Abstract
Introduction
Endemic in Latin America, Chagas disease is now becoming a serious global health problem, and yet has no financial viability for the pharmaceutical industry and remains incurable. In 2012, two antimycotic drugs inhibitors of fungal sterol 14α-demethylase (CYP51) – posaconazole and ravuconazole – entered clinical trials. Availability of the X-ray structure of the orthologous enzyme from the causative agent of the disease, protozoan parasite Trypanosoma cruzi, determined in complexes with posaconazole as well as with several experimental protozoa-specific CYP51 inhibitors opens an excellent opportunity to improve the situation.
Areas covered
This article summarizes the information available in PubMed and Google on the outcomes of treatment of the chronic Chagas disease. It also outlines the major features of the T. cruzi CYP51 structure and the possible structure-based strategies for rational design of novel T. cruzi specific drugs.
Expert opinion
There is no doubt that screenings for alternative drug-like molecules as well as mining the T. cruzi genome for novel drug targets are of great value and might eventually lead to groundbreaking discoveries. However, all newly identified molecules must proceed through the long, expensive and low-yielding drug optimization process, and all novel potential drug targets must be validated in terms of their essentiality and druggability. CYP51 is already a well-validated and highly successful target for clinical and agricultural antifungals. With minimal investments into the final stages of their development/trials, T. cruzi-specific CYP51 inhibitors can provide an immediate treatment for Chagas disease, either on their own or in combination with the currently available drugs.
Keywords: Chagas disease, drug discovery, sterol 14α-demethylase (CYP51) inhibitors, sterol biosynthesis, structure-based drug design, Trypanosoma cruzi
1. Introduction
Chagas disease, also known as American trypanosomiasis, is a life-long infection, anthropozoonosis caused by the unicellular flagellate protozoan parasite Trypanosoma cruzi (class Kinetoplastida) [1–3]. The pathogen, first described in 1909 by Carlos Chagas, has a complex life cycle, involving four developmental stages: proliferative epimastigote and infective metacyclic trypomastigote in hematophagous triatomine vectors (‘kissing bugs’); and proliferative intracellular amastigotes and infective bloodstream trypomastigotes in humans and a variety of mammalian hosts (> 150 species) that form the disease reservoir. The insect stages of T. cruzi develop in the kissing bug hindgut and are transmitted to mammals in the bug feces deposited during blood feeding through the itchy bite wound or mucous membranes. In mammals, parasites soon exit the bloodstream and invade the cytoplasm of host cells. Although various cell types are infected, the chronic disease is associated mainly with cardiac and enteric tissues. Non-vector-borne routes of T. cruzi transmission include congenital (from mother to child, 1 – 10% of newborn babies in some endemic countries [4]), and transfusion of infected blood, organ transplantation, ingestion of food or liquid contaminated with T. cruzi, the latter being generally associated with massive parasitic infestation, resulting in more severe acute clinical presentation and high mortality [5,6].
Clinically, the disease is characterized by three phases: acute, indeterminate and chronic. During the acute phase, T. cruzi can easily be detected in blood. Then the parasitemia goes down, making the diagnostics more complicated. The severity of the acute phase, which lasts for 4 – 8 weeks, depends on different factors, such as the parasite load and strain, patient immune response and varies from a mild and often unrecognized flu-like illness to symptoms of hepatosplenomegaly, meningoencephalitis and myocarditis, fatality rate being < 5%. The indeterminate phase is generally asymptomatic and comprises a period that may last for 10 – 20 years. Then, ~ 30% of infected patients develop the chronic form of the disease, which may be cardiac (arrhythmias, congestive heart failure, cardiac enlargement, thromboembolism and sudden cardiac death), digestive (megaoesophagus and mega colon) or cardiodigestive [1,7–9]. About 60% of patients with chronic Chagas disease die 7 months to 2 years after the onset of symptoms [10]. The risk of the disease activation sharply increases in immunodeficient patients (immunosuppressant therapies [11], HIV coinfections [12]).
Chagas disease has been afflicting humans in South America for at least 9,000 years, 41% prevalence rate in tissue specimens from human mummies being reported [13]. Sadly enough, the infection still remains endemic in 18 countries, where it represents one of the leading causes of morbidity, long-term disability and mortality [14]. In the late 1970s, 20% of the Bolivian population was estimated to be infected with T. cruzi, and rural areas provided prevalence rates from 40 to 80% [15]. Over the past decades, however, the WHO statistics have certainly improved. While in 1990 WHO reported 18 million infected, 700,000 new cases and 50,000 deaths per year in South and Central America, by 2000 the corresponding numbers declined to 200,000 new cases and 21,000 deaths per year [16] and only ~ 10 million infected were reported in 2010 [17]. This improvement must be largely due to extensive vector and donor blood bank control programs in most endemic areas [1,6], but also because of the change in the prevailing view on the nature of the disease.
For several decades drug development for Chagas was basically halted by the ‘outstanding influence’ [2] of so-called neurogenic postulate followed by autoimmune hypothesis, both of which were essentially based on the data obtained due to insufficient sensitivity of the techniques employed and stated that the chronic form of Chagas is not to be considered as an infectious disease but rather as an inflammatory disease/imbalanced immune response. Consequently, specific antiparasitic treatment was declared irrelevant and abandoned, and only the treatment aimed to mitigate the symptoms (such as antiarrhythmic drugs, pacemakers or heart transplantation [1]) remained to be provided. For example, according to the autoimmune hypothesis, the parasite only triggers the autoimmune response in some hosts, and then its persistence does not play a role in the disease pathogenesis. Therefore, even successful antiparasitic treatment may not lead to any improvement in the clinical outcome [2,18].
Fortunately, however, more recent studies, using more sensitive methods demonstrated clear correlation between the inflammatory processes and the presence of the parasite [19], thus supporting the alternative opinion that eradication of T. cruzi from the infected patients is a prerequisite to cure the disease [20–23], etc.). The prevailing view has begun to change; nevertheless, the damage was already done. The disease acquired the status of incurable, and consequently, has been highly neglected, which in turn delayed the development of i) specific etiological treatment, ii) efficient diagnostics and iii) cure evaluation criteria [9]. Within the past years, certain progress has been achieved in all these three fields. This article will only concentrate on the first aspect: specific antiparasitic chemotherapy, mostly focusing on CYP51 inhibitors.
Other modern strategies to develop antitrypanosomal treatment of Chagas disease include phenotypic (whole organism) screening of libraries of biologically active small molecules for new chemotypes with trypanocidal activity, fast in vivo screening of the compounds identified in vitro, mining the T. cruzi genome for new drug targets; searching for efficient inhibitors of potential targets (proteins/DNA) that have been confirmed as essential for the parasite survival. These approaches are highly promising and may eventually lead to pioneering discoveries. But the lack of knowledge of the molecular target/mechanism for the compounds identified via phenotypic screening can make the lead optimization process highly challenging. On the other hand, target selection is a minefield of potential issues, because not only should the target be essential, but it should also be druggable and available to assay in vitro. Thus, the process will certainly take time and requires significant financial investments. Pharmaceutical companies, however, remain reluctant to invest resources in the development of antichagasic drugs, especially because of the lack of assurances that they can make a return on their investment.
