnocline deep water causes sudden (within 1–2 minutes) drops of up to 10°C in temperature, of 0.6 units in pH and increases of up to 9.4 µmol in NOx l−1 [31]. LAIW impact on these reefs varies seasonally. LAIW are stronger during the calm northeast (NE) monsoon (January through April) [31]. During the southwest (SW) monsoon (May through October) strong winds cause mixing and resuspension of bottom sediments [32]. Both LAIW and SW monsoon act from the same W to SW direction. Hence, in the Similan Islands, ocean facing W reefs are exposed to both monsoon and LAIW, while the east (E) reefs are sheltered from both phenomena. As a result, the islands feature a peculiar reef development which is restricted to the E sides, whereas a true framework is lacking on the W sides [31], [32], [36]. The development is in contrast to most other corals reefs, where growth is most pronounced on the windward sides [37], except in upwelling areas where coral growth appears to be suppressed in a similar fashion [28]. Although the observed asymmetry has been attributed to the combined effects of monsoon and LAIW [31], [32], experimental evidence for a reduction in coral growth is so far lacking. Parallel studies showed a higher biomass, protein content and nutritional status in exposed corals [38], [39], and a better survival during periods of restricted photosynthesis [38]. This could suggest a potentially higher energy allocation to calcification. On the other hand, calcification may be more energy-demanding and therefore reduced [9], [40], due to the frequent exposure to low pH waters [31]. The combination of seasonally enriched nutrient conditions and enhanced exposure to storm and high wave energy along the W areas might further undermine the development of a stable carbonate reef framework as coral growth may not compensate for both, framework destruction by bioerosion and wave energy [41].
The present study explores coral growth and bioerosion on dead skeletal substrate under marked differences in LAIW and monsoon exposure. We hypothesize that LAIW and monsoon differentially affect shallow (∼7 m), deep (∼20 m), west (W) and east (E) carbonate production and erosion, representing areas of strong monsoon (W 7 m), strong LAIW (W 20 m), moderate (E 20 m) and low (E 7 m) LAIW impact, respectively. Coral growth may be reduced at the exposed W compared to the sheltered E due to the combined effect of low temperature, low pH and high nutrients depressing growth [42]–[45]. We hypothesize further that bioerosion will be enhanced due to nutrient stimulated boring filter-feeders [18], [46] and increased grazing by excavating herbivores taking advantage of nutrient-enhanced macroalgal growth [23]. Other calcifiers (collectively referred to “fouling community” in the following) may on the one hand benefit from LAIW-enhanced supply of food [38], but also suffer from reduced CaCO3 precipitation rates.
Materials and Methods
Experimental setup
The massive coral Porites lutea (Milne Edwards and Haime 1851) was used as a model organism for this study. It is the dominant reef-building coral in the Thai Andaman Sea [36], [47], [48] and the coral of choice for numerous physiological and ecological investigations in the Andaman Sea and elsewhere [47]–[49]. In addition P. lutea in this region was shown to be associated with the same algal symbiont (C15) [50] excluding possible symbiont-related differences in growth. Coral growth in this study is defined as the net calcium carbonate accretion of a living, healthy and unscathed coral nubbin. Although neither biological erosion due to excavating grazers or boring filter feeders nor biological dissolution due to microbial activities or endolithic algae can be ruled out, we consider these factors negligible in a healthy coral [2], [51]. Bioerosion is the carbonate loss due to biological processes. It can be determined from the post-exposure net mass loss of carbonate blocks by subtracting mass accretion due to fouling organisms. Carbonate dissolution in aragonite saturated waters with pH>7.4 can be ruled out [13]. The pH drops due to LAIW are only intermittent (15 to 30 minutes) and usually above the critical value [31].
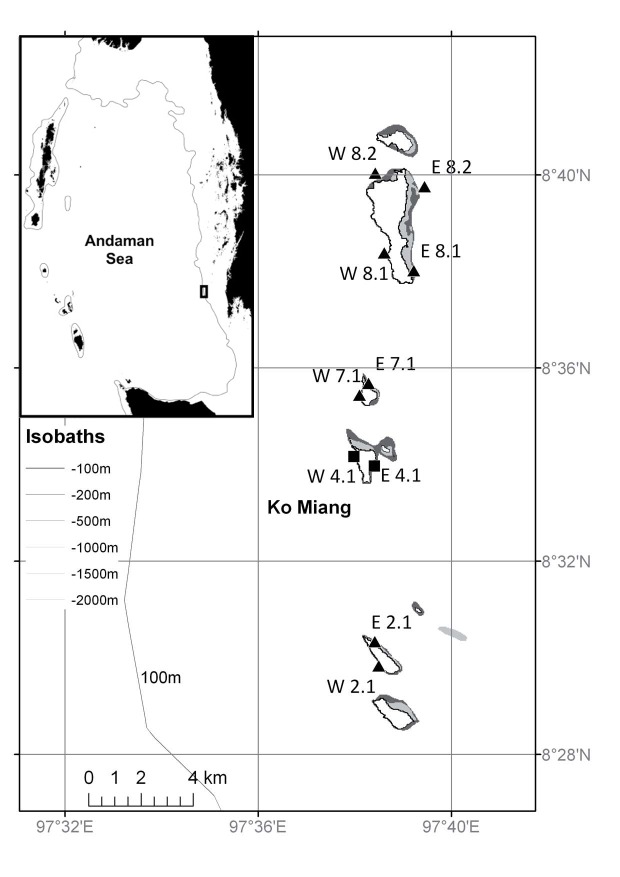
To investigate the effect of LAIW and monsoon on coral growth and bioerosion, coral nubbins and coral carbonate blocks from a shallow (7 m) sheltered (E) site were transplanted to 20 Similan Island sites: shallow (7 m, monsoon effect) and deep (20 m, LAIW effect) sites on the exposed (W) and sheltered (E) sides of 5 different locations in the Similan Islands archipelago (Fig. 1). Despite the limitations of such a one-level experimental design with all experimental coral nubbins originating from the same location in contrast to a complete cross-transplantation design, sampling restrictions in the National Park did not allow for another approach. But the results indicate (see below) that coral origin had no significant effect on mortality (Table S1, S2), as opposed to findings in other shallow to deep transplant experiments [52]. We assessed transplanted (E 20 m, W 7 m, W 20 m) and control growth (E 7 m) and bioerosion after 12 and 21 months exposure periods between February 2007 and November 2008, coinciding with high and low LAIW activity years [39]. The study in the Similan National Park was carried out with the official research permit of the National Research Council of Thailand (NRCT, permit numbers: TS0907.1/12593 and NRCT002.3/03231).
Figure 1. Locations of study sites in the Similan Islands, Andaman Sea, Thailand.
Symbols show locations of temperature loggers and growth rate experiments (▴) and of bioerosion experiments (▪). (Figure inset courtesy A. Buschmann, AWI. Data source: http://www.ngdc.noaa.gov/mgg/shorelines/gshhs.html).
Temperature
Temperature loggers (TidbiT v2, Onset computers; resolution 0.2°C) collecting data at 6 min intervals were deployed along with the transplants. Intercalibration of the TidbiT loggers with a high precision digital thermometer (Amarell ama-digit ad 3000 th) showed low deviations between instruments (<0.3°C) compared to the up to 10°C variations measured within the respective instrument records (see results). Because the arithmetic mean is sensitive to extreme values, temperature data are reported as the most frequent (or mode) values along with the corresponding ranges. As a measure for LAIW frequency and intensity, we integrated the temperature anomalies (measured value versus daily mode temperature) over time yielding a cumulative cooling index in degree days (DD in [°C d], cf. [34]). The temperature anomalies were multiplied by the sampling interval in days (the sampling interval of 6 minutes equaled to a 0.0042 interval in days). A value encapsulating LAIW impact was obtained by summing up all negative temperature anomalies for each time period of interest (12 months: February 2007 to February 2008 and 21 months February 2007 to November 2008; see below).
