Abstract
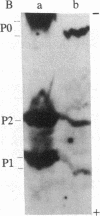
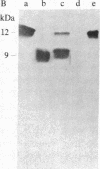
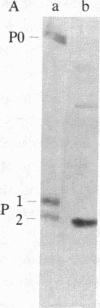
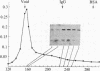
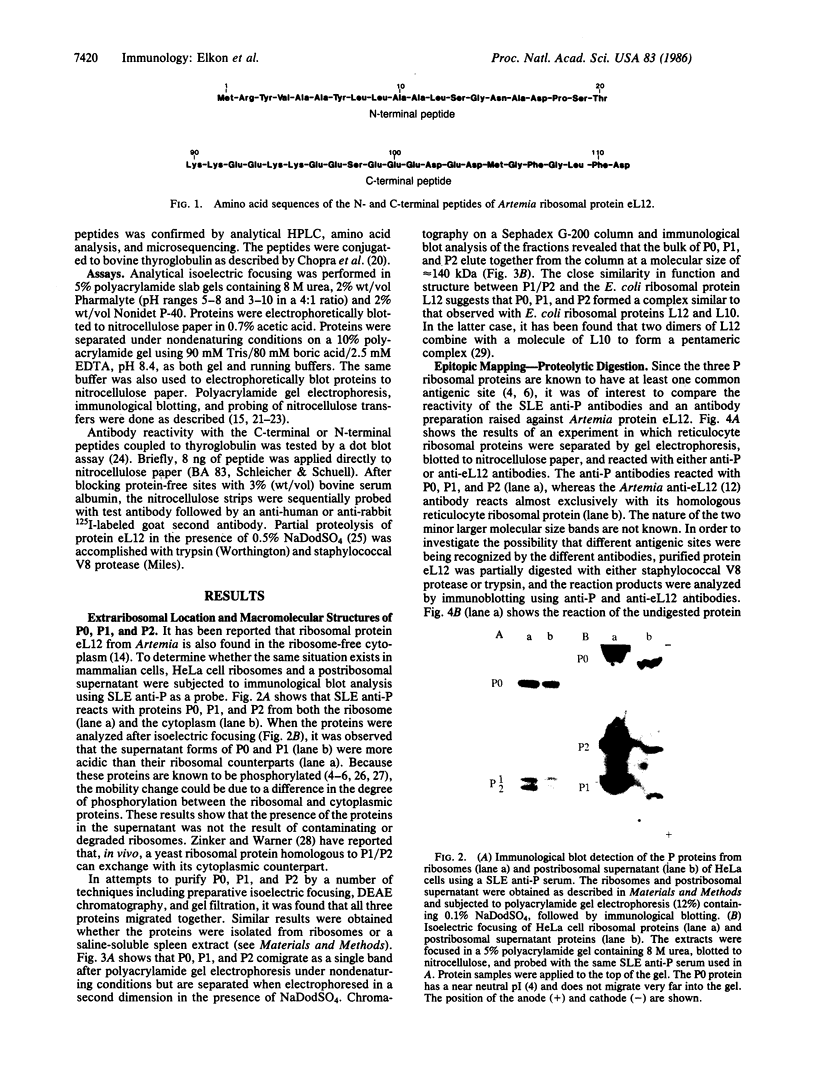
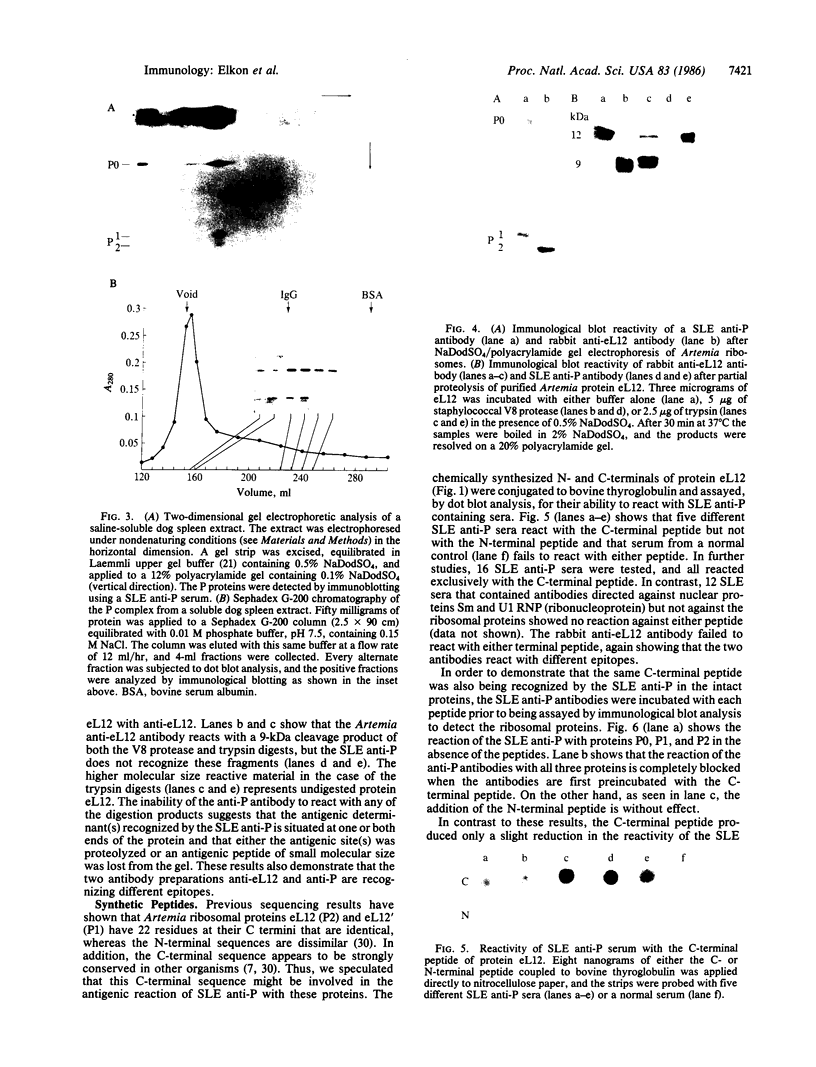
The characteristics of eukaryotic ribosomal proteins P0, P1, and P2 (P proteins) and their antigenic determinants were studied using the sera of patients with systemic lupus erythematosus (SLE). P0, P1, and P2 were isolated as a macromolecular complex by preparative isoelectric focusing and anion-exchange chromatography in the presence of 6 M urea. The apparent molecular size of the complex was 140 kDa as determined by gel filtration on a Sephadex G-200 column. P0 may, therefore, be the eukaryotic equivalent of Escherichia coli ribosomal protein L10. In addition, all three P proteins were detected in the postribosomal supernatant of HeLa cells, and P0 and P1 were found to be more acidic than their ribosome-bound counterparts. Partial proteolysis experiments revealed that SLE anti-P sera recognized one or both ends of the P2 equivalent protein from Artemia salina (eL12). Sixteen SLE sera containing antibodies to P0, P1, and P2 reacted with a carboxyl-terminal peptide 22 amino acids in length of eL12 and not with an amino-terminal peptide of 20 amino acids. Even though the carboxyl-terminal peptide completely inhibited the ability of the antiserum to react with all three proteins on an immunological blot, the same peptide produced only small decreases in binding of the SLE antibody to the native, nondenatured P proteins. These findings indicate that SLE anti-P antibodies react with a single sequential (linear) antigenic determinant on all three P proteins, but that additional antibodies recognize a conformational determinant(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Pluijms W., Möller W. The primary structure of ribosomal protein eL12/eL12-P from Artemia salina 80 S ribosomes. FEBS Lett. 1979 Aug 1;104(1):85–89. doi: 10.1016/0014-5793(79)81089-3. [DOI] [PubMed] [Google Scholar]
- Brot N., Weissbach H. Chemistry and biology of E. coli ribosomal protein L12. Mol Cell Biochem. 1981 Apr 13;36(1):47–63. doi: 10.1007/BF02354831. [DOI] [PubMed] [Google Scholar]
