Abstract
In this brief review, the development of breast cancer is discussed from the vantage of phenotypic differentiation, similar to what has been considered over the years for leukemias and melanomas, both of which express easily visible differentiation markers (Hart and Easty, 1991; Clarke et al., 1995; Lynch, 1995; Sachs, 1996; Sledge, 1996). The review is divided into a theoretical background for human breast differentiation and a discussion of recent experimental results in our laboratories with differentiation of breast epithelial cells.
In the theoretical background, in situ markers of differentiation of normal breast and carcinomas are discussed with emphasis on their possible implications for tumor therapy. So far, most of the emphasis regarding differentiation therapy of tumors has been focused on the possible action of soluble factors, such as colony-stimulating factors in leukemias and retinoic acids in solid tumors (Lotan, 1996; Sachs, 1996). However, an emerging and promising new avenue in this area appears to point to additional factors, such as the cellular form and extracellular matrix (ECM) (Bissell et al., 1982; Bissell and Barcellos-Hoff, 1987; Ingber, 1992). The recent interest in these parameters has evolved along with an increasing understanding of the molecular composition of the ECM, and of the molecular basis of the classical findings that normal cells—in contrast to tumor cells—are anchorage dependent for survival and growth (Folkman and Moscona, 1978; Hannigan et al., 1996). We now know that this is the case for epithelial as well as fibroblastic cells, and that interaction with ECM is crucial for such regulation. Indeed, ECM and integrins are emerging as the central regulators of differentiation, apoptosis, and cancer (Boudreau et al., 1995; Boudreau and Bissell, 1996; Werb et al., 1996; Bissell, 1997; Weaver, et al., 1997).
In the experimental part, we elaborate on our own recent experiments with functional culture models of the human breast, with particular emphasis on how “normal” and cancer cells could be defined within a reconstituted ECM. Special attention is given to integrins, the prominent ECM receptors. We further discuss a number of recent experimental results, all of which point to the same conclusion: namely that phenotypic reversion toward a more normal state for epithelial tumors is no longer an elusive goal. Thus “therapy by differentiation” could be broadened to include not only blood-borne tumors, but also solid tumors of epithelial origin.
I. INTRODUCTION
Globally, breast cancer is the most frequent form of cancer affecting women (Wolff et al., 1996). Approximately one out of eight women will be diagnosed with this disease if they live long enough, and the incidence rates have been increasing by 2% per year over the past decade (Feuer et al., 1993; Wolff et al., 1996). Among the identified risk factors for breast cancer development are early menarche and late menopause (Kelsey and Berkowitz, 1988; Snedeker and Diagustine, 1996). A large placenta during fetal life also correlates with higher risk of breast cancer in adult life (Ekbom et al., 1995). Conversely, pregnancy after teens, before age 35, and the associated period of lactation have been identified as preventive factors (Kelsey and Berkowitz, 1988; Snedeker and Diagustine, 1996). Because the lactational state represents the ultimate level of differentiation in the human breast, it is arguable that repeated periods of tissue-specific differentiation constitute a physiological mechanism for overruling the accumulation of carcinogenic and cancer-promoting events during a lifetime (Russo et al., 1982; Snedeker and Diagustine, 1996). If this were true, it would be tempting to speculate that the signals responsible for maturation, structure formation, and tissue-specific function could integrate to counteract tumor growth and progression even in the advanced stage, where tumor cells are generally considered to be beyond normal cell regulation. In other words, although tumorigenesis has been viewed as the result of accumulation of multistep genetic aberrations (Klein and Klein, 1985; Bishop, 1987; Weinburg, 1989), and mutation is the accepted paradigm of cancer research (Bishop, 1991), would it be still possible even after the tumor is fully developed to revert the phenotype through manipulation of the microenvironment toward the differentiation of normal breast? If we consider differentiation to be synonymous with controlled behavior, growth arrest, minimal cell motility, limited life span, and structural integrity, this then is the opposite of cancer, as Pierce, Bissell, and others have postulated (Bissell, 1981; Pierce and Speers, 1988; Zutter et al., 1995; Campisi, 1996). Certainly, efforts to differentiate cancer cells have been exercised at the experimental as well as at the therapeutic level as possible chemoprevention (Noda, 1993; Lotan, 1996), with reasonable success achieved for blood tumors such as leukemias, for which the maturation pathways in hematopoiesis have been studied exhaustively (Sachs, 1996, and references herein).