In the meantime, Chagas disease is becoming a severe global medical challenge, mainly due to human/vector migration [24], but also because of the lack of awareness and diagnostics in nonendemic areas [25,26]. Some estimates indicate that there are up to 1 million cases of Chagas disease in the United States [27], with as many as 267,000 cases in Texas alone [28]. The majority of those infected are reported to be immigrants from Latin America [29]. Yet it is well known that infected insects are widely spread across the southern states of the country, largely feeding on wild animals (in 2007, almost 30% of the armadillos and 38% of the opossums in Louisiana were infected with T. cruzi [27]), but occasionally affecting domestic dogs (e.g., there were 537 confirmed canine cases of Chagas disease between 1993 and 2007 in Texas [30]), and humans [31,32]. Many cases of autochthonous vector-borne transmission in the United States are presumed to occur undetected, sustaining the risk of infection through blood and organ donation and from mother to child [29]. More than 5,500 individuals infected with T. cruzi were reported in Canada, > 3000 in Japan, > 1500 in Australia [33] and > 80,000 in Spain [34]. In Europe, it is well recognized that the majority of patients remain untreated, the index of undiagnosed cases being 94 – 96% [26,35]. For millions of those who are now infected, dreading the onset or worsening of symptoms, by far of greatest importance is availability of any options for immediate treatment.
2. Current clinical etiological treatment for Chagas disease
Currently, only two drugs are available for therapeutic use against T. cruzi in humans: nifurtimox and benznidazole [29]. Both of them are nitroheterocyclic compounds (Figure 1A), discovered in 1965 and in 1971, respectively [36]. Nifurtimox is a 5-nitrofuran derivative; benznidazole is a 2-nitroimidazole derivative. As most nitroheterocycles, they both function as prodrugs and must undergo activation of their nitro group within the pathogen before mediating their cytotoxic effects, most likely via damage to DNA and other macromolecules [37–39].
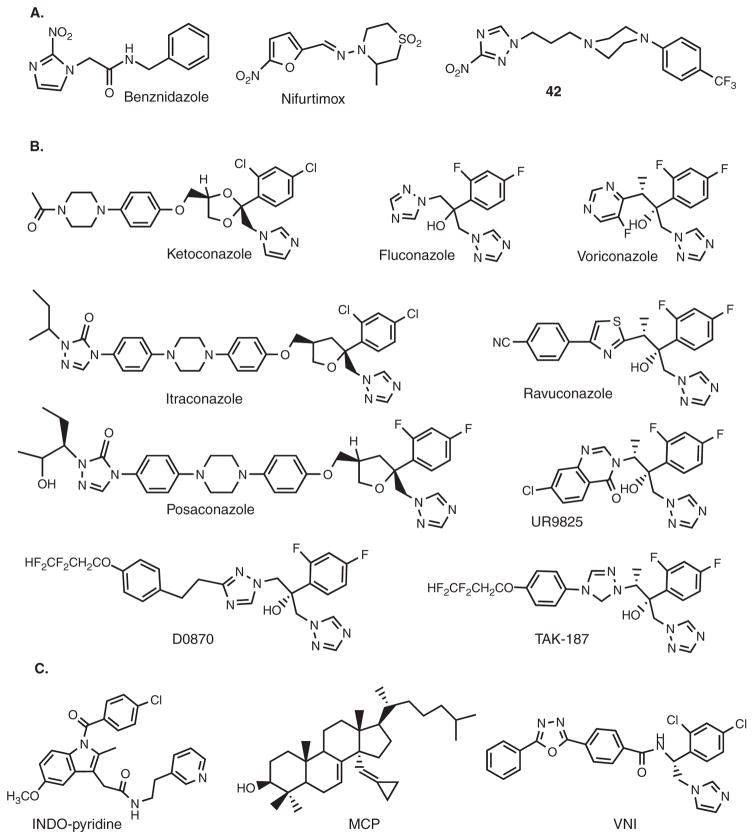
Figure 1. Compounds active against T. cruzi.
A. Nitroheterocycles: antichagasic drugs benznidazole and nifurtimox and an experimental compound 42 in ref [43]. B. Antifungal drugs and drug candidates. C. Examples of experimental inhibitors of T. cruzi CYP51.
Unfortunately, both benznidazole and nifurtimox have variable efficiency in different T. cruzi strains [40] and considerable adverse side effects, especially in adults, which frequently result in treatment discontinuation [9]. Particularly, 50 – 70% of patients treated with nifurtimox suffer from gastrointestinal disorders, neurologic toxicity occurs in up to 50% of patients. Benznidazole is better tolerated than nifurtimox and, therefore, it is recommended as the first-choice treatment [8], although it may produce severe dermatitis and sometimes bone marrow disorders [41].
Neither drug is approved by the US Food and Drug Administration (FDA), but both can potentially be obtained by special request from the Center for Disease Control and used under investigational protocols [29]. According to recommendations in 2005 and 2007, antitrypanosomal treatment is now strongly advised for all cases of acute, congenital and reactivated infection, for all children with infection, and for patients up to 18 years of age with chronic disease. Drug treatment should generally be offered to adults aged 19 – 50 years without advanced Chagas heart disease, and is optional for those > 50 years. There are still debates on whether the drugs should be used in the late chronic stage, mainly due to their adverse side effects and difficulties in cure assesment. Treatment failures are still widely reported. However, by now, there are also multiple reports indicating that treatment with benznidazole/nifurtimox can prevent and even reverse the development of chronic symptoms of the disease [1,4,7,9,20,42]. More extended trials have been proposed [9]. Attempts to improve the drug properties through altered formulations, etc. are also being undertaken [8]. Moreover, de novo design and broad testing of safer nitroheterocycles, for example, 3-nitrotriazole amines (Figure 1A) is currently supported by the Drugs for Neglected Disease initiative (DNDi) and appears to be rather promising [43].