- Cenatiempo Y., Twardowski T., Redfield B., Reid B. R., Dauerman H., Weissbach H., Brot N. Simplified in vitro system for study of eukaryotic mRNA translation by measuring di- and tripeptide formation. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3223–3226. doi: 10.1073/pnas.80.11.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Nelson J. C., Solomon D. H., Beall G. N. Production of antibodies specifically binding triiodothyronine and thyroxine. J Clin Endocrinol Metab. 1971 Mar;32(3):299–308. doi: 10.1210/jcem-32-3-299. [DOI] [PubMed] [Google Scholar]
- Christian C. L., Elkon K. B. Autoantibodies to intracellular proteins. Clinical and biologic significance. Am J Med. 1986 Jan;80(1):53–61. doi: 10.1016/0002-9343(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Culhane L. Partial immunochemical characterization of the Ro and La proteins using antibodies from patients with the sicca syndrome and lupus erythematosus. J Immunol. 1984 May;132(5):2350–2356. [PubMed] [Google Scholar]
- Elkon K. B., Jankowski P. W. Fine specificities of autoantibodies directed against the Ro, La, Sm, RNP, and Jo-1 proteins defined by two-dimensional gel electrophoresis and immunoblotting. J Immunol. 1985 Jun;134(6):3819–3824. [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Heckman K. J., Lee J. C., Tan E. M. Identification of ribosomal protein autoantigens. J Immunol. 1985 Oct;135(4):2378–2384. [PubMed] [Google Scholar]
- Gohill J., Cary P. D., Couppez M., Fritzler M. J. Antibodies from patients with drug-induced and idiopathic lupus erythematosus react with epitopes restricted to the amino and carboxyl termini of histone. J Immunol. 1985 Nov;135(5):3116–3121. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Gudkov A. T., Behlke J., Vtiurin N. N., Lim V. I. Tertiary and quaternary structure for ribosomal protein L7 in solution. FEBS Lett. 1977 Oct 1;82(1):125–129. doi: 10.1016/0014-5793(77)80901-0. [DOI] [PubMed] [Google Scholar]
- Hardy S. J. The stoichiometry of the ribosomal proteins of Escherichia coli. Mol Gen Genet. 1975 Oct 3;140(3):253–274. doi: 10.1007/BF00334270. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Horak I., Schiffmann D. Acidic phosphoproteins of the 60-S ribosomal subunits from HeLa cells. Eur J Biochem. 1977 Oct 3;79(2):375–380. doi: 10.1111/j.1432-1033.1977.tb11818.x. [DOI] [PubMed] [Google Scholar]