Coral growth rates
For the growth experiment, a total of 200 Porites lutea nubbins was collected chiseling 20 nubbins from each of 10 large mother colonies at the sheltered E side of the Similan island Ko Similan (8°38′21.43″N 97°38′59.73″E) from 7 m depth. The nubbins were transferred to a laboratory where they were kept in flow-through reef water aquaria for 1 day to measure and weigh them. Growth rates were determined gravimetrically (cf. buoyant weight technique [53]) using a high precision microbalance (Sartorius ME-235S, precision 0.01 mg, Fig. S1) by comparing the weights of each individual nubbin before and after the 12 month growth period. Initially, P. lutea nubbins were measured twice, before and after attaching them to individual numbered holders using fast drying concrete (Fig. S2). The weight of each nubbin was recorded along with the corresponding weight of the nubbin attached to its holder. We used a skeletal density of 1.41 g cm−3 for P. lutea determined after Davies [53] to calculate the dry mass of each nubbin. Coral nubbins were approximately 3 to 5 cm in diameter, and had a mean mass of 42.9 g (Table S2).
The nubbins were distributed in a way that each of the 20 sites (5 locations×2 sides×2 depths, Fig. 1) received 1 representative of all 10 mother colonies. Nubbin sizes were randomized to avoid bias (Fig. 2). Hence the resulting 10 nubbins per site represented one genetically identical population. By this a systematic error due to possibly existing cryptic species [54] within the original P. lutea community could be avoided. The nubbins, each one on its individual holder, were attached to acrylic racks (Fig. S2) fixed on dead coral substrate. The experiment was started between February 20th and 24th 2007 and ended between February 22nd and 27th 2008. Logistic constraints did not allow revisiting the sites on a regular basis for maintenance. To avoid artefacts due to fouling, the nubbins were therefore left unprotected, even though this increased the risk of losing nubbins due to fish predation [55] or waves. The high number of nubbins deployed ensured, however, a sufficient number of replicates for statistical analyses after the exposure period (Table S2). At the end of the exposure period the corals were retrieved and returned to the laboratory. Dead nubbins covered with fish bite marks where discarded. Only undamaged and living nubbins were used for the analysis. Nubbin holders were cleaned of epiphytes and the nubbins on their holders weighed, first in seawater and subsequently in air after drying at 60°C for 24 h. Growth rates (g yr−1) were calculated as change in mass over the 1 year duration of the experiment, assuming no significant mass change in the plastic holders which were free of bite marks after the exposure. There were no differences in the initial nubbin weights between exposure treatments (side and depth, Student's t-tests, Table S3). Assuming no significant size-dependent differences in area-specific growth [56] we normalized the final weights to the initial weights and expressed the mean growth rates as % yr−1.
Figure 2. Experimental design of Porites lutea growth rate experiment.

For the experiment in each case 20 coral nubbins were collected from 10 mother colonies at a sheltered east site in 7(close to E 8.1, cf. Fig. 1) and transplanted to the west and east sides of 5 islands at 2 depths (7 m and 20 m) from February 2007 to February 2008 along the Similan Islands (cf. Fig. 1).
Bioerosion rates
Bioerosion was assessed by deploying bioerosion-free dead coral skeleton and measuring the weight differences before and after the exposure. A total of 72 rectangular blocks of 8×8×1.5 cm3 were cut from the skeleton of a dead P. lutea colony (Fig. S3) collected from the fringing reef close to Phuket Marine Biological Centre at the southern tip of Phuket peninsula (7°47′58.76″N, 98°24′31.14″E, Fig. 1). Only immaculately clean blocks from the inner portions of the colony were selected. The blocks were soaked in running fresh water, dried, perforated in the center with a 5 mm drill for later attachment, measured to the closest mm, and weighed to the closest 0.01 g. Groups of blocks were mounted on PVC racks yielding a total of 12 racks holding 6 blocks each. For each rack, blocks were fixed at a distance of 15 cm to each other and 2 cm to the rack (Fig. S4), using stainless steel screws and bolts. Limitations on the amount of material restricted the spatial scale of the bioerosion experiment to the eastern and western side of the central Similan island Ko Miang (Fig. 1). Previous studies showed the island to represent very well the general oceanographic and reef ecological conditions of the Similan Island archipelago, reflecting the environmental conditions, coral cover and framework distribution [31]. At each of the 4 sites (W×E, 20×7 m), 3 racks were attached to dead coral substrate in a distance of 20 m to each other. The racks were deployed mid of March 2007. Half of the blocks (i.e. 3 blocks per rack) were retrieved after 12 months (mid of March 2008), the remainder after 21 months (mid of November 2008). The block numbers to be sampled were chosen randomly before collection to avoid personal bias. Immediately after collection the blocks were bleached to remove organic material, rinsed in fresh water, dried and weighed as above. Care was taken not to lose any material during handling. Carbonate accretion due to calcareous fouling organisms (serpulids, bivalves, balanids and corals growing particularly on the undersides of the blocks, cf. Fig. S5, S6) was calculated from their skeletal volumes (cm3) and respective skeletal densities taken from the literature [47], [48], [53], [57] (Fig. S5). Volumes were estimated from length measurements carried out under a stereomicroscope applied to geometric approximations of the skeletal structures. Tubes of serpulids and balanids were represented by truncated hollow cones with the tube walls accounting for 40% of the tube volume, respectively [57]. The volume of bivalve shells was calculated by multiplying the area of the shell (approximated by the planar projection of the shell on a surface) with the shell thickness. The volume of small dome-shaped corals was calculated as 3/8 π r3, where r is the radius of the coral skeleton. For branching corals, the volume was calculated by the sum of branch segments approximated by cylinders (π r2 l, where l is the length of the branch segment). Serpulids, balanids and bivalves were assumed to have a skeletal density of 2.7 g cm−3 [39], corals' density was taken as 1.4 g cm−3 after Davies [53] and in accordance with previous bulk density determinations [47], [48]. Bioerosion values for both exposure periods (12 and 21 months) were normalized to one year and expressed as kg m−2 [5], [7].
Statistical analysis
For statistical analyses the software Statistica v 9 was used. Data were tested for normal distribution and homogeneity of variances with Kolmogorov-Smirnov and Levene's test, and square root transformed if necessary. The effect of exposure (W and E) and depth (7 and 20 m) on rates of coral growth and bioerosion was analyzed using analysis of covariance (ANCOVA), with side and depth as the treatment factors and the initial weight of coral nubbins and dead skeleton blocks as the respective covariate. Posthoc, pair wise comparisons of the adjusted group means were performed via Tukey HSD-tests. Possible site or rack effects between coral fragments exposed at the same site on the same rack were considered by taking into account the factor site as random factor into the analysis. This factor was statistically insignificant (see results) and allowed to pool the data. The effect of exposure (W and E) and depth (7 and 20 m) on the temperature conditions (monthly mode, maxima, minima and negative cumulative temperature anomalies (calculated as degree days, °C d) was tested with a non-parametric Kruskal-Wallis ANOVA and median test followed by multiple comparisons of mean ranks. Both growth rate data and bioerosion data were related to the temperature conditions (cumulative °C d) at each site. General linear models were fitted to the data with growth rate and bioerosion as dependent and temperature as independent variables. If not stated otherwise data are displayed as means (± SE).