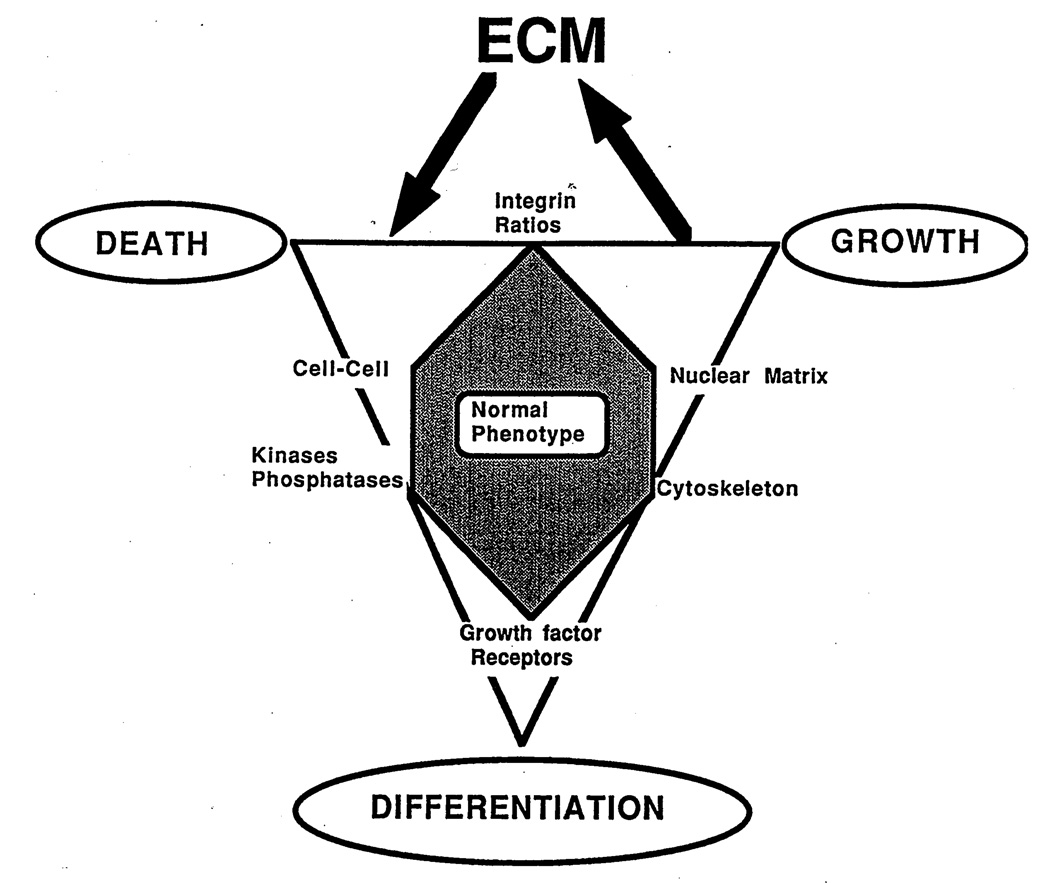
The groundbreaking discoveries of oncogenes and tumor suppressor genes and the new understanding of breast cancer biology in general have not translated equally well into therapy (Sledge, 1996). The very fact that breast cancer has such a diverse array of phenotypes in terms of histology and biology, ranging from most anaplastic to differentiated tubular carcinomas that are often difficult to discriminate from benign lesions, indicates that breast carcinomas have an intrinsic ability to assume considerable phenotypic maturation. Here we argue the case for the microenvironment as an active participant not only in normal differentiation (see Fig. 1), but also during induction and progression of cancer, with a view to find novel avenues for therapeutic intervention for solid tumors (Bissell, 1981; Petersen et al., 1992, 1995; Rønnov-Jessen, 1996; Rønnov-Jessen et al., 1996; Weaver et al., 1997).
Fig. 1.
Diagram illustrating the normal phenotype of a cell in a triangle between growth, death, and differentiation. The extracellular matrix (ECM) is proposed to play an important role via integrin-mediated signaling.