- Itoh T. Primary structure of yeast acidic ribosomal protein YP A1. FEBS Lett. 1980 May 19;114(1):119–123. doi: 10.1016/0014-5793(80)80873-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin A., Wittmann-Liebold B., McNally J., Wool I. G. The primary structure of the acidic phosphoprotein P2 from rat liver 60 S ribosomal subunits. Comparison with ribosomal 'A' proteins from other species. J Biol Chem. 1982 Aug 10;257(15):9189–9197. [PubMed] [Google Scholar]
- MacConnell W. P., Kaplan N. O. The activity of the acidic phosphoproteins from the 80 S rat liver ribosome. J Biol Chem. 1982 May 25;257(10):5359–5366. [PubMed] [Google Scholar]
- Möller W., Castleman H., Terhorst C. P. Characterization of an acidic protein in 50 s ribosomes of E. coli. FEBS Lett. 1970 Jun 8;8(4):192–196. doi: 10.1016/0014-5793(70)80261-7. [DOI] [PubMed] [Google Scholar]
- Nye L., Pontes de Carvalho L. C., Roitt I. M. Restrictions in the response to autologous thyroglobulin in the human. Clin Exp Immunol. 1980 Aug;41(2):252–263. [PMC free article] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B. Small-angle X-ray scattering and crosslinking study of the proteins L7/L12 from Escherichia coli ribosomes. FEBS Lett. 1976 Jul 1;66(1):48–51. doi: 10.1016/0014-5793(76)80582-0. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hardy S. J., Liljas A. The ribosomal protein L8 is a complex L7/L12 and L10. FEBS Lett. 1976 Apr 15;64(1):135–138. doi: 10.1016/0014-5793(76)80267-0. [DOI] [PubMed] [Google Scholar]
- STURGILL B. C., CARPENTER R. R. ANTIBODY TO RIBOSOMES IN SYSTEMIC LUPUS ERYTHEMATOSUS. Arthritis Rheum. 1965 Apr;8:213–218. doi: 10.1002/art.1780080205. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Maassen J. A., Möller W. Involvement of 50S ribosomal proteins L6 and L10 in the ribosome dependent GTPase activity of elongation factor G. Biochem Biophys Res Commun. 1973 Jul 2;53(1):90–98. doi: 10.1016/0006-291x(73)91405-8. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R. Copies of proteins L7 and L12 and heterogeneity of the large subunit of Escherichia coli ribosome. J Mol Biol. 1975 Jun 15;95(1):1–8. doi: 10.1016/0022-2836(75)90330-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., Vidales F. J., Ballesta J. P. Functional role of acidic ribosomal proteins. Interchangeability of proteins from bacterial and eukaryotic cells. Biochemistry. 1981 May 26;20(11):3263–3266. doi: 10.1021/bi00514a043. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Wittmann-Liebold B., Möller W. 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2 , isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):13–19. doi: 10.1111/j.1432-1033.1972.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Ramjoué H. P., Kuster H., Liverani D., Gordon J. Monoclonal antibodies against eucaryotic ribosomes. Use to characterize a ribosomal protein not previously identified and antigenically related to the acidic phosphoproteins P1/P2. J Biol Chem. 1982 Nov 10;257(21):12709–12715. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K., Collatz E., Todokoro K., Ulbrich N., Lightfoot H. N., Wool I. G. Isolation of eukaryotic ribosomal proteins. Purification and characterization of the 60 S ribosomal subunit proteins La, Lb, Lf, P1, P2, L13', L14, L18', L20, and L38. J Biol Chem. 1978 Feb 10;253(3):946–955. [PubMed] [Google Scholar]
- Wong K. P., Paradies H. H. Shape properties of proteins L7 and L12 from E. coli ribosomes. Biochem Biophys Res Commun. 1974 Nov 6;61(1):178–184. doi: 10.1016/0006-291x(74)90550-6. [DOI] [PubMed] [Google Scholar]
- Zinker S., Warner J. R. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J Biol Chem. 1976 Mar 25;251(6):1799–1807. [PubMed] [Google Scholar]
- van Agthoven A. J., Maassen J. A., Möller W. Structure and phosphorylation of an acidic protein from 60S ribosomes and its involvement in elongation factor-2 dependent GTP hydrolysis. Biochem Biophys Res Commun. 1977 Aug 8;77(3):989–998. doi: 10.1016/s0006-291x(77)80075-2. [DOI] [PubMed] [Google Scholar]
- van Agthoven A., Kriek J., Amons R., Möller W. Isolation and characterization of the acidic phosphoproteins of 60-S ribosomes from Artemia salina and rat liver. Eur J Biochem. 1978 Nov 15;91(2):553–565. doi: 10.1111/j.1432-1033.1978.tb12709.x. [DOI] [PubMed] [Google Scholar]