Results
Temperature
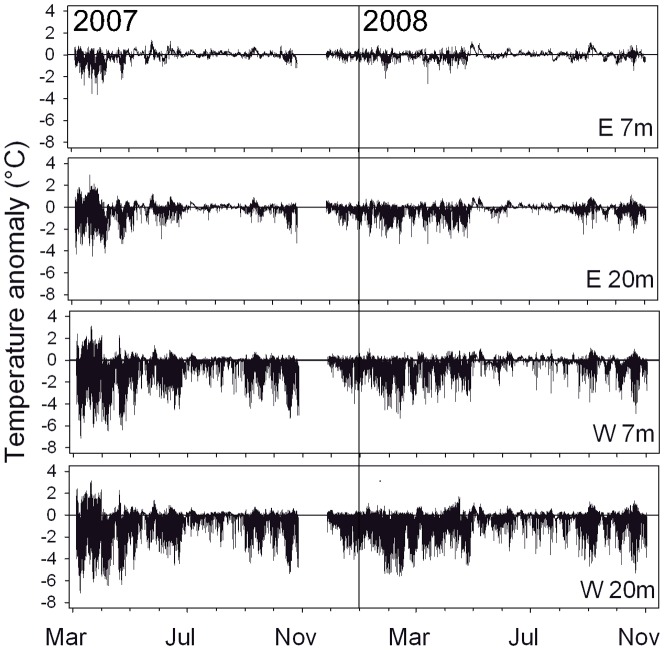
Temperature variations showed the strongest presence of large amplitude internal waves (LAIW) between February and April (Fig. 3) corresponding to the weak north east (NE) monsoon (e.g. W 20 m: monthly ΔT = 8.6±0.2°C in March 2007). The lowest LAIW activity was found between July and November (e.g. W 20 m: monthly ΔT = 3.8±0.3 in 2007) corresponding to the strong south west (SW) monsoon. Significant interannual differences were also evident (W 20 m: monthly ΔT = 6.0±0.1°C in 2008, i.e. a 2.6°C smaller range than in 2007) (Table 1). Temperatures varied significantly between, but not within the levels of the two factors depth (levels: 7 m, 20 m) and exposure (levels: W, E) ([31], this study]. The temperature anomalies split by years (2007 and 2008), exposure (W versus E) and depth (7 and 20 m) are shown in Fig. 4 A and B. Differences between monthly mode and maximum values were small (Fig. 4 A, mode: ΔT<3.6°C, maxima: ΔT<3.0°C) compared to the differences between mode and minimum values (Fig. 4 A, ΔT>8.0°C). Exposure and depth differences were significant for the minima (but not for modes and maxima) (Table 1) with lowest values at W 20 m (24.5±0.2°C compared to 25.6±0.2°C for W 7 m, 27.1±0.1°C for E 20 m, and 27.9±0.1°C for E 7 m) resulting in increased temperature ranges with depth and exposure, particularly in 2007 (Table 1, Fig. 3 and 4). The cumulative negative temperature anomalies which consider both, the intensity and frequency of temperature drops below the daily running mode, were most pronounced in W 20 m and decreased with decreasing exposure down to the lowest values at E 7 m (Table 1, Fig. 4 C). They did not show a statistically detectable difference between the years as found for the temperature minima and ranges (2007 and 2008).
Figure 3. Temperature anomalies at Similan island Ko Miang.
Study sites E 4.1 and W 4.1, period March 2007 to November 2008. Anomalies were calculated relative to mode values (gap in data due to exceeded storage capacity).
Table 1. Differences in monthly temperature values at Similan Islands between depths and sides.
| df | N | Mode | maxima | Minima | range | df | N | cum. negative anomaly (°C d) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 vs 2008 | 1 | 440 | Kruskal Wallis: | n.s. | n.s. | * | * | 1 | 40 | n.s. | ||||||||||||||
| 2007 | 3 | 220 | Kruskal Wallis: | n.s. | Kruskal Wallis: | * | Kruskal Wallis: | *** | Kruskal Wallis: | *** | 3 | 20 | Kruskal Wallis: | *** | ||||||||||
| W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | ||||||||||
| W 20 m | W 20 m | W 20 m | W 20 m | W 20 m | ||||||||||||||||||||
| W 7 m | n.s. | W 7 m | n.s. | W 7 m | n.s. | W 7 m | n.s. | W 7 m | n.s. | |||||||||||||||
| E 20 m | n.s. | n.s. | E 20 m | n.s. | n.s. | E 20 m | *** | *** | E 20 m | *** | *** | E 20 m | n.s. | n.s. | ||||||||||
| E 7 m | n.s. | n.s. | n.s. | E 7 m | * | n.s. | * | E 7 m | *** | *** | ** | E 7 m | *** | *** | n.s. | E 7 m | *** | n.s. | n.s. | |||||
| 2008 | 3 | 220 | Kruskal Wallis: | *** | Kruskal Wallis: | *** | Kruskal Wallis: | ** | Kruskal Wallis: | ** | 3 | 20 | Kruskal Wallis: | *** | ||||||||||
| W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | W 20 m | W 7 m | E 20 m | ||||||||||
| W 20 m | W 20 m | W 20 m | W 20 m | W 20 m | ||||||||||||||||||||
| W 7 m | * | W 7 m | n.s. | W 7 m | n.s. | W 7 m | n.s. | W 7 m | n.s. | |||||||||||||||
| E 20 m | n.s. | n.s. | E 20 m | n.s. | n.s. | E 20 m | *** | *** | E 20 m | *** | *** | E 20 m | ** | n.s. | ||||||||||
| E 7 m | *** | n.s. | n.s. | E 7 m | ** | n.s. | n.s. | E 7 m | *** | *** | ** | E 7 m | *** | *** | n.s. | E 7 m | *** | n.s. | n.s. | |||||
Comparisons of monthly mode, maxima, minima, range = maximum – minimum values, and negative cumulative temperature anomalies (calculated as degree days, °C d). Kruskal-Wallis ANOVA and median test followed by multiple comparisons of mean ranks for all groups (year: February 2007–February 2008 (2007) versus November 2007–November 2008 (2008), side: west (W) versus east (E), and depth: 20 m versus 7 m). (df = degrees of freedom; N = sample size; p = probability level; significance levels are *0.05>P≥0.01, **0.01>P≥0.001, ***P<0.001, n.s.: not significant).
Figure 4. Temperature data for all Similan Islands sites.
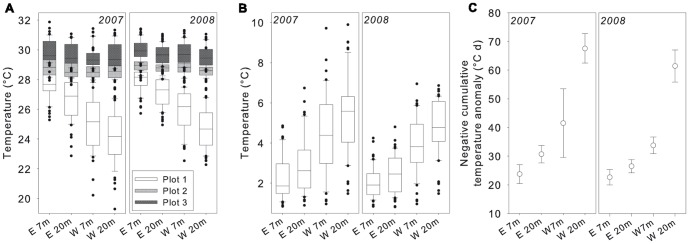
Central tendency box plots (median with 25th and 75th percentile and non-outlier range) with extremes (dots) (A, B) and scatter plots with standard deviation (SD, whiskers) for the study periods [Feb 2007 to Feb 2008 (2007) and Nov 2007 to Nov 2008 (2008)], sides (east, E and west, W) and depths (7 and 20 m), featuring overall monthly minimum, mode and maximum temperatures (A), monthly temperature ranges (B), and negative cumulative temperature anomalies (calculated as degree days, °C d; calculation details see methods) (C).
Coral growth rate
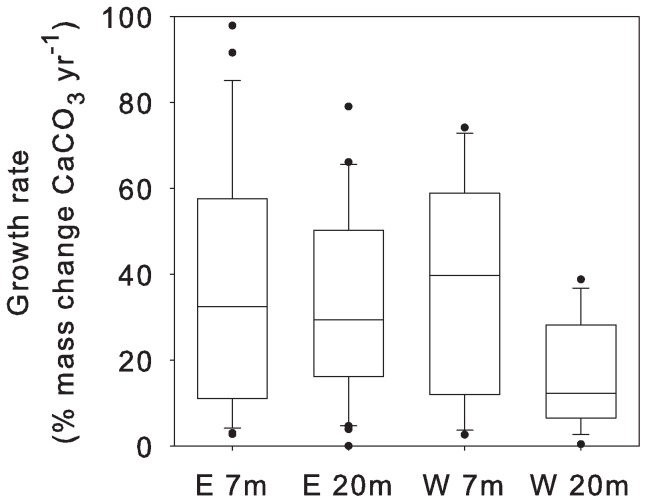
ANCOVA results for the growth rates of P. lutea nubbins revealed significant effects of exposure and depth (p<0.038, Table 2, Fig. 5). Pair wise comparisons showed suppressed coral growth at the LAIW-impacted sites (W 20 m: 16.3±0.2% yr−1) compared to the monsoon-exposed (W 7 m: 36.2±0.4%) and sheltered sites (E 7 m and E 20 m: 36.7±0.2% and 36.5±0.2%, respectively, Tukey HSD, Table 2).