That the differentiated state is indeed a relevant end point to pursue in terms of therapy is supported by the fact that highly differentiated tumors have a remarkably better prognosis when compared to less differentiated tumors (Tabar et al., 1996). This in itself is not a novel or surprising finding. These statements have been made often over the years. Thus, what is new in this area? First, the plasticity of tumors is better documented. There is now evidence that otherwise cohesive, differentiated tumor cells become transiently more aggressive on stimulation with exogenous factors, such as hepatocyte growth factor (Birchmeier and Behrens, 1994), and that noncohesive, invasive cells, on the other hand, may be reverted by, e.g., insulin-like growth factor I, tangeretin, retinoic acid, and tamoxifen (Bracke et al., 1996). In other words, invasion and metastasis are not irreversible next steps on a one-way progression ladder even for epithelial cancers. Second, adhesion molecules and extracellular matrix in general are becoming recognized as important modulators of normal and malignant phenotypes (Petersen et al., 1992; Bracke et al., 1996; Jones et al., 1996; Varner and Cheresh, 1996; Weaver et al., 1997). Third, we now know that the surrounding stromal cells are extensively recruited, as the tumor develops, to convey the formation of microenvironmental conditions resembling those of chronic wounds, the implications of which are only beginning to be understood (Clarke et al., 1995; Rønnov-Jessen et al., 1995, 1996, and references therein). Thus, stromal cells, which are expected to be devoid of genotypic aberrations (although this may need further scrutiny in the future), actively contribute to tumor development and progression, and consequently, peritumoral fibroblasts may prove instrumental in future attempts to regulate the behavior of the tumor.
II. MARKERS OF BREAST DIFFERENTIATION
A. Morphology
The human breast is divided longitudinally into main ducts (lactiferous ducts), of which there are 10–12, each ending in the nipple, branching interlobular ducts, and terminal duct lobular units, also referred to as alveoli (for review, see Rønnov-Jessen et al., 1996). Each alveolus is the origin of further acinus formation during pregnancy and lactation (Battersby and Anderson, 1988). Thus, rather than being formed at each pregnancy from budding ducts all over again, as is the case of mice, the human acinus develops by expansion and multiplication of existing alveoli (Ferguson and Anderson, 1983). This has made the search for “stem” cells less feasible in humans compared to mice (see below). However, a transverse section through a duct or an alveolus in the human or mouse mammary gland reveals the same fundamental structures, i.e., an inner continuous layer of luminal epithelial (glandular) cells and an outer more or less continuous layer of myoepithelial cells (Rønnov-Jessen et al., 1996). This basic pattern exists irrespective of the lactational status of the gland (Umemura et al., 1996). The interrelationship between luminal epithelial cells and myoepithelial cells continues to be a mystery. Basically, they originate from the same developmental origin—the ectoderm. It would, however, be a misunderstanding to assume that, because of their stromallike phenotype, myoepithelial cells are derived from mesenchymal cells. These cells are always on the epithelial side of the basement membrane, as opposed to the true stromal cells, and so far there has been little published evidence for a stromal-to-epithelial conversion as an explanation for their occurrence in vivo (Deugner et al., 1995).
We would like to propose that the precise structural composition of the mammary gland as a double-layered tube has to be considered the ultimate level of organization. So far, it has not been possible to model this structure outside an organism, and thus we do not know how crucial this is for maintenance of function or lack thereof during cancer development. There is some evidence to suggest that the myoepithelial cells may have two opposite functions during tissue remodeling and resting conditions. In the former, they serve to pave the way for emerging ductules in the stroma, consistent with the fact that they express a number of proteases (Niranjan et al., 1995). Under resting conditions, myoepithelial cells or their products may contribute to maintain the differentiation of luminal cells or the luminal-derived carcinoma cells, and thus, may elicit a “tumor-suppressive” function (Bani et al., 1994; Liu et al., 1996).
Clearly, myoepithelial cells are altogether missing or are less differentiated in the full-blown invasive breast cancer in most species (Rudland et al., 1995). Here the basement membrane is lost, and cancer cells express markers for luminal epithelial cells rather than myoepithelial cells (for review, see Rønnov-Jessen et al., 1996). Is it meaningful then, even theoretically, to believe that a cancer cell and its progeny can be reverted all the way back to a double layered tube, if the cancer—monoclonal as it may be—originates from a predetermined luminal epithelial cell?