3. Screening of existing antifungal drugs
Azole derivatives have served as the most successful antifungal drugs for more than four decades [44–46]. They act by blocking ergosterol biosynthesis in fungi via inhibition of the cytochrome P450 enzyme, called sterol 14α-demethylase (CYP51). CYP51 is not only essential for sterol biosynthesis but also happened to be highly druggable. While the electronegative nitrogen of the azole heterocyclic ring forms a coordination bond with the catalytic heme iron, decreasing its reduction potential, the noncoordinated, hydrophobic portion of the azole-containing molecule forms multiple van der Waals contacts with the amino acid residues inside the hydrophobic substrate binding cavity of the enzyme, preventing substrate binding and metabolism. Together, the Fe–N coordination and hydrophobic interactions with the apoprotein moiety can ensure tight binding and very strong, often functionally irreversible inhibitory effect on the catalysis [47]. It is well known that small azole derivatives, such as imidazole or phenyl-imidazole, have little influence on CYP51 activity; whereas the effects of larger molecules strongly depend on their composition [48]. It is noteworthy that all antifungal azole drugs were discovered empirically in cellular studies, based on antifungal effects they produced upon screening. Their mechanism of action was proven by sterol analysis (accumulation of the 14α-methylated sterol precursors) [49]. Testing their potencies as CYP51 inhibitors have not been included into the drug discovery process because, being highly hydrophobic membrane-bound proteins, fungal CYP51s are rather difficult to handle and assay in vitro. Absence of structural information on any fungal CYP51 complicates further drug development. As a result, there are currently only five azoles approved for clinical systemic use: ketoconazole, itraconazole, posaconazole, fluconazole and voriconazole (Figure 1B). Some other closely related compounds, such as ravuconazole, TAK-187 and UR9825 (Figure 1B) are under trials/development.
Similar to fungi, T. cruzi is completely dependent on the endogenously produced sterols (ergosterol and its 24-alkylated derivatives) [50] that are vital for the parasite membranes, cell division, growth and development processes [47]. Therefore, the idea of screening the existing antifungal agents as potential drugs for specific etiological treatment of Chagas disease was very reasonable, especially because they are already on the market and are much safer than benznidazole or nifurtimox.
The first report showing that two topical antifungal drugs, miconazole and econazole, inhibit growth of T. cruzi (Tulahuen strain) was published in 1981 [51]. In 1984, antiparasitic effect of ketoconazole was reported in vivo in mouse models of Chagas disease induced by four different strains of the parasite [52], including the Y strain that is less sensitive to nitroderivatives [40]. In these experiments, at 60 mg/kg/bid dosage given for 7 days ketoconazole protected mice against death, even if the treatment was started at parasitemia onset. Parasitological cure was not achieved, presumably due to the short (4 h) life time of the drug in mouse plasma. Because the incidence of side effects in humans treated with ketoconazole for up to years was known to be low, the authors suggested that the drug should be tested as a therapeutic agent for Chagas disease [52]. However, testing of ketoconazole in humans demonstrated that it was only able to eradicate parasitemia from two of eight treated chronic chagasic patients [53]. A few years later, itraconazole, a triazole derivative of ketoconazole was found to display better results. Treatment of infected mice with 120 mg itraconazole/kg/day for 7 – 9 weeks resulted in the parasitological cure as determined by negative hemocultures and subinoculations, negative serology for T. cruzi, and absence of parasites in histological sections following completion of therapy [54]. Treatment of humans with chronic Chagas disease with itraconazole produced parasitological cure in 53% and normalization of electrocardiogram (ECG) in 48% of patients (four years follow-up period) [55]. Further, at 20 years’ follow-up, 54.3% of the patients were negative in all the parasitological tests used, and 60% of these cases had normal ECG traces [56]. Fluconazole did not cure mice upon treatment with the 200 mg/kg daily dosage for 30 days [57], which in fact is in agreement with its relatively weak inhibitory potency as T. cruzi CYP51 inhibitor in vitro [58,59]. Its close-derivative voriconazole, however, has been recently reported to reveal some suppressive effect in a mouse model (75% survival rate at the 40 mg/kg/day dosage administered for 30 days; Tulahuen strain of T. cruzi) [60].
Several antifungal drug candidates, such as TAK-187, UR9825 [61,62], also show strong potential for the treatment of Chagas disease. Most potent of them, D0870, cured both short- and long-term experimental Chagas disease in mice [63], yet unfortunately failed in clinical trials as an antifungal drug due to cardiotoxicity (inhibitory effect on the hERG channel causing QTc prolongation [64]). Finally, in 2012, posaconazole (the derivative of itraconazole) and ravuconazole (the derivative of voriconazole) entered clinical trials for Chagas disease in Spain, Bolivia and Argentina.
Undoubtedly, successful repurposing of clinical antifungal drugs for Chagas disease would be the most cost-efficient way to provide an immediate treatment that is needed urgently. However, their second-use application has certain limitations. Thus, ravuconazole requires further optimization as it only produces suppressive effect in animal models of Chagas disease, probably largely due to its pharmacokinetics, particularly low bioavailability [65,66]. Posaconazole, which has been extensively studied as antichagasic drug candidate by J. A. Urbina’s team and was proved to be highly potent in vivo against the infection caused by multiple, including several nitroderivative-resistant strains of T. cruzi [66], is unfortunately highly expensive due to its complex and low-yielding synthetic scheme [67] and might have issues with bioavailability. Although posaconazole cured a chronic chagasic patient [68], the hospital patient cost was 8,000 euro [69], and the authors reporting the success of the drug indicate that ‘its current cost is too high for its widespread use in endemic countries’ [68]. Other agents with the same mechanism of action but lower potential cost are needed [69].
4. Experimental inhibitors of T. cruzi CYP51 and CYP51-structure-based drug design and development
Contrary to fungal CYP51s, sterol 14α-demethyases from three Trypanosomatidae pathogens, T. cruzi, T. brucei and Leishmania infantum (25% average amino acid sequence identity to fungal orthologs) have been well characterized biochemically and structurally [58,59,70–72]. Studies on inhibition of their activity in the reconstituted catalytic reaction in vitro revealed that susceptibilities of the protozoan CYP51s to antifungal drugs often differ significantly from those of fungal orthologs, and led to identification of several novel experimental inhibitory scaffolds (Figure 1C) [58,73–75], including indomethacin amid derivatives (INDO-pyridine scaffold) [75], substrate analog 14α-methylenecyclopropyl-24,25-dihydrolanosterol (MCP) [74] and carboxamide containing β-phenyl imidazoles (VNI scaffold) [58]. INDO-pyridine derivatives are interesting because of their potential dual action in vivo: as nonazole heme binding CYP51 inhibitors and as COX2 inhibitors (anti-inflammatory effect). MCP is the first example of an effective mechanism-based CYP51 inhibitor that acts as a T. cruzi CYP51 selective suicide substrate. Testing of their effects in animal models of Chagas disease is currently in progress, while VNI has recently been proven to be able to cure, with 100% cure rate and 100% survival, both the acute and chronic forms of Chagas disease in mice [76]. Nontoxic, orally bioavailable, with favorable pharmacokinetics, no mutagenicity or influence on the hERG channel, easy to synthesize and modify, VNI is an excellent drug candidate, clearly deserving broader attention to accelerate its progress towards clinical trials.