Table 2. Analysis of covariance (ANCOVA) for coral growth experiment.
| Factor | df | MS | F | p |
| initial weight | 1 | 3.296 | 3.829 | * |
| side & depth | 3 | 4.172 | 1.192 | *** |
| Site | 4 | 0.684 | 0.197 | 0.932 |
| side & depth*island | 7 | 3.614 | 4.120 | 0.379 |
| Error | 76 | 0.861 | ||
| Tukey HSD, significantly different, pairwise comparisons: | p | |||
| W 20 m<E 20 m | ** | |||
| W 20 m<E 7 m | ** | |||
Coral growth data square-root-transformed and analysed for east (E) and west (W) of Similan Islands from February 2007 to February 2008. Side (E and W) and depth (7 and 20 m) as treatment factors, and initial weight of coral nubbins as covariate; factor site (rack) included as random factor; posthoc, pair wise comparisons of the adjusted group means via Tukey HSD-tests. (df = degrees of freedom; MS = means square; F = F-value; p = probability level, significance levels are *0.05>P≥0.01, **0.01>P≥0.001, ***P<0.001).
Figure 5. Coral growth rates (mass change of calcium carbonate, CaCO3) of Porites lutea nubbins.
Nubbins were transplanted from sheltered east (E) 7 m to 7 and 20 m depth at E and west (W) sides of Similan Islands (Central tendency box plots: median with 25th and 75th percentile and non-outlier range, extremes: dots) between February 2007 and February 2008 (number of replicates: E 7 m: 27, E 20 m: 33, W 7 m: 13, W 20 m: 19).
Rates of bioerosion
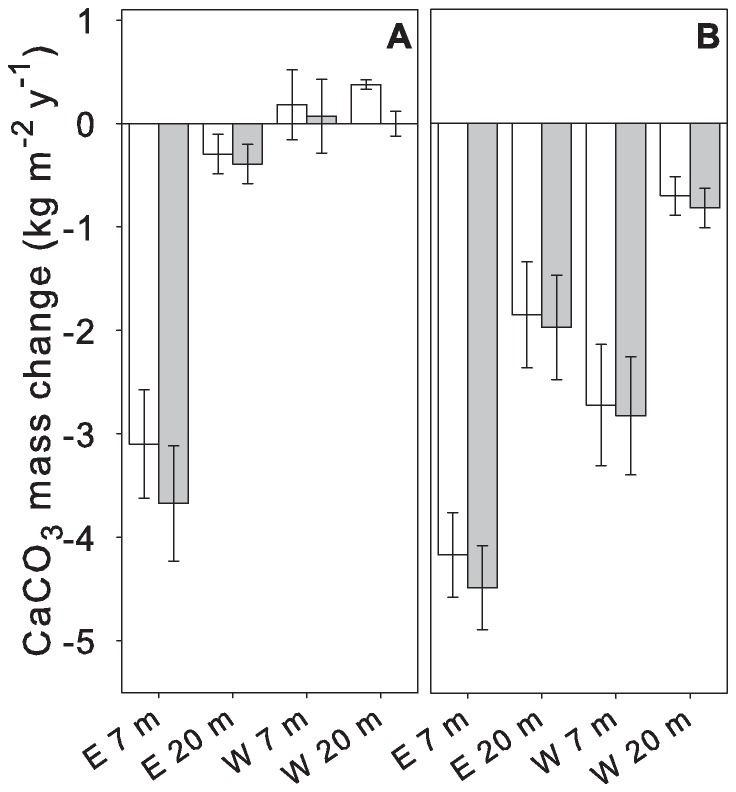
ANCOVA results for bioerosion rates revealed significant effects of side and depth for both exposure periods (Table 3). Irrespective of exposure length, bioerosion was highest in E 7 m (Fig. 6). After 12 months exposure bioerosion was 9-fold higher in E 7 m than in E 20 m (−3.7±0.6 kg CaCO3 m−2 in 7 m compared to −0.4±0.2 kg CaCO3 m−2 in 20 m), and almost 40-fold higher than in W 7 m and 20 m where a carbonate loss was barely detectable (0.1±0.4 kg CaCO3 m−2 in W 7 m and −0.01±0.1 kg CaCO3 m−2 in W 20 m). The longer exposure (21 months) caused no change in the annual bioerosion in E 7 m (−4.2±0.4 kg CaCO3 m−2), but resulted in a more than 4-fold increase in E 20 m (−1.9±0.5 kg CaCO3 m−2), and a pronounced carbonate loss in W (W 7 m: −2.7±0.6 kg CaCO3 m−2 and W 20 m: −0.7±0.2 kg CaCO3 m−2, respectively). Again, erosion rates were highest in E 7 m (2-fold higher than in E 20 m and 5-fold than in W 20 m, respectively), followed by W 7 m with 3-fold higher erosion compared to W 20 m (Table 3 B). Biological carbonate accretion on the skeletal blocks was negligible compared to the net carbonate loss irrespective of exposure time, except for W 20 m after 12 months (net carbonate loss versus gross mass change: Student's t-test: p<0.01, Table S4, Fig. 6, Table S5, S6). Compared to the negligible 12 months exposure erosion rates in W carbonate accretion was comparatively high (Fig. 6). In all other cases the differences between net carbonate loss (corrected for the accretion due to fouling organisms) and gross mass change was less than 25% (24.3% in E 20 m after 12 months), in most cases between 4 and 7% (at E 7 and 20 m and W 7 m after 21 months exposure, Fig. 6, Table S4, S5, S6).
Table 3. Analysis of covariance (ANCOVA) for bioerosion experiment.
| A | 12 months exposure | ||||
| Factor | df | MS | F | p | |
| initial weight | 1 | 19.498 | 91.636 | *** | |
| Side | 1 | 5.901 | 27.732 | *** | |
| depth | 1 | 2.933 | 13.785 | *** | |
| side*depth | 1 | 3.794 | 17.830 | *** | |
| Error | 31 | 0.213 | |||
| Tukey HSD, significantly different, pairwise comparisons: | |||||
| E 7 m<E 20 m | *** | ||||
| E 7 m<W 7 m | *** | ||||
| E 7 m<W 20 m | *** | ||||
Bioerosion data (corrected for bioaccretion) square-root-transformed and analysed for east (E) and west (W) of Similan island Ko Miang. Side (E and W) and depth (7 and 20 m) as treatment factors, and initial weight of skeletal substrates as covariate; posthoc, pair wise comparisons of the adjusted group means via Tukey HSD-tests. (df = degrees of freedom; MS = means square; F = F-value; p = probability level, significance levels are **0.01>P≥0.001, ***P<0.001). Results for experimental period from February 2007 to February 2008, 12 months (A), and from February 2007 to November 2008, 21 months (B).
Figure 6. Calcium carbonate (CaCO3) mass change accretion (>0) and bioerosion (<0).
Measured mass change (white columns) and calculated mass change (grey columns, corrected for accretion due to fouling organisms) of CaCO3 blocks at east (E) and west (W) side of Similan island Ko Miang. Error bars: ±1 SE of mean. Results from 12 months exposure (February 2007 to February 2008) (A), and 21 months exposure (February 2007 to November 2008) (B) normalized to 1 year (number of replicates in each case: 9).
Coral growth and bioerosion in relation to temperature anomalies
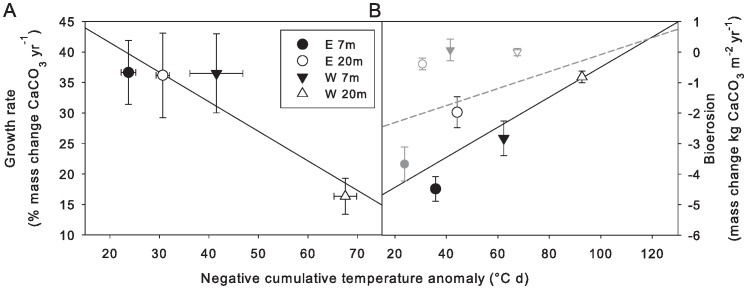
Both coral growth and bioerosion rates revealed differences between sides and depths. In order to explore the potential role of LAIW in explaining the differences, general linear models (GLM) were calculated with coral growth or bioerosion as dependent variable and temperature anomalies calculated for the respective exposure periods as independent variables. The results show that both coral growth (r2 = 0.46, p = 0.039) and bioerosion after 12 (r2 = 0.30, p<0.001) and 21 months exposure (r2 = 0.59, p<0.001) are inversely related to LAIW (Fig. 7).