Understanding the evolution of lineage has been considered important in the search for the cellular origin of cancer in any tissue. The problem, as mentioned above, is that, because we know little about the precise interrelationship between luminal epithelial and myoepithelial cells, it is difficult to search for a “predetermined” luminal epithelial cell, assuming this exists (Rønnov-Jessen et al., 1996). In the mouse mammary gland, it is believed that the end buds, the epithelial cords budding from the ducts, contain the stem cells (Williams and Daniel, 1983). The end buds comprise two epithelial cell types: an outer layer of cap cells and an inner solid mass of body cells. Looking at the dynamics in the growing end bud by time-lapse video microscopy and thymidine labeling, it has been reported that the basal cap cells give rise to myoepithelial cells, by maturation along the subtending duct, as well as to body cells, which comprise the later luminal epithelial cells by migration into the central cord of epithelial cells (Williams and Daniel, 1983; Dulbecco et al., 1982). Thus, cap cells have been ascribed stem cell-like properties, which fits well with their low level of differentiation. Therefore, in the mouse, fully developed myoepithelial cells of the duct presumably derive from cap cells in the end buds, although definitive proof is lacking even here. The transitional phenotypes are located in the interlobular duct and are referred to as basal cells with intermediate levels of differentiation (Sonnenberg et al., 1986). Numerous attempts have been made to extrapolate these results to the human breast, but there is no solid evidence for the presence of end buds or cap cells in the human mammary gland. The presumed cellular equivalent, the so-called clear cells, have been found throughout the glandular tree (for review, see Rønnov-Jessen et al., 1996). Novel data that human breast cancers in fact widely do express the most obligate myoepithelial marker, the oxytocin receptor, are an indication that cancer cells may possess the ability to differentiate more along this lineage than previously believed (Bussolati et al., 1996). That breast carcinoma cells, in addition, respond to oxytocin by differentiation and growth inhibition suggests that the myoepithelial phenotype should also be considered in assays of breast cancer differentiation (Cassoni et al., 1996).
To clarify the discussion concerning the morphology and the existence of putative stem cells in the human breast, measures to purify the different lineages and to regenerate the double-layered duct/alveolus would be extremely useful.
B. Estrogen Receptors
Another trait that needs to be addressed if one is to understand the origin of carcinoma cells and how they relate to any normal counterpart, is estrogen receptor (ER) status. It has been quite a task to extract sensible information about ER function under normal and malignant conditions based on the standard tissue culture assays (Bissell and Werb, 1995). The fact is, that if measured biochemically, the estrogen receptor is expressed in about 60% of breast carcinomas but is hardly measurable in normal breast tissue (Ricketts et al., 1991; Khan, 1995). By this definition then, the ER-negative carcinomas should be the most highly differentiated tumors in that, in this respect, they would resemble normal tissue the most! This of course is not the case. Rather, all evidence from histopathological and clinical trials point to the opposite scenario: moderate ER expression correlates with morphologically differentiated tumors and better survival (Tavassoli and Man, 1995; Joslyn et al., 1996). Therefore, the ER-positive breast carcinoma cell appears to be a relatively differentiated cell. Furthermore, if one accepts that normal breast tissue is essentially devoid of ERs, then ER-positive carcinomas would have to arise from a multipotent stem cell similar to those of teratocarcinomas (Sell and Pierce, 1994). However, once immunocytochemical assays became available, it was discovered that the sensitivity of the original biochemical ER analysis was too low to measure the level of ER expression in normal breast tissue (Petersen et al., 1987; Khan, 1995). Using the assay, the level of expression in normal breast tissue from reduction mammoplasties was found to be an average of 7% of the luminal epithelial cells, and the stroma was devoid of receptor expression (Petersen et al., 1987; Khan, 1995). Based on studies in the mouse, it had been hypothesized previously that the ERs in the stroma signal to the epithelium. However, these studies were conducted with labeled ligands that gave too high a background (Underwood, 1983). More recent data on mice agree with those of human studies in that it is now known that stroma is indeed ER negative or weakly ER positive (Haslam and Shyamala, 1981; Haslam and Nummy, 1992; Sapino et al., 1993).