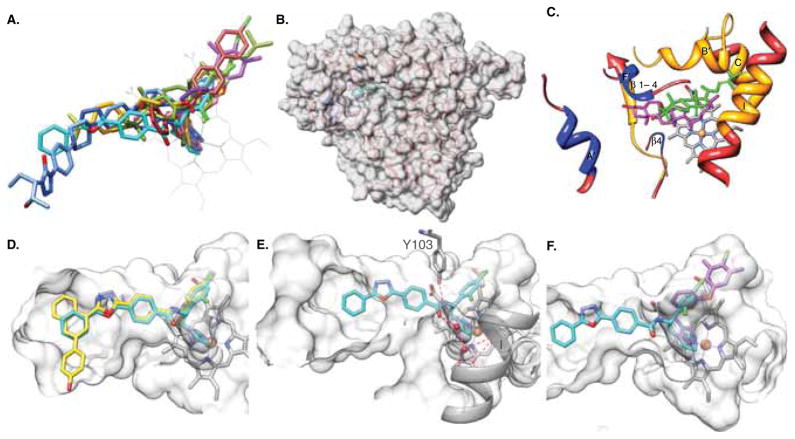
CYP51 structural studies not only explained the potencies of VNI and other T. cruzi CYP51 inhibitors [70,77,78] but also uncovered the molecular basis for the CYP51 exceptional druggability and outlined directions that could be undertaken to rationally design even better drugs. First and foremost, contrary to many, particularly human drug-metabolizing CYPs, which due to their enormous substrate promiscuity display high structural plasticity, CYP51s structure, and especially its substrate binding cavity, is highly rigid [78]. This rigidity must be essential for the conserved CYP51 catalytic function, in order to preserve the enzyme’s strict substrate specificity and the rare amongst P450s three-step stereospecific reaction. During the CYP51 reaction, the sterol molecule must be maintained in the proper catalytic position during the three cytochrome P450 monooxygenation cycles while its 14α-methyl group is successively converted into the 14α-alcohol, the 14α-aldehyde derivative and finally the C–C bond is cleaved with the release of the 14α-demethylated product and formic acid [47]. As a result of this rigidity, ligand must be accommodated inside the enzyme binding cavity, the best topological fit defining the tightest binding and, accordingly, the strongest inhibitory potency (Figure 2A). Second, the substrate access channel in the CYP51 structures, both ligand-free and bound to different ligands, remains open and well defined (Figure 2B), bordered by helices A′, F″ and the tip of the beta 4 hairpin (these secondary structural elements are enlarged and marked in Figure 2C). Finally, the CYP51 substrate binding cavity extends deeper inside the P450 molecule than in other CYPs, reaching helix C and the N-terminal portion of helix I (Figure 2C).
Figure 2. T. cruzi CYP51 structure as a template for rational drug design.
The orientation of the protein in all panels is similar (~ distal view of a P450 molecule). A. CYP51-structure-based inhibitory pharmacophore including imidazoles VNI (cyan, PDB ID [3gw9]), VNF (brick, [3skw]), and NEE (purple, [4h6o]); triazoles posaconazole (blue, [3k1o]), and fluconazole (pink, [3lfd]); pyridine derivative UDO (goldenrod, [3zg2]) and substrate analog MCP (olive, [3p99]). B. Overall semitransparent surface representation of the CYP51 molecule, the enlarged view of the secondary structural elements forming the substrate access channel and the binding cavity is seen in c. C. The three secondary structural elements forming the entrance into the substrate binding channel are colored in blue. The CYP51 binding cavity (orange) protrudes deeper inside the enzyme molecule. T. cruzi CYP51 substrate eburicol (olive) is shown in comparison with cholesterol (magenta) in the superimposed CYP11A1 [3mzs]. The 14α atom of eburicol is positioned directly above the heme iron, while in CYP11A1, the P450 which is functionally closest to CYP51 and also catalyzes a three-step reaction cleaving the side chain of cholesterol at C22; it is the cholesterol C22 atom that is positioned above the hem. D. A hypothetical three-ring structure (yellow) that may fit into the area around the entrance into the CYP51 substrate access channel. E. VNI in the CYP51 substrate binding cavity; the hydrogen bond network is shown as red dashed lines. F. VNI and NEE in the superimposed CYP51 co-structures showing that the VNI β-phenyl ring arm can be elongated. The binding cavity in d, e, and f, d, is shown in semitransparent surface representation.
Using the VNI scaffold as an example, I will outline our understanding on how these three major CYP51 structural features can be rationally used to accelerate drug development process. VNI in the CYP51 binding cavity is oriented similar to posaconazole (Figure 2A). The imidazole ring nitrogen (N3) coordinates to the heme iron, a 2,4-dichlorinated β-phenyl ring is projected into the deepest portion of the enzyme substrate binding cavity, and a 3-ring linear polycycle is positioned along the substrate access channel and connected to the rest of the inhibitor molecule via a carboxamide fragment (Figure 2E). The carbonyl oxygen of this fragment forms a hydrogen bond with the catalytic water molecule, thus connecting the inhibitor with the CYP51 I-helix and disrupting the proton delivery route in the enzyme. The amide nitrogen of the fragment forms a hydrogen bond with the OH group of Y103, thus repositioning the side chain of this functionally essential residue, whose OH group in the enzymatically active CYP51 enzymes provides support to the heme being H-bonded with the porphyrin ring D propionate [71]. Based on our previous experience, location of the phenyl ring in the β-position relative to the azole ring as well as the presence/position of the Cl atoms in the ring are essential for the potency of this scaffold [58], while replacement of the chlorine atom with fluorine as well as replacement of the imidazole ring with the triazole ring (examples are shown in [79]) worsens the pharmacokinetics (Villalta and Lepesheva, unpublished). Thus the central core of the scaffold, including the imidazole ring, the β-phenyl ring, and the carboxamide fragment should be preserved.
Other portions of the molecule could potentially be derivatized to further increase the potency and enhance drug-like properties of the resulting compounds. Modifications can be performed in two regions (Figure 2C and F), either on the same molecule (as long as it complies with Lipinski rules/ADME considerations) or separately, with the aim to produce two alternative drug candidates; the latter might be advantageous in the case of acquired resistance. Extension of the VNI long arm (Figure 2C) may mimic the surface-binding sub-site, which is seen around the access channel entrance in the T. cruzi CYP51 co-structure with posaconazole [3k1o] [70]. Filling the deepest portion of the CYP51 binding cavity (Figure 2F) appears to be especially promising i) because the molecules much smaller than VNI (VNF [70] and later NEE [77]) that are accommodated here (Figure 2A) were proven to be highly potent as T. cruzi CYP51 inhibitors; ii) because this portion of the cavity is CYP51-specific, which may potentially mean substantial decrease of the influence on other human CYPs, for example, as it was observed upon addition of another aromatic ring to the β-phenyl ring of VNI: the modification resulted in > 8-fold increase in the IC50 for CYP3A4 inhibition [79].