Figure 7. Coral growth and bioerosion as functions of temperature anomaly in west (W) and east (E), 7 m and 20 m depth.
Coral growth rate of Porites lutea nubbins at all study sites (n = 20, df = 1, F-value = 4.4, r2 = 0.46, p = 0.039) along the Similan island chain after 12 months exposure (February 2007 to February 2008; see Fig. 4) (A), and bioerosion (calculated mass change in calcium carbonate (CaCO3) corrected for accretion due to fouling organisms) on CaCO3 blocks at the central Similan island Ko Miang (see Fig. 1) after 12 months (grey depiction; February 2007 to February 2008; n = 4, degrees of freedom = 1, F-value = 14.4, r2 = 0.30, p<0.001) and 21 months exposure normalized to 1 year (February 2007 to November 2008; n = 4, degrees of freedom = 1, F-value = 17.9, r2 = 0.58, p<0.001) (see Fig. 5; <0, bioerosion) (B) as functions (GLM) of the cumulative negative temperature anomaly (calculated as °C d) of the respective time period. All values are plotted as mean ± SE.
Discussion
Environmental conditions
The abrupt temperature variations observed in the Similan Islands are characteristic for internal waves in coastal waters, in contrast to the much more gradual changes (hours) induced by changing tides [58]. The temperature excursions on this short time scale rival in magnitude the seasonal or monthly temperature variations at higher latitude reefs [41], [42], [59], underscoring the severity of these events and the potentially important role of cold shocks (up to 10°C in the present study) on coral metabolism and growth. The particularly strong temperature fluctuations during spring 2007 corresponded to the positive 2007 Indian Ocean Dipole (IOD) [60] with a shallow pycnocline guiding internal waves well onto the shelf, while spring 2008 corresponds to a deeper pycnocline with less pronounced temperature fluctuations around the islands. Previous studies showed the temperature drops to be associated with corresponding drops in oxygen concentrations and pH, down to 12% saturation and 0.6 units below ambient, respectively [31]. Nutrient concentrations were found to increase to up to 12-fold for nitrate and nitrite, 5-fold for silicate and over 20-fold for phosphate [31]. Although temperature variations were also measured on the E side of the islands, they were markedly lower than on the W sides of the islands (Fig. 3, 4). The environmental contrasts between W and E are also reflected in the monsoon regime causing strong swell on W but only weak wave action on the E sides of the islands [32]. Monsoon-enhanced sedimentation, surface wave action and LAIW impact were found to collectively determine reef development in the Similan Islands [32] explaining a complex, dense reef framework along the sheltered E sides contrasting the only scattered coral communities along the exposed W sides of the islands [31]. The direct relationship between LAIW intensity and depth [31] and the inverse relationship between monsoonal surface wave impact and depth [61] show that both phenomena act from opposite directions, with LAIW shaping the deeper and monsoon the shallower reef areas.
Coral growth
The coral nubbins of P. lutea showed a reduced growth in W 20 m (Fig. 5, Table 2) supporting our hypothesis of LAIW suppressed growth, compared to the monsoon affected (W 7 m) and sheltered (E 7 m, E 20 m) sites. This may be due to the combined effect of unfavourable environmental conditions, notably lower temperature, light and pH in W 20 m compared to the other sites [31]. The GLM analysis shows that coral growth rates correlated well with temperature anomaly (Fig. 7), underscoring the potential role of LAIW in suppressing coral growth. The growth rates in W 7 m are important in assessing the relative importance of monsoon and LAIW for reef growth [32] by showing that monsoon exposure did not limit skeletal growth. It is likely that the high water motion here enhanced metabolic rates and calcification [62] and prevented a stressful permanent sedimentation on the coral surfaces.
Coral growth rates vary in response to a number of extrinsic factors, such as temperature [56], [63], aragonite saturation state [9], [12], [16] or nutrient supply [45]. Growth rates may vary even more between species [64] and within species [47] due to intrinsic factors which are so far not well understood. Early ontogeny may play an important role in determining whether skeletogenesis is slow or rapid, and it is possible that recruitment in LAIW-exposed versus –sheltered or deep versus shallow habitats may involve selection for differential growth. To test whether such a bias has occurred in our study, compromising the validity of our results in coral growth, we analysed for side- and depth-differences in another performance indicator: coral survival. It is well known that coral transplantation from shallow to deep habitats can cause coral mortality in corals that, similar to Porites lutea, are unable to adapt the composition of their zooxanthellae symbionts [52]. Mortalities in our study were, however, not significantly different between deep and shallow corals (Table S1, ANOVA, p>0.05), and coral growth was indistinguishable between E 7 m (source population) and deep (Table 2), suggesting that coral origin did not compromise our findings.
Coral growth depends on light availability due to the direct linkage of calcification to the photosynthetic energy supply by the zooxanthellae [40], [65]. Despite comparable light levels in W and E 20 m depths [31] (C. Jantzen, unpublished data) growth rates in E 20 m were significantly higher than in W 20 m and comparable - if not higher - than in E 7 m – in spite of 3-fold lower light levels. Differences in light levels are therefore unlikely to explain the drastic E-W differences in coral growth at 20 m - emphasizing the importance of LAIW - notably temperature and aragonite saturation.
Coral growth is strongly dependent on temperature. Extended periods of low temperature stress (<19°C) were found to affect coral photosynthesis, tissue maintenance and growth [66]. Cold temperature can reduce the photosynthetic efficiency [67] and coral calcification [63]. Although extended periods of cold temperatures are known to have a negative effect on coral metabolism and growth [42], [67], it is not known so far if and to what extent repeated short-term temperature drops affect coral physiology and growth. But the effects on corals may be similar as described in several studies on daily temperature variations in shallow habitats affecting coral metabolism and heat stress. Although these studies deal with positive temperature anomalies it is likely that the mechanisms evoked by the thermal history [68], [69] including acclimation [70] and adaptation strategies [71] are similar to corals exposed to temperature drops. The results in this study indicate that step changes in temperature conditions of 6 to 9°C at supertidal frequencies (Fig. 3 and 4) could account in large parts to the reduced growth performance of the corals in W 20 m. Calcification in corals is further known to depend on high aragonite saturation to maintain a high saturation state at the site of calcification [9], [12], [40]. The temperature related drops in pH of 0.2 to 0.5 units below ambient [31] exceed natural swings of pH in other reef areas which show diurnal changes between 7.9 and 8.1 [72]. Weekly to seasonal changes between upwelling and non-upwelling periods in the eastern tropical Pacific are generally below 0.2 pH units [73]. The step changes in pH conditions due to LAIW in our study may thus require an especially high energetic effort to sustain the microenvironment beneath the coral tissue needed for calcification [9], [12]. As P. lutea is mixotrophic, LAIW-enhanced fluxes of both inorganic and organic materials may provide additional energy [45], [72], as suggested by the higher nutritional status of P. lutea in W compared to E [39]. Similarly, Leichter and Genovese [34] reported positive growth of the coral Madracis mirabilis under the influence of daily upwelling due to internal waves and suggested that the lower light conditions might be offset by the higher input of particle and nutrient fluxes. However, the surplus energy in our study does not seem to be sufficient to keep calcification at comparable levels, and the drop in calcification in W 20 m shows that the positive LAIW effects (higher nutritional status of the corals) are outweighed by the negative effects of i.a. temperature and pH. The effect may be much stronger in other corals, particularly the species rare or absent in W [31], as suggested by numerous studies describing massive Porites as highly tolerant to different environmental stressors including sedimentation, high nutrition and low pH conditions [21], [74]–[76].