If breast cancer were to occur in a stochastic manner within the epithelium with an initial ER level of approximately 7%, then it is puzzling that as many as 60% of the tumors are ER-positive. One possibility is that at least 60% of breast epithelial cells could turn on the ER if “activated” by an as yet unknown mechanism that may be operative in the early stages of malignancy. It is also likely that the 7% is an average value for all breast epithelium (Petersen et al., 1987). Thus, Ricketts et al. (1991) reported that the total cellular pool may be divided into classes of nonexpressors or low and high expressors, respectively. Therefore, another possibility could be that even though the high expressors represent only a small percent of the total normal cellular population (16%), they may be at a significantly higher risk of developing breast cancer and thereby account for the 60% prevalence of ER-positive tumors. In this respect, it is interesting that ER positivity is recorded in up to 80% of in situ carcinomas and that ER expression is highest in lesions that are associated with invasive carcinoma. Once the ER-positive tumor is developed, however, ER expression is gradually lost with progression as a result of selection of “less differentiated” ER-negative cells (Robertson, 1996).
The appreciation of ER expression as a differentiated trait has been somewhat confounded by the large body of information gathered from experimental cancers in nude mice. Here, estrogen acts analogous to a tumor promotor, because ER-positive tumor cells will not develop into tumors unless estrogen is provided either from the mouse or exogenously through the diet (Soule and McGrath, 1980). This, and the fact that estradiol increases the incidence of mammary tumors in mice and rats (Snedeker and Diagustine, 1996), raise an interesting question, namely whether estrogen acts as a carcinogen for normal cells (Khan, 1995). This question has been difficult to answer in a physiological setting because normal breast epithelial cells—as mentioned above—should definitely not be considered a homogeneous population in terms of ER expression. The picture has remained confusing because thus far no one has been successful in maintaining the ER expression under experimental conditions (Rønnov-Jessen et al., 1996). Thus, to understand how to maintain ER in “normal” cells in culture remains a major future challenge.
One approach to overcome the difficulties of maintaining ER-positive normal cells in short-term culture has been to transfect the ER into established normal breast epithelial cell lines (Zajchowski et al., 1993). Surprisingly, in these cell lines, the response to estrogen is the direct opposite of what is observed with tumor cells and normal cells in short-term culture; they are in fact growth inhibited (Zajchowski et al., 1993). One of our laboratories has transfected a spontaneously immortalized, intrinsically ER-negative breast epithelial cell line with an ER construct and obtained the same experimental outcome, i.e., the cells were indeed growth inhibited (Lundholt et al., 1996).
From these experiments, we are left with the question of whether the paradoxical response in normal, transfected cells is due to the fact that these cells are intrinsically different from ER-positive tumor cells, or whether the transfected receptor lacks additional sequences that usually give the growth signal. Unfortunately, it appears that the latter may be the correct answer, because if overtly malignant, ER-negative breast carcinoma cells are transfected in a similar manner, they, too, behave like normal transfected cells—that is, they are growth inhibited by estrogen (Levenson and Jordan, 1994; Petrangeli et al., 1994). It therefore seems reasonable to conclude that transfection of an apparently functional ER, eliciting all the downstream events that can be measured biochemically (Lundholt et al., 1996), does not evoke the same fundamental growth response as in nontransfected cell lines. Thus, much more needs to be learned about normal ER function and lineage evolution during tumor development. This means that we must also learn about how tissue-specific gene expression is maintained in the normal breast, i.e., we must dissect the elements of microenvironmental control (Bissell and Barcellos-Hoff, 1987).