Weaker influence on other human CYPs is certainly desirable, although its importance for the drugs aimed at infectious diseases (especially for poor people who are unlikely to permanently use a large number of additional medications) seems to be exaggerated: Drug-metabolizing CYPs are not essential, besides they are inducible and their activities are being restored as soon as the administration of a drug is stopped [80]. Temporary inhibition of human CYP51 is also unlikely to be a real problem, since humans can consume cholesterol from the diet, and inhibitors of human CYP51 were once even considered as potential cholesterol-lowering drugs, although such studies were abandoned, mainly because of high potency of statins, the drugs that block sterol biosynthesis upstream the CYP51 reaction [81].
High heterogeneity of the T. cruzi population is another reason why further development of CYP51 inhibitors might be helpful. More than 70 different strains of the parasite (www.dbbm.fiocruz.br/TcruziDB/strain.html, 01/02/2013), which are now joined into six major groups (TcI – TcVI), vary broadly in their sensitivities to clinical nitroheterocyclyc drugs benznidazole and nifurtimox [40] and, apparently, to antifungal azoles as well, including D0870 and posaconazole [82,83]. The CYP51 gene sequences from different T. cruzi strains also revealed surprising variability: for example, CYP51s in Colombiana and Sylvio (TcI group) have 8 and 6 amino acid differences from CYP51 in Tulahuen (TcVI) [84]. Expression of these genes is currently in progress and hopefully may provide an explanation to the altered sensitivities of these T. cruzi strains to azole inhibitors. Given that CYP51 is regarded as the housekeeping gene (e.g., CYP51 in human differs from the orthologs in macaca and dog only in 3 and 8 amino acid residues, respectively), this finding agrees with the hypothesis that different T. cruzi lineages might be evolutionarily very distant (diverged up to 88 million years ago [85]), implying that sensitivity to other potential drug targets is likely to have even higher variability across the parasite strains.
5. Other potential drug targets
Currently, CYP51 inhibitors are the most advanced drug candidates for etiological treatment of Chagas disease [9,17,61]. Several other enzymes involved into the sterol biosynthetic pathway have potential to serve as possible future targets for antitrypanosomal chemotherapy. The most apparent examples are HMG-CoA reductase, which in humans is the clinical target for statins, farnesyl diphosphate synthase, the target for bisphosphonates, squalene synthase, which is inhibited by quinuclidine derivatives and sterol 24-methyltransferase, inhibited by azasterols [47]. So far, however, antiparasitic activity of these inhibitors in vivo has only been reported when used in combination with antifungal azoles, often producing synergistic effect [61] and thus suggesting that blockage of sterol biosynthesis at different stages might be highly advantageous, not only for Chagas disease treatment, but also against other sterol-dependent human pathogens, including fungi. Synergetic antiparasitic effect of an antiarrhythmic drug amiodaron with posaconazole appears to be a particularly interesting example, as amiodaron, which was recently found to inhibit lanosterol synthase, is most frequently used in chronic cardiac Chagas disease patients [86]. This opens the possibility of combining two drugs already approved for clinical use.
A number of alternative potential targets from other metabolic pathways that are crucial for T. cruzi survival and intracellular development have been reported, such as thiol-dependent redox metabolism (trypanothione reductase), glycolysis (glyceraldehyde-3-phosphate dehydrogenase); pentose phosphate pathway, purine salvage pathway and nucleotide synthesis, cysteine proteases (cruzain) and kinetoplastid DNA binders (aromatic diamidines) [87]. The success here is difficult to predict, particularly due to the lack of a centralized program which could perform any standardized comparative evaluation. For example, kDNA binders do not receive much publicity, although they could be highly attractive because they are specific for Kinetoplastid pathogens and, in addition to pronounced antiparasitic effects in vivo, induce the decrease in cardiac inflammation [88]. In contrast, broadly advertized cruzain inhibitor K777 was reported to be receiving an Investigational New Drug status [69], apparently despite of its hepatotoxicity [61] and only suppressive antiparasitic effect in mice and dogs. Thus, a long and uneasy path involving validation of druggability, lead optimization and so on lies ahead of alternative drug targets. Right now CYP51 inhibitors are much closer to offering a cure to Chagas disease.
6. Conclusion
Both screening and design of drugs for the treatment of Chagas disease are promising. Further, they could and should be mutually beneficial. It is the lack of effort coordination that severely halts the progress. Including promising drug candidates such as VNI and its derivatives into the sphere of interest of a pharmaceutical company, foundation or a partnership that claim to be devoted to the success of drug discovery for neglected tropical diseases and have a well developed integrated strategy evaluating their medicinal chemistry, safety, pharmacokinetics, pharmacodynamics, etc. [89]. would be highly helpful in advancing the leading compounds to clinical trials.
It will take years of follow-up until the results on posaconazole/ravuconazole clinical trials become available. In the meantime, several additional phenotypic high throughput screenings (HTS) will be completed, bringing new hits and new hopes. In is noteworthy that the statements (which now appear in the reports of some HTS-conducting teams) about excluding from their libraries those hits that ‘look like CYP51 inhibitors’ do not sound especially reasonable. On the contrary, those hits should be thoroughly studied as they have a higher probability of success vs. attrition. In fact, optimization of one HTS hit (plant antifungal agent fenarimol) by DNDi [90] has led to the development of two highly promising and potent nonazole drug candidates, whose costructures with T. cruzi CYP51 (PDB ID 3zg2 and 3zg3) [91] will facilitate the finishing steps in the drug discovery process. Impartial and comprehensive comparative preclinical testing of the most advanced experimental CYP51 inhibitors appears to be a good way to offer safer and affordable specific treatment for Chagas disease when the outcomes of clinical trials of posaconazole and ravuconazole become clearer.
7. Expert opinion
The major advance in the field is certainly the growing appreciation that Chagas disease is a persistent parasitic infection which should be treated as an infectious disease and requires parasitological cure, regardless of the stage, in order to prevent the development of clinical symptoms. Also, highly encouraging is the information on the efficacy of current, although not optimal and largely underutilized, antiparasitic drugs in achieving the cure, reducing the disease severity and even reversing chronic clinical symptoms by killing the parasite. The major weaknesses appear to result from the long-term neglect and gross underfunding. They include lack of easily available and reliable diagnostic procedures, shortages in the drug availability and, most important, the absence of a unanimous opinion on criteria for cure (e.g., if serological tests are used as the main assessment of cure, long-lasting immune memory requires lengthy follow-up periods in order to indicate that the treatment worked). Development of standardized sensitive quantitative PCR analyses and protocols to conduct preclinical animal studies which would allow a reliable comparison of new drug efficacies seems to be most promising.