Bioerosion
Bioerosion was highest in E and lowest in W (Fig. 6) and showed an inverse relationship with temperature anomaly (Fig. 7) refuting our hypothesis of LAIW- (i.e. nutrient-) enhanced bioerosion. However, bioerosion has also been shown to be related to coral cover [2], [14], in line with the observation of higher reef development in E [31], [32]. This is because higher coral cover provides more habitats for internal and external bioeroders, notably living space for boring mussels and sponges, and hiding space for a wealth of external bioeroders such as sea-urchins and fish [2].
The estimation of bioerosion as carbonate loss always implicates possible sources of error due to the difficult estimation of the simultaneously occurring carbonate accretion [5]–[8], [17]–[19]. Internal carbonate accretion on bore holes and channels as well as accretion due to coralline algae was neglected. The comparatively short exposure time of less than 2 years and the marginal actual accretion coming with it justified this approach [5], [7]. The calculation of the skeletal volumes and densities of the newly grown carbonate structures [57] implicated the risk of false estimates because we purely calculated the growth and did not directly measure it. Nevertheless for this study it was the most appropriate and exact method in contrast to direct weight measurements because it was impossible to accurately separate the newly built carbonate structures from the original skeletal blocks. As biological carbonate accretion on the skeletal blocks was shown to be negligible compared to bioerosion (Fig. 6, S6) [5], [7], [19] the possible methodical errors were likely equally insignificant.
Erosion rates in E for both exposure periods and in W shallow after 21 months were similar or higher than rates measured elsewhere in offshore coral reefs denoted as healthy and balanced in their carbonate budget with grazing fish as the main bioeroders [5], [7], [8], [18]. Bioerosion in W 20 m was low even after 21 months exposure contrasting findings in the eastern tropical Pacific where external bioerosion was a function of upwelling intensity [19]. The better light conditions in 7 m depth may explain the higher bioerosion compared to the 20 m sites, where light may have enhanced both, internal bioerosion by favouring phototrophic microborers [77] but also external bioerosion by fostering algal growth [78] and, hence, grazers [77]. After 12 months exposure, skeletal blocks were virtually free of grazing traces and bite marks, and after 21 months W blocks still showed much less external damage than E blocks after 12 months (Fig. S3). Perhaps the duration of the experiment was too short for internal bioerosion to take its toll: in spite of an enhanced supply of plankton [38] likely favouring bioeroding filter feeders [2], [18], [46] it may have taken several months for the boring community to infest the blocks [2], [7], [18]. A lag effect is also suggested by the increase in bioerosion rates after 21 months, only after a community of internal bioeroders may have become established [7].
Conclusions and outlook
This is the first study indicating evidence for LAIW-depressed coral growth. The results support previous findings that LAIW can inhibit the development of a complex carbonate framework [31], [32] and by this play an important yet largely unexplored role in controlling reef development outside established upwelling regions [19], [23]. They also suggest that surface swell does not suppress coral growth in monsoon areas. Despite the limited growth performance and reduced reef framework coral communities along the W Similans were found to be especially diverse and species rich [31]. This is in contrast to typical upwelling regions [79] or natural CO2 vents were only some very robust species dominate the communities [21]. LAIW exposed coral communities therefore offer the possibility to study the tipping points and limits of various coral species to acclimatize or adapt to different natural stress factors simultaneously entrained by LAIW [30], [31], [80] and relevant for future climate scenarios [25]–[27]. Research in this field has to be extended including especially sensitive taxa studying the effects of co-varying stress factors such as temperature, pH and nutrient concentrations. Although the potential of corals to acclimatize still needs to be ascertained, LAIW-exposed corals direct a higher proportion of their energy allocation more to LAIW acclimation rather than growth which may therefore provide an insurance to vagaries of temperature variations in a changing climate.
Supporting Information
Microbalance and weighing construction for buoyant weight technique. (After Davies [36]).
(TIF)
Coral nubbins of Porites lutea on individual transplant holders. Nubbins with holders attached to racks built of acrylic glass and metal rocks (a), and coral nubbins on holders right after recollection with epiphytes (b, c) and with epiphytes removed (d).
(TIF)
Examples for changes on dead coral skeleton during exposure period. Four different rectangular blocks (ordered among one another) of dead coral skeleton of Porites lutea before (East 20 m: A, D, West 20 m: G, J), after 12 months (East 20 m: B, C, West 20 m: E, F) and after 21 months (East 20 m: H, I, West 20 m: K, L) exposure. The second column (B, E, H, K) shows the upper sides, the third column (C, F, I, L) the under sides of the skeletal blocks. The first two rows show blocks right after collection (B, C, E, F), the lower two rows show blocks after drying them (H, I, K, L).
(TIF)
Bioerosion racks made of PVC-tubes and metal bars. Each rack with six dead coral blocks of Porites lutea attached. View from above the setup (A), and from diagonally below (B).
(TIF)
Calcium carbonate accretion due to fouling organisms on dead skeletal blocks. Shown are calcium carbonate precipitating organisms on dead skeletal blocks of Porites lutea: from top to bottom: Balanids (A–D), serpulids (E–H), bivalves (I–L), and corals (M–O).
(TIF)
Comparison of total accretion due to fouling organisms and accretion by different groups of carbonate producers. Total accretion (upper panels) and accretion by different groups of carbonate producers (lower panels, as fractions of total accretion) on dead coral substrates at island Ko Miang. Error bars: ±1 SE of mean. Results from 12 months exposure (February 2007 to February 2008) (A), and 21 months exposure (February 2007 to November 2008) (B) (number of replicates in each case: 9).
(TIFF)
Analysis of variance (ANOVA) of coral nubbin mortality.
(DOCX)
Surface area, initial (start) and end air weights of coral nubbins of Porites lutea .
(DOCX)
Comparison of initial weights of coral nubbins between different sides and depths at Similan Islands.
(DOCX)
Comparison of measured mass change and calculated mass change of calcium carbonate (CaCO3) blocks in east and west of Similan island Ko Miang.
(DOCX)
Comparison of accretion due to fouling organisms at east (E) and west (W) side of Similan island Ko Miang.
(DOCX)
Comparison of accretion due to fouling organisms on dead skeletal blocks between 12 and 21 months exposure.
(DOCX)
Acknowledgments
The authors would like to thank N. Phongsuwan for his help and assistance in coral sampling, handling and exposure along the Similans, S. Khokiattiwong, W. Thubthimsang and the staff of the Phuket Marine Biological Center (PMBC) as well as the Similan Island National Park staff for the very kind field assistance and support in logistic issues. We thank L. Fillinger and A. Buschmann for their assistance in drawing figure 1
Funding Statement
Funding for this study was provided by the German Research Foundation (RI 1074/7-1: Ocean-Reef Coupling in the Andaman Sea), the National Research Council of Thailand and the German Federal Ministry for Education and Research (Grant number: 03F0608B, Bioacid 3.2.3, Coral calcification in marginal reefs). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Goreau TF (1963) Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef builders. Ann NY Acad Sci 109: 127–167. [DOI] [PubMed] [Google Scholar]
- 2.Glynn PW (1997) Bioerosion and coral reef growth: a dynamic balance. In: Birkeland C, editor. Life and death of coral reefs. Chapman and Hall. pp. 68–95. [Google Scholar]
- 3. Scoffin TP, Stearn CW, Boucher D, Frydl P, Hwakins CM, et al. (1980) Calcium carbonate budget of a fringing reef on the west coast of Barbados. Bull Mar Sci 30: 475–508. [Google Scholar]
- 4. Hutchings PA (1986) Biological destruction of coral reefs. Coral Reefs 4: 239–252. [Google Scholar]
- 5. Tribollet A, Golubic S (2005) Cross-shelf differences in the pattern and pace of bioerosion of experimental carbonate substrates exposed for 3 years on the northern Great Barrier Reef, Australia. Coral Reefs 24: 422–434. [Google Scholar]
- 6. Davies PS, Hutchings PA (1983) Initial colonization, erosion and accretion on coral substrate. Coral Reefs 2: 27–35. [Google Scholar]
- 7. Osorno A, Peyrot-Clausade M, Hutchings PA (2005) Patterns and rates of erosion in dead Porites across the Great Barrier Reef (Australia) after 2 years and 4 years of exposure. Coral Reefs 24: 292–303. [Google Scholar]
- 8. Pari N, Peyrot-Clausade M, Le Campion-Alsumard T, Hutchings PA, Chazottes V, et al. (1998) Bioerosion of experimental substrates on high islands and on atoll lagoons (French Polynesia) after two years of exposure. Mar Ecol Prog Ser 166: 119–130. [Google Scholar]
- 9. Marubini F, Ferrier-Pagès C, Furla P, Allemand D (2008) Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs 27: 491–499. [Google Scholar]
- 10. Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pol Bul 50: 125–146. [DOI] [PubMed] [Google Scholar]
- 11.Fabricius KE (2011) Factors determining the resilience of coral reefs to eutrophication: a review and conceptual model. In: Coral Reefs: An ecosystem in transition. pp. 493–505.