C. Integrins
The organization of epithelial cells into branching ducts and alveoli depends on the action of various soluble factors, cell–cell, and cell–ECM interactions, and the establishment of epithelial polarity. The epithelial cell membrane is segregated into specialized apical and basolateral domains separated by tight junctions, and the cytoskeleton is organized into fibers terminating at junctional complexes and transmembrane integrin heterodimers facing the basement membrane (Taylor-Papadimitriou et al., 1983; Eaton and Simons, 1995). The largest class of ECM receptors, the integrins, are now known to be the cellular antennas that sense the subtleties of the ECM and convert the chemistry of the microenvironment into subcellular signaling, which is then translated into form and function. Normal human breast epithelial cells express at least two β-integrins (β1 and β4) and four α-integrins (αl, α2, α3, α6) (Bergstraesser and Weitzman, 1994; Glukhova et al., 1995). The expression of integrins in breast cancer is usually altered quantitatively and/or qualitatively (Zutter et al., 1990; Mechtersheimer et al., 1993). Based on their pairing and assumed function under normal and malignant conditions, the integrins will be described as (1) α6β4 in the normal breast, (2) α6β4 in breast cancer, and (3) β1-integrins in the normal breast and in breast cancer.
1. α6β4 IN NORMAL BREAST
Expression of the α6β4-integrin as a dimer in the human mammary gland has not been analyzed in great detail until recently because of lack of relevant antibodies. However, when studied individually in the normal breast, both β4 and α6 are expressed primarily by myoepithelial cells (Berdichevsky et al., 1994). This should not be surprising, because α6β4 is expressed generally by basal cells such as those in the skin or the bronchial tree (Carter et al., 1990; Sheppard, 1996). With regard to luminal epithelial cells, it is generally assumed that β4-integrin is expressed at the basolateral surface of the cell membrane in vivo. However, at the light microscopic level, it is difficult to determine whether the observed β4-integrin staining derives from myoepithelial or luminal cells (Koukoulis et al., 1991; Natali et al., 1992). With regard to α6-integrin, there is no clear consensus on the luminal epithelial expression pattern. It has been described either as strictly limited to the basal cell surface (Natali et al., 1992), variably expressed also at the lateral membranes (Mechtersheimer et al., 1993), or homogeneously expressed on the entire cell surface (Friedrichs et al., 1995). The apparent wider subcellular distribution of α6-integrin should be seen in light of the fact that, in addition to β4-integrin, α6 also pairs with β1-integrin (Sonnenberg et al., 1990). It is important to realize, however, that α6-integrin will preferentially pair with β4-integrin if the latter is present (Cress et al., 1995; Schoenenberger et al., 1994). The α6β4 pairing leads to formation of stable anchoring contacts or hemidesmosomes to the basement membrane, typical of polarized, resting cells with little or no motile behavior (Carter et al., 1990; Cress et al., 1995). The quiescent state of the cells is most likely signaled via β4-mediated induction of p21/WAFl/CIPl, an inhibitor of the cyclin-dependent kinases (Clarke et al., 1995). This fits nicely with the fact that myoepithelial cells under normal conditions are virtually noncycling (Rønnov-Jessen et al., 1996). As expected from these studies, p21/WAFl/CIPl under normal conditions is expressed at least occasionally in some myoepithelial cells (Barbareschi et al., 1996).
2. α6β4 IN BREAST CANCER
At first glance, the reports on α6β4-integrin expression in cancer seems quite confusing. Clearly, whereas it is up-regulated in some forms of cancers, including squamous cell carcinomas of the skin (Giancotti and Mainiero, 1994), astrocytomas (Previtali et al., 1996), and thyroid carcinomas (Serini et al., 1996), other cancers, such as those of breast and prostate, are almost completely devoid of α6β4 as determined by β4 staining (Cress et al., 1995; Koukoulis et al., 1991). It is noteworthy, however, that the up-regulation of α6β4 in some cancers, e.g., in squamous carcinomas, may be a product of the inability of the tumor cells to differentiate and to become α6β4 negative as they would during the process of normal skin differentiation (Giancotti and Mainiero, 1994). However, if α6β4 is indeed up-regulated specifically, it may be instrumental in the malignant phenotype in a manner different from that governing the normal conditions as outlined above. Thus, some of the reported oncogenic activities of α6β4 include tyrosine phosphorylation of Shr/Grb2 and induction of the ras pathway (Mainiero et al., 1995), phosphorylation of pl85HER2 (Campiglio et al., 1994), EGF-induced deterioration of adhesive structures (Mainiero et al., 1996), and escape from cell death mechanisms (Dowling et al., 1996).