The fact that the doctors are now recommended to prescribe specific treatment for Chagas disease is encouraging, not only for the infected patients, but also for further search for new, better and safer drugs. Positive results will eventually overcome the low impetus that is now based on the lack of belief that those drugs will be widely used even if proven efficacious. As detailed above, the availability of T. cruzi CYP51 structure makes structure-based rational lead optimization quite straightforward, although multidisciplinary approach involving chemistry, biochemistry, structural and cellular biology, pharmacology and toxicology, as well as comprehensive testing of the best drug candidates preformed by a centralized unbiased organization is required. Also, a better knowledge on the biology and genetics of various T. cruzi strains, which eventually might even be distinguished as different species, is needed. As for now, for the infections caused by the drug-resistant strains of the parasite, such as Colombiana, combination therapy that would include CYP51 inhibitors that are much more potent against multiplying intracellular amastigotes than against trypomastigotes [58,76] and nitroderivatives should be seriously considered. This will allow the decrease in the dosage of, for example, benznidazole and thus alleviation of its side-effects.
Because several organizations, including DNDi, have been formed that invest into screenings and the programs for drug discovery and implementations [89], there is no doubt that future directions will involve high throughput searches for new active compounds of alternative mechanisms of action as well as mining the T. cruzi genome for novel potential alternative drug targets. The efforts might eventually lead to important discoveries, advancing the basic knowledge on antimicrobial chemotherapy. New sets of potent drugs supported by efficient vector control programs will hopefully work together so that the devastating disease could be eliminated.
Article highlights.
Broader use of antiparasitic therapy to treat intermediate and chronic forms of Chagas disease brings positive results, thus supporting the concept that it should be treated as an infection and can be cured.
Current clinical drugs, benznidazole and nifurtimox, however, are rather toxic and therefore better agents are needed.
To date, inhibitors of sterol 14α-demethylase (CYP51) are the most advanced new drug candidates: two antifungal azoles, posaconazole and ravuconazole are being evaluated in clinical trials.
Repurposing of clinical antifungal drugs that are already on the market for another indication is the most cost-efficient way to add to the arsenal available for antichagasic chemotherapy; however, being selected as inhibitors of fungal CYP51, they have certain limitations.
Availability of the X-ray structure of T. cruzi CYP51, in complexes with novel experimental protozoa-specific CYP51 inhibitors that are active in vivo makes lead optimization process rather straightforward.
Including novel drug candidates, such as VNI and its derivatives, into the sphere of interest of drug developing companies would be highly helpful in advancing their passage towards clinical trials.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
GI Lepesheva declares support in the form of a grant from the US National Institutes of Health (R01 GM067871).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1•.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2012;375:1388–402. doi: 10.1016/S0140-6736(10)60061-X. The paper provides an interesting update on the current status of Chagas disease. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi MdL, Benvenuti LA, Martins Reis M, et al. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res. 2003;60:96–107. doi: 10.1016/s0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 3.Tanowitz HB, Kirchhoff LV, Simon D, et al. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–19. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto Dias JC. The treatment of Chagas disease (South American trypanosomiasis) Ann Intern Med. 2006;144:772–4. doi: 10.7326/0003-4819-144-10-200605160-00012. [DOI] [PubMed] [Google Scholar]
- 5.Pereira KS, Schmidt FL, Guaraldo AM, et al. Chagas’ disease as a foodborne illness. J Food Prot. 2009;72:441–6. doi: 10.4315/0362-028x-72.2.441. [DOI] [PubMed] [Google Scholar]
- 6.Coura JR, Borges-Pereira J. Chagas disease: what is known and what should be improved: a systemic review. Rev Soc Bras Med Trop. 2012;45:286–96. doi: 10.1590/s0037-86822012000300002. [DOI] [PubMed] [Google Scholar]
- 7.Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;6:583–7. doi: 10.1097/01.qco.0000247592.21295.a5. [DOI] [PubMed] [Google Scholar]
- 8.Salomon CJ. First century of chagas’ disease: an overview on novel approaches to nifurtimox and benznidazole delivery systems. J Pharm Sci. 2012;101:888–94. doi: 10.1002/jps.23010. [DOI] [PubMed] [Google Scholar]
- 9•.Bern C. Antitrypanosomal therapy for chronic Chagas’ disease. N Engl J Med. 2011;364:2527–34. doi: 10.1056/NEJMct1014204. The paper summarized the information on the current diagnostics and clinical etiological treatment of Chagas disease. [DOI] [PubMed] [Google Scholar]
- 10.Prata A. Chagas’ disease. Infect Dis Clin North Am. 1994;8:61–76. [PubMed] [Google Scholar]
- 11.Altclas J, Sinagra A, Dictar M, et al. Chagas disease in bone marrow transplantation: an approach to preemptive therapy. Bone Marrow Transplant. 2005;36:123–9. doi: 10.1038/sj.bmt.1705006. [DOI] [PubMed] [Google Scholar]
- 12.Sartori AMC, Ibrahim KY, Nunes, et al. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann Trop Med Parasitol. 2007;101:31–50. doi: 10.1179/136485907X154629. [DOI] [PubMed] [Google Scholar]
- 13.Aufderheide AC, Salo W, Madden M, et al. A 9,000-year record of Chagas’ disease. Proc Natl Acad Sci USA. 2004;101:2034–9. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Bustamante JM, Tarleton RL. Methodological advances in drug discovery for Chagas disease. Expert Opin Drug Discov. 2011;6:653–61. doi: 10.1517/17460441.2011.573782. The review outlines new approaches for fast in vivo screening of new antitrypanosomal drug candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastien J. The kiss of keath: Chagas’ disease in the Americas. Univ. of Utah Press; Salt Lake City: 1998. [Google Scholar]
- 16.WHO Technical Report Series No 905 Press Release. World Health Organization; Geneva: 2002. Second report of the WHO expert committee on the control of Chagas disease; p. 85. Available from: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=10&codcch=905#. [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) Chagas disease: Control and Elimination. UNDP/World Bank/WHO; 2010. They post their annual reports at: http://www.who.int/topics/chagas_disease/en/ [Google Scholar]
- 18.Urbina JA. Parasitological cure of Chagas disease: is it possible? Is it relevant? Mem Inst Oswaldo Cruz. 1999;94:349–55. doi: 10.1590/s0074-02761999000700068. [DOI] [PubMed] [Google Scholar]