- 12. Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, et al. (2009) Net loss of CaCO3 from coral reef communities due to human induced seawater acidification. Biogeoscience Discussion 6: 2163–2182. [Google Scholar]
- 13. Rodolfo-Metalpa R, Houlbrèque F, Tambutté E, Boisson F, Baggini C, et al. (2011) Coral and mollusc resistance to ocean acidification adversely affected by warming. Nature Climate Change 10.1038/NCLIMATE1200 [DOI] [Google Scholar]
- 14. Hallock P (1988) The role of nutrient availability in bioerosion: consequences to carbonate build-ups. Palaeogeogr Palaeoclimatol Palaeoecol 63: 275–291. [Google Scholar]
- 15. Carriero-Silva M, McClanahan T, Kiene W (2005) The role of inorganic nutrients and herbivory in controlling microbioerosion of carbonate substrate. Coral Reefs 24: 214–221. [Google Scholar]
- 16. Cohen AL, Holcomb M (2009) Why corals care about ocean acidification – uncovering the mechanism. Oceanography 22: 118–127. [Google Scholar]
- 17. Rose CS, Risk MJ (1985) Increase in Cliona delitrix infestation of Montastrea cavernosa heads on an organically polluted portion of the Grand Cayman fringing reef. Mar Ecol 6: 345–363. [Google Scholar]
- 18. Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M, Cuet P (2002) The effects of eutrophication-related alterations to coral reef communities on agents and rates of bioerosion (Reunion Island, Indian Ocean). Coral Reefs 21: 375–390. [Google Scholar]
- 19. Reaka-Kudla M, Feinglod JS, Glynn W (1996) Experimental studies of rapid bioerosion of coral reefs in the Galapagos Islands. Coral Reefs 15: 101–107. [Google Scholar]
- 20. Manzello DP (2009) Reef development and resilience to acute (El Niño warming) and chronic (high-CO2) disturbances in the eastern tropical Pacific: a real-world climate change model. Proc 11th Int Coral Reef Symp 25: 1299–1303. [Google Scholar]
- 21. Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, et al. (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change 10.1038/NCLIMATE1122 [DOI] [Google Scholar]
- 22. Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC (2010) Ocean Warming slows coral growth in the central Red Sea. Science 329: 322–325. [DOI] [PubMed] [Google Scholar]
- 23. Cook Mc (1999) Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18: 357–367. [Google Scholar]
- 24. Hallock P, Schlager W (1986) Nutrient excess and the demise of coral reefs and carbonate platforms. Palaios 1: 389–398. [Google Scholar]
- 25. Hoegh-Guldberg O, Mumby PJ, Hotten AJ, Steneck RS, Greenfield P, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 26. Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, et al. (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321: 560–563. [DOI] [PubMed] [Google Scholar]
- 27. Pandolfi JJ, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333: 418–422. [DOI] [PubMed] [Google Scholar]
- 28. Riegl B (2003) Climate change and coral reefs: different effects in two high-latitude areas (Arabian Gulf, South Africa). Coral Reefs 22: 433–446. [Google Scholar]
- 29. Riegl B, Piller WE (2003) Possible refugia for reefs in times of environmental stress. Int J Earth Sci 92: 520–531. [Google Scholar]
- 30. Leichter JJ, Deane GB, Stokes MD (2005) Spatial and temporal variability of internal wave forcing on a coral reef. J Phys Oceanogr 35: 1945–1962. [Google Scholar]
- 31. Schmidt GM, Phongsuwan N, Jantzen C, Roder C, Khokiattiwong S, et al. (2012) Coral community composition and coral reef development at the Similan Islands, Andaman Sea, in response to strong environmental variations. Mar Ecol Prog Ser 456: 113–126. [Google Scholar]
- 32. Wall M, Schmidt GM, Janjang P, Khokkiattiwong S, Richter C (2012) Differential impact of monsoon and large amplitude internal waves on coral reef development in the Andaman Sea. PLOS ONE 7: e50207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkeland C (1997) Life and death of coral reefs. Chapman & Hall. Int Thomson Publishing
- 34. Leichter JJ, Genovese SJ (2006) Intermittent upwelling and subsidized growth of the scleractinian coral Madracis mirabilis on the deep fore-reef slope of Discovery Bay, Jamaica. Mar Ecol Prog Ser 316: 95–103. [Google Scholar]
- 35.Jackson CR (2004) An atlas of internal solitary-like waves and their properties. 2nd Edition. Office of Naval Research. Global Ocean Associates. Alexandria. VA. USA
- 36.Phongsuwan N, Yamarunpattana C, Paokanta Y, Areechon P (2008) Status of Coral Reefs in the Surin and Similan Archipelagos, Thailand. In: Obura DO, Tamelander J, Linden O, editors. Ten years after bleaching - facing the consequences of climate change in the Indian Ocean. CORDIO Status report 2008. Mombasa
- 37.Veron JEN (2000) Corals of the World. Australian Institute of Marine Science. Townsville. Australia
- 38. Roder C, Fillinger L, Jantzen C, Schmidt GM, Khokiattiwong S, et al. (2010) Trophic response of corals to large amplitude internal waves. Mar Ecol Prog Ser 412: 113–128. [Google Scholar]
- 39. Roder C, Jantzen C, Schmidt GM, Kattner G, Phongsuwan N, et al. (2011) Metabolic plasticity of the corals Porites lutea and Diploastrea heliopora exposed to large amplitude internal waves. Coral Reefs 30: 57–69. [Google Scholar]
- 40. Gattuso JP, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Am Zool 39: 160–183. [Google Scholar]
- 41. Riegl B (2001) Inhibition of reef framework by frequent disturbance: examples from the Arabian Gulf, South Africa, and the Cayman Islands. Palaeo 175: 79–101. [Google Scholar]
- 42. Coles SL, Fadlallah UH (1991) Reef coral survival and mortality at low temperatures in the Arabian Gulf: new species specific lower temperature limits. Coral Reefs 9: 231–237. [Google Scholar]
- 43. Marubini F, Atkinson MJ (1999) Effects of lowered pH and elevated nitrate on coral calcification. Mar Ecol Prog Ser 188: 117–121. [Google Scholar]
- 44. Marubini F, Bernett H, Langdon C, Atkinson MJ (2001) Dependance of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa . Mar Ecol Prog Ser 220: 153–162. [Google Scholar]
- 45. Ferrier-Pagès C, Gattuso JP, Dallot S, Jaubert J (2000) Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19: 103–113. [Google Scholar]
- 46. Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida reef tract suggest land-based nutrient inputs. Mar Pollut Bull 51: 570–579. [DOI] [PubMed] [Google Scholar]
- 47. Scoffin TP, Tudhope AW, Brown BE, Chansang H, Cheeney RF (1992) Coral Reefs (1992) Patterns and possible environmental controls of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 11: 1–11. [Google Scholar]
- 48. Tanzil JTI, Brown BE, Tudhope AW, Dunne RP (2009) Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand between 1984 and 2005. Coral Reefs 28: 519–528. [Google Scholar]
- 49. Titlyanov EA, Yakovleva IM, Titlyanova TV (2007) Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J Exp Mar Biol Ecol 342: 282–291. [Google Scholar]
- 50. LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, et al. (2010) Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium . J Biogeogr 37: 785–800. [Google Scholar]
- 51. Le Campion-Alsumard T, Golubic S, Hutchings P (1995) Microbial endoliths in skeletons of live and dead corals: Porites lobata (Moorea, French Polynesia). Mar Ecol Prog Ser 117: 149–157. [Google Scholar]
- 52. Baker AC (2001) Ecosystems: Reef corals bleach to survive change. Nature 411: 765–766. [DOI] [PubMed] [Google Scholar]
- 53. Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101: 389–395. [Google Scholar]
- 54.Forsman ZH (2003) Phylogeny and phylogeography of Porites & Siderastrea (Scleractinia: Cnidaria) species in the Caribbean and Eastern Pacific based on the nuclear ribosomal ITS region. PhD Thesis. University Houston. 160 p.