Nevertheless, in vivo β4-integrin is down-regulated in invasive breast cancer as compared to normal breast tissue (Koukoulis et al., 1991). In comparing the different reports in the literature concerning α6-integrin in breast cancer, it is clear that this integrin is not down-regulated to the same extent as β4, because it is expressed in at least 50–70% of breast carcinomas compared to reports of β4 expression in the range of 0–22% of all carcinomas [compare Friedrichs et al. (1995) with Koukoulis et al. (1991) and Taylor-Papadimitriou and Alford 1995)]. The significance of this difference again should be viewed in light of the level of β1-integrin expression in the normal breast and breast cancers (see below) and the fact that β1-integrin is the other integrin that α6 may pair with, as mentioned above (Sonnenberg et al., 1990).
3. β1-INTEGRINS IN NORMAL BREAST AND BREAST CANCER
The hypothesis that ECM, and hence its receptors, have information and direct tissue-specific form and function was set forth a decade and a half ago (Bissell et al., 1982). That this is indeed the case has been amply demonstrated for many tissues but especially the mammary gland of rodents and more recently humans (for reviews, see Adams and Watt, 1993; Lin and Bissell, 1993; Hay, 1995; Roskelley and Bissell, 1995; Rønnov-Jessen et al., 1996; Bissell, 1997). β1-Integrin has been shown to be involved in the regulation of milk protein gene expression (Streuli et al., 1991) as well as apoptosis (Howlett et al., 1995; Boudreau et al., 1995). Knockouts of β1-integrin are embryonically lethal (Fassler and Meyer, 1995; Stephens et al., 1995). In 1994, Matlin and collaborators (Schoenenberger et al., 1994) put forth an imaginative hypothesis that stated that if phenotype, in casu, morphogenesis should depend only qualitatively on the expression of integrins at the cell surface, then minor changes would not be expected to be important. On the other hand, if a correct phenotype depends on the exact balance of various integrins at the plasma membrane, then a change in the relative amounts of individual integrins would be quite significant (Schoenenberger et al., 1994). Because α6-integrin may pair with β4 as well as with β1, and the ratio between α6 and β4 appears to increase with cancer development, it becomes essential to know what happens to the level and location of β1 in cancer cells (Weaver et al., 1997). The equation needs to also include α2 and α3, the other two integrin subunits compatible with β1.
In the race to be the β1 partner on the cell surface, it has been reported that α2 wins over α3, which in turn wins over α6, but that there is usually an excess of β1 on the cell surface of nontransformed cells (Schoenenberger et al., 1994). In the normal human breast, α2-integrin is expressed equally well on the basolateral cell surface of both luminal and myoepithelial cells, whereas α3 is slightly weaker in luminal epithelial cells (Koukoulis et al., 1991; Glukhova et al., 1995; Taylor-Papadimitriou and Alford, 1995). Looking at mRNA expression (which is not as informative) and in at least one case of protein staining, α2 shows the same tendency as α3 in that there is a little higher expression in myoepithelial cells compared to luminal epithelial cells (Koukoulis et al., 1991; Zutter et al., 1993). The expression of α2 and α3 at the cell–cell junctions as well as at the cell–ECM junctions reflects their assumed function in directing morphogenesis and maintaining cell survival (Berdichevsky et al., 1994; Howlett et al., 1995).