- 19•.Tarleton RL, Zhang L, Downs MO. “Autoimmune rejection” of neonatal heart transplants in experimental Chagas disease is a parasite-specific response to infected host tissue. Proc Natl Acad Sci USA. 1997;94:3932–7. doi: 10.1073/pnas.94.8.3932. This work has proven that the chronic form of Chagas should be treated as an infectious disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viotti R, Vigliano C, Armenti H, et al. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–62. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 21.Andrade SG, Stocker-Guerret S, Pimentel AS, et al. Reversibility of cardiac fibrosis in mice chronically infected with Trypanosoma cruzi, under specific chemotherapy. Mem Inst Oswaldo Cruz. 1991;86:187–200. doi: 10.1590/s0074-02761991000200008. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan D, Ferrari I, Bergami PL, et al. Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus. Proc Natl Acad Sci USA. 1997;94:10301–6. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Docampo R, Schmuñis GA. Sterol biosynthesis inhibitors: potential chemotherapeutics against chagas disease. Parasitol Today. 1997;13:129–30. doi: 10.1016/s0169-4758(97)01021-1. [DOI] [PubMed] [Google Scholar]
- 24.Leslie M. Drug developers finally take aim at a neglected disease. Science. 2011;333:933–5. doi: 10.1126/science.333.6045.933. [DOI] [PubMed] [Google Scholar]
- 25.Tarleton RL, Reithinger R, Urbina JA, et al. The challenges of Chagas disease - grim outlook or glimmer of hope? PLoS Med. 2007;4:e332. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein N, Hurwitz I, Durvasula R. Globalization of Chagas disease: a growing concern in nonendemic countries. Epidemiol Res Int. 2012:1–13. Article ID 136793. [Google Scholar]
- 27•.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. Globalization of Ghagas disease problem (USA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Hanford EJ, Zhan FB, Lu Y, et al. Chagas disease in Texas: recognizing the significance and implications of evidence in the literature. Soc Sci Med. 2007;65:60–79. doi: 10.1016/j.socscimed.2007.02.041. Globalization of Ghagas disease problem (USA) [DOI] [PubMed] [Google Scholar]
- 29.Bern C, Kjos S, Yabsley MJ, et al. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24:655–81. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry MA, Weatherhead JE, Hotez PJ, et al. Childhood parasitic infections endemic to the United States. Pediatr Clin North Am. 2013;60:471–85. doi: 10.1016/j.pcl.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 32•.Barry MA, Bezek S, Serpa JA, et al. Neglected infections of poverty in Texas and the rest of the United States: management and treatment options. Clin Pharmacol Ther. 2012;92:170–81. doi: 10.1038/clpt.2012.85. Globalization of Ghagas disease problem (USA) [DOI] [PubMed] [Google Scholar]
- 33.Coura JR, Viñas PA. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 34.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 35•.Basile L, Jansa JM, Carlier Y, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16:1–10. Globalization of Ghagas disease problem (Europe) [PubMed] [Google Scholar]
- 36.Mady C, Ianni BM, de Souza JLJ. Benznidazole and Chagas disease: can an old drug be the answer to an old problem? Expert Opin Investig Drugs. 2008;17:1427–33. doi: 10.1517/13543784.17.10.1427. [DOI] [PubMed] [Google Scholar]
- 37.Raether W, Hanel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol Res. 2003;S1:S19–39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- 38.Mejia AM, Hall BS, Taylor MC, et al. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J Infect Dis. 2012;206:220–8. doi: 10.1093/infdis/jis331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson SR, Taylor MC, Horn D, et al. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci USA. 2008;105:5022–7. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–9. doi: 10.1016/0035-9203(87)90020-4. First report showing that drug susseptibility varyes significally among different T. cruzi strains. [DOI] [PubMed] [Google Scholar]
- 41.Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis) Hum Exp Toxicol. 2006;8:471–9. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 42.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment. A nonrandomized trial. Ann Intern Med. 2006;144:724–34. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulou MV, Trunz BB, Bloomer WD, et al. Novel 3-nitro-1H-1,2,4-triazole-based aliphatic and aromatic amines as anti-Chagasic agents. J Med Chem. 2011;54:8214–23. doi: 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett JE. Chemotherapy of systemic mycoses. N Engl J Med. 1974;290:320–3. doi: 10.1056/NEJM197402072900607. [DOI] [PubMed] [Google Scholar]
- 45.Maertens JA. History of the development of azole derivatives. Clin Microbiol Infect. 2004;10:101–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 46.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, et al. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010;9:719–27. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 47.Lepesheva GI, Villalta F, Waterman MR. Targeting Trypanosoma cruzi sterol 14α-demethylase (CYP51) Adv Parasitol. 2011;75:65–87. doi: 10.1016/B978-0-12-385863-4.00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Correia MA, Ortiz de Montellano PR. Inhibition of cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: structure, mechanism, and biochemistry. Plenum Publishing Corp; New York: 2005. pp. 246–322. [Google Scholar]
- 49.Vanden Bossche H, editor. Mode of action of pyridine, pyrimidine and azole antifungals. Ellis Horwood; Chichester: 1988. pp. 79–119. [Google Scholar]
- 50.Roberts CW, McLeod R, Rice DW, et al. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol Biochem Parasitol. 2003;126:129–42. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 51•.Docampo R, Moreno SN, Turrens JF, et al. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol Biochem Parasitol. 1981;3:169–80. doi: 10.1016/0166-6851(81)90047-5. First report on antitrypanosomal activity of an antifungal azole. [DOI] [PubMed] [Google Scholar]
- 52.McCabe RE, Remington JS, Araujo FG. Ketoconazole inhibition of intracellular multiplication of Trypanosoma cruzi and protection of mice against lethal infection with the organism. J Infect Dis. 1984;150:594–601. doi: 10.1093/infdis/150.4.594. [DOI] [PubMed] [Google Scholar]
- 53.Brener Z, Cançado JR, Galvao LM, et al. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem Inst Oswaldo Cruz. 1993;88:149–53. doi: 10.1590/s0074-02761993000100023. [DOI] [PubMed] [Google Scholar]
- 54.McCabe RE, Remington JS, Araujo FG. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg. 1986;35:280–4. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- 55.Apt W, Aguilera X, Arribada A, et al. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59:133–8. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- 56.Apt W, Arribada A, Zulantay I, et al. Treatment of Chagas’ disease with itraconazole: electrocardiographic and parasitological conditions after 20 years of follow-up. J Antimicrob Chemother. 2013;68(9):2164–9. doi: 10.1093/jac/dkt135. [DOI] [PubMed] [Google Scholar]
- 57.Campos R, Amato Neto V, Moreira AA, et al. Evaluation of the therapeutic activity of fluconazole in acute experimental infection caused by Trypanosoma cruzi. Rev Hosp Clin Fac Med Sao Paulo. 1992;47:174–5. [PubMed] [Google Scholar]