- 55. Rotjan RD, Lewis SM (2008) Impact of coral predators on tropical reefs. Mar Ecol Prog Ser 367: 73–91. [Google Scholar]
- 56. Edmunds PJ (2008) The effects of temperature on the growth of juvenile scleractinian corals. Mar Biol 154: 153–162. [Google Scholar]
- 57.Hassan M (1997) Modification of carbonate substrata by bioerosion and bioaccretion on coral reefs of the Red Sea. PhD Thesis. University of Kiel. 126 p.
- 58. Scotti A, Pineda J (2004) Observation of very large and steep internal waves of elevation near the Massachusetts coast. Geophys Res Let 10.1029/2004GL021052 [DOI] [Google Scholar]
- 59. Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: Where do we draw the line? Amer Zool 39: 146–159. [Google Scholar]
- 60. Behera SK, Luo JJ, Yamagata T (2008) Unusual IOD event in 2007. Geophys Res Let 35: L14S11. [Google Scholar]
- 61. Storlazzi CD, Brown EK, Field ME, Rodgers K, Jokiel PL (2005) A model for wave control on coral breakage and species distribution in the Hawaiian Islands. Coral Reefs 24: 43–55. [Google Scholar]
- 62. Dennison WC, Barnes DJ (1988) Effect of water motion on coral photosynthesis and calcification. J Exp Mar Biol Ecol 115: 67–77. [Google Scholar]
- 63. Al-Horani FA (2005) Effects of changing seawater temperature on the photosynthesis and calcification in the scleractinian coral Galaxea fascicularis, measured with O2, Ca2+ and pH microsensors. Sci Mar 69: 347–354. [Google Scholar]
- 64. Buddemeier RW, Maragos JE (1974) Radiographic studies of reef coral exoskeletons: Rates and patterns of coral growth. J Exp Biol Ecol 14: 179–200. [Google Scholar]
- 65. Chalker BE, Taylor DL (1975) Light-enhanced calcification and the role of oxidative phosphorylation in calcification of the coral Acropora cervicornis . Proc R Soc Lond, B 190: 323–331. [DOI] [PubMed] [Google Scholar]
- 66. Crossland CJ (1984) Seasonal variations in the rates of calcification and productivity in the coral Acropora formosa on a high-latitude reef. Mar Ecol Prog Ser 15: 135–140. [Google Scholar]
- 67. Saxby T, Dennison WC, Hoegh-Guldberg O (2003) Photosynthetic response of the coral Montipora digitata to cold temperature stress. Mar Ecol Prog Ser 248: 85–97. [Google Scholar]
- 68. Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, et al. (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLOS ONE 10.1371/journal.pone.0033353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 70. Mayfield AB, Chan PH, Putnam HM, Chen CS, Fan TY (2012) The effects of a variable temperature regime on the physiology of the reef-building coral Seriatopora hystrix: results from a laboratory-based reciprocal transplant. J Exp Biol 10.1242/jeb.071688 [DOI] [PubMed] [Google Scholar]
- 71. Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, et al. (2010) Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Molecular Biol 19: 1705–1720. [DOI] [PubMed] [Google Scholar]
- 72. Bates NR, Samuels L, Merlivat L (2001) Biogeochemical and physical factors influencing seawater fCO2, and air-sea CO2 exchange on the Bermuda coral reef. Limnol Oceanogr 46: 833–846. [Google Scholar]
- 73. Manzello DP (2010) Ocean acidification hot spots: Spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnol Oceanogr 55: 239–248. [Google Scholar]
- 74. Cortés J, Risk MJ (1985) A reef under siltation stress: Cahuita, Costa Rica. Bull Mar Sci 36: 339–356. [Google Scholar]
- 75. Tomascik T, Sander F (1987) Effects of eutrophication on reef-building corals. Mar Biol 94: 53–75. [Google Scholar]
- 76. Alution S, Boberg J, Nyström M, Tedengren M (2001) Effects of multiple stressors copper and reduced salinity on the metabolism of the hermatypic coral Porites lutea. Mar Environ Res 52: 289–299. [DOI] [PubMed] [Google Scholar]
- 77. Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M (1995) Bioerosion rates on coral reefs: interactions between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeo 113: 189–198. [Google Scholar]
- 78. McCook LM, Jompa J, Diaz-Pulido G (2001) Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19: 400–419. [Google Scholar]
- 79. Jiménez C, Bassey G, Segura A, Cortés J (2010) Characterization of the coral communities and reefs of two previously undescribed locations in the upwelling region of gulf of Papagayo (Costa Rica). Rev Cienc Mar Cost 2: 95–108. [Google Scholar]
- 80. Leichter JJ, Stewart HL, Miller SL (2003) Episodic nutrient transport to Florida coral reefs. Limnol Oceanogr 48: 1394–1407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microbalance and weighing construction for buoyant weight technique. (After Davies [36]).
(TIF)
Coral nubbins of Porites lutea on individual transplant holders. Nubbins with holders attached to racks built of acrylic glass and metal rocks (a), and coral nubbins on holders right after recollection with epiphytes (b, c) and with epiphytes removed (d).
(TIF)
Examples for changes on dead coral skeleton during exposure period. Four different rectangular blocks (ordered among one another) of dead coral skeleton of Porites lutea before (East 20 m: A, D, West 20 m: G, J), after 12 months (East 20 m: B, C, West 20 m: E, F) and after 21 months (East 20 m: H, I, West 20 m: K, L) exposure. The second column (B, E, H, K) shows the upper sides, the third column (C, F, I, L) the under sides of the skeletal blocks. The first two rows show blocks right after collection (B, C, E, F), the lower two rows show blocks after drying them (H, I, K, L).
(TIF)
Bioerosion racks made of PVC-tubes and metal bars. Each rack with six dead coral blocks of Porites lutea attached. View from above the setup (A), and from diagonally below (B).
(TIF)
Calcium carbonate accretion due to fouling organisms on dead skeletal blocks. Shown are calcium carbonate precipitating organisms on dead skeletal blocks of Porites lutea: from top to bottom: Balanids (A–D), serpulids (E–H), bivalves (I–L), and corals (M–O).
(TIF)
Comparison of total accretion due to fouling organisms and accretion by different groups of carbonate producers. Total accretion (upper panels) and accretion by different groups of carbonate producers (lower panels, as fractions of total accretion) on dead coral substrates at island Ko Miang. Error bars: ±1 SE of mean. Results from 12 months exposure (February 2007 to February 2008) (A), and 21 months exposure (February 2007 to November 2008) (B) (number of replicates in each case: 9).
(TIFF)
Analysis of variance (ANOVA) of coral nubbin mortality.
(DOCX)
Surface area, initial (start) and end air weights of coral nubbins of Porites lutea .
(DOCX)
Comparison of initial weights of coral nubbins between different sides and depths at Similan Islands.
(DOCX)
Comparison of measured mass change and calculated mass change of calcium carbonate (CaCO3) blocks in east and west of Similan island Ko Miang.
(DOCX)
Comparison of accretion due to fouling organisms at east (E) and west (W) side of Similan island Ko Miang.
(DOCX)
Comparison of accretion due to fouling organisms on dead skeletal blocks between 12 and 21 months exposure.
(DOCX)