- 58.Lepesheva GI, Ott RD, Hargrove TY, et al. Sterol 14 alpha-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chem Biol. 2007;14:1283–93. doi: 10.1016/j.chembiol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lepesheva GI, Zaitseva NG, Nes WD, et al. CYP51 from Trypanosoma cruzi: a phyla-specific residue in the B’ helix defines substrate preferences of sterol 14alpha-demethylase. J Biol Chem. 2006;281:3577–85. doi: 10.1074/jbc.M510317200. [DOI] [PubMed] [Google Scholar]
- 60.Gulin JEN, Eagleson MA, Postan M, et al. Efficacy of voriconazole in a murine model of acute Trypanosoma cruzi infection. J Antimicrob Chemother. 2013;68:888–94. doi: 10.1093/jac/dks478. [DOI] [PubMed] [Google Scholar]
- 61.Urbina JA. New insights in Chagas’ disease treatment. Drug Fut. 2010;35:409–19. [Google Scholar]
- 62.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Urbina JA, Payares G, Molina J, et al. Cure of short- and long-term experimental Chagas’ disease using D0870. Science. 1996;273:969–71. doi: 10.1126/science.273.5277.969. [DOI] [PubMed] [Google Scholar]
- 64.Williams KJ, Denning DW. Termination of development of D0870. J Antimicrob Chemother. 2001;47:720–1. doi: 10.1093/oxfordjournals.jac.a002691. [DOI] [PubMed] [Google Scholar]
- 65.Diniz Lde F, Caldas IS, Guedes PM, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54:2979–86. doi: 10.1128/AAC.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbina JA. Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):311–18. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- 67.Dobish MC, Villalta F, Waterman MR, et al. Organocatalytic, enantioselective synthesis of VNI: a robust therapeutic development platform for Chagas, a neglected tropical disease. Org Lett. 2012;14:6322–5. doi: 10.1021/ol303092v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Pinazo MJ, Espinosa G, Gallego M, et al. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg. 2010;82:583–7. doi: 10.4269/ajtmh.2010.09-0620. Cure of a chronic chagasic patient with posaconazole. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clayton J. Chagas disease: pushing through the pipeline. Nature. 2010;465:S12–15. doi: 10.1038/nature09224. [DOI] [PubMed] [Google Scholar]
- 70.Lepesheva GI, Hargrove TY, Anderson S, et al. Structural insights into inhibition of sterol 14 alpha-demethylase in the human pathogen Trypanosoma cruzi. J Biol Chem. 2010;285:25582–90. doi: 10.1074/jbc.M110.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lepesheva GI, Park HW, Hargrove TY, et al. Crystal structures of Trypanosoma brucei sterol 14 alpha-demethylase and implications for selective treatment of human infections. J Biol Chem. 2010;285:1773–80. doi: 10.1074/jbc.M109.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hargrove TY, Wawrzak Z, Liu J, et al. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14α-demethylase (CYP51) from Leishmania infantum. J Biol Chem. 2011;286:26838–48. doi: 10.1074/jbc.M111.237099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lepesheva G, Hargrove T, Kleshchenko Y, et al. CYP51: a major drug target in the cytochrome P450 superfamily. Lipids. 2008;43:1117–25. doi: 10.1007/s11745-008-3225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hargrove TY, Wawrzak Z, Liu J, et al. Structural complex of sterol 14α-demethylase (CYP51) with 14α-methylenecyclopropyl-Δ7-24, 25-dihydrolanosterol. J Lipid Res. 2012;53:311–20. doi: 10.1194/jlr.M021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konkle ME, Hargrove TY, Kleshchenko YY, et al. Indomethacin amides as a novel molecular scaffold for targeting Trypanosoma cruzi sterol 14 alpha-demethylase. J Med Chem. 2009;52:2846–53. doi: 10.1021/jm801643b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Villalta F, Dobish MC, Nde PN, et al. VNI cures acute and chronic experimental Chagas disease. J Infect Dis. 2013;208:504–11. doi: 10.1093/infdis/jit042. Curative effect of a new experimental T.cruzi-specific CYP51 inhibitor VNI in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andriani G, Amata E, Beatty J, et al. Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. J Med Chem. 2013;56:2556–67. doi: 10.1021/jm400012e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr Top Med Chem. 2011;11:2060–71. doi: 10.2174/156802611796575902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Hargrove TY, Kim K, de Nazaré Correia Soeiro M, et al. CYP51 structures and structure-based development of novel, pathogen-specific inhibitory scaffolds. Int J Parasitol Drugs Drug Resist. 2012;2:178–86. doi: 10.1016/j.ijpddr.2012.06.001. Initial steps of CYP51 structure-based VNI scaffold development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guengerich FP. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006;8:E101–11. doi: 10.1208/aapsj080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta. 2007;1770:467–77. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molina J, Brener Z, Romanha AJ, et al. In vivo activity of the bis-triazole D0870 against drug-susceptible and drug-resistant strains of the protozoan parasite Trypanosoma cruzi. J Antimicrob Chemother. 2000;46:137–40. doi: 10.1093/jac/46.1.137. [DOI] [PubMed] [Google Scholar]
- 83.Molina J, Martins-Filho O, Brener Z, et al. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother. 2000;44:150–5. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soeiro MdNC, de Souza EM, da Silva CF, et al. Antiparasitic activity of sterol 14α-demethylase (CYP51) inhibitor VNI against drug-resistant strains of Trypanosoma cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother. 2013;57(9):4151–63. doi: 10.1128/AAC.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawashita SY, Sanson GFO, Fernandes O, et al. Maximum-likelihood divergence date estimates based on rRNA gene sequences suggest two scenarios of Trypanosoma cruzi intraspecific evolution. Mol Biol Evol. 2001;18:2250–9. doi: 10.1093/oxfordjournals.molbev.a003771. [DOI] [PubMed] [Google Scholar]
- 86.Benaim G, Sanders JM, Garcia-Marchán Y, et al. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem. 2006;49:892–9. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 87•.Soeiro MN, de Castro SL. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin Ther Targets. 2009;13:105–21. doi: 10.1517/14728220802623881. The paper provides a deetailed review of potential alternative drug targets in T. cruzi. [DOI] [PubMed] [Google Scholar]
- 88.Soeiro MNC, Werbovetz K, Boykin DW, et al. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology. 2013;140:929–51. doi: 10.1017/S0031182013000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chatelain E, Ioset JR. Drug discovery and development for neglected diseases: the DNDi model. Drug Des Devel Ther. 2011;5:175–81. doi: 10.2147/DDDT.S16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keenan M, Abbott MJ, Alexander PW, et al. Analogues of fenarimol are potent inhibitors of Trypanosoma cruzi and are efficacious in a murine model of Chagas disease. J Med Chem. 2012;55:4189–204. doi: 10.1021/jm2015809. [DOI] [PubMed] [Google Scholar]
- 91.Hargrove TY, Wawrzak Z, Alexander P, et al. T. cruzi CYP51 complexes with two pyridine-based drug candidates for Chagas disease: structural basis for pathogen-selectivity. J Biol Chem. 2013 doi: 10.1074/jbc.M113.497990. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]