In breast carcinomas, α2-integrin has been reported to be absent or close to absent in about 50% of the cases (Mechtersheimer et al., 1993; Taylor-Papadimitriou and Alford, 1995). Also, around 40% of breast carcinomas show decreased levels or absence of α3 (Mechtersheimer et al., 1993). For comparison, β1-integrin is not down-regulated at the protein level to the same extent and in one report no decrease was found in the majority of carcinomas (Mechtersheimer et al., 1993; Zutter et al., 1993). The equation may thus be concluded as follows: with little or no β4, significant reductions in α2 and α3, and available β1, there is room for pairing of α6 with β1 in breast cancer as opposed to what is seen in normal cells. This fits nicely with a reported reciprocal expression of α2β1 with α6β1 in around 50% of breast cancers (Oda et al., 1994). The α6β1 phenotype is particularly characteristic of one histological variant of breast cancer, that of the invasive lobular cancer, which shows little morphological differentiation (Koukoulis et al., 1993). The conclusion of the equation is compatible with observations on prostatic carcinomas whereby the α6β4 pathway is essentially bypassed in favor of the α6β1 pathway (Cress et al., 1995). The biological significance of this observation is supposed to involve the ability of cells to migrate and metastasize. Whereas α6β4 is associated with stable anchoring contacts, α6β1 colocalizes with focal contacts at the cellular protrusions (Cress et al., 1995; Koukoulis et al., 1991, 1993). In this respect, it is also interesting that the nonmalignant HBL-100 breast epithelial cells, although clearly not normal, almost exclusively express α6β4, whereas the highly malignant breast carcinoma cell line, MDA-MB435, shows the opposite constitution. The invasive phenotype of the latter may in fact be dramatically reduced by dominant negative knockout of α6β1 [compare Sonnenberg et al. (1990) with Shaw et al. (1996)]. A related phenomenon has been reported for a colonic carcinoma cell line in which progression from a poorly tumorigenic variant to a more aggressive variant was associated primarily with an increase in β1 and a parallel decrease in β4 integrin expression (Lopez-Conejo et al., 1996). It is important, however, to realize that a shift in β1 relative to β4 integrin in itself may not be sufficient to trigger an aberrant phenotype. In this respect it is quite intriguing that a tumorlike shift in the ratio between β1 and β4 integrins is already grossly apparent at the level of noninvolved breast tissue from the majority of cancer patients (Jones et al., 1992). The importance of the relative ratios of integrins at the cell surface in determining the phenotype of normal and malignant cells was tested most recently in our laboratories and will be discussed in more detail below (Weaver et al., 1997).
III. CULTURE MODELS OF MAMMARY GLAND DIFFERENTIATION
All cancer biology, indeed all biology, relies on well-characterized model systems. When dealing with human cells, the only option is to explant the relevant cells and develop culture assays aimed at answering specific questions. Classical cancer research has benefited from monolayer culture assays of immortality, transformation, and progression (Nettesheim and Barrett, 1985; Harris, 1987). However, for epithelial cells, these assays do not measure differentiation beyond the level of squamous metaplasia (Bissell, 1981; Masui et al., 1986). Morphological differentiation appears to require more elaborate model systems.
A. Basement Membrane (EHS) Assay
We have searched for a set of criteria to define safely “normal” or “near-normal” for cultured human breast epithelial cells, without incorporating the complexity of the myoepithelial cells until such time that we know more about them. Analogous to mouse mammary epithelial cells (Barcellos-Hoff et al., 1989; Aggeler et al., 1991), normal luminal epithelial cells, when confronted with a physiologically relevant reconstituted basement membrane (EHS; Matrigel, Collaborative Research), were shown to recapitulate a number of normal features, independent of the presence of a basal layer of myoepithelial cells. First, they rapidly (within 7–10 days) organized a near-perfect acinus-like sphere (Petersen et al., 1992). Some of these structures may well have been clonal because they were preceded by an initial burst of growth that declined to almost zero once the final size (<50 µm) was reached. The growth arrest was not the result of suboptimal culture conditions, because the same medium readily supported growth of similar cells in monolayer culture or tumor cells in EHS (Petersen et al., 1992). Second, when sectioned, these structures contained true acini with a central lumen formed by apical cell membranes and cells with basally located nuclei. The growth arrest was synchronized with acinus formation. We determined the mean number of cells in an equatorial section of the spheres to be around 8 cells, and thus similar to that seen in acini in vivo. Because an important measure of differentiation in the mammary gland is the ability to polarize the cellular axis in a correct manner, we stained the cells for an integral, apical-specific protein, sialomucin, as well as the basement membrane component, type IV collagen. The localization of both proteins was found to be comparable to what is found in acini in vivo, i.e. apical deposition of sialomucin and basal deposition of type IV collagen at the cell–ECM junction (Petersen et al., 1992). It is interesting to note that both the final size of spheres (50 µm), the number of cells in aggregates (~8), the initial growth burst, the time before growth arrest (7 days), and the apical lumen formation and basolateral type IV collagen deposition have all been found previously for Madin–Darby canine kidney (MDCK) cells in collagen cultures, implying that these are fundamental epithelial and-glandular behavior in the presence of relevant three-dimensional structures (Wang et al., 1990). The fact that ER-negative epithelial cells were able to form almost normal acini on cultivation in a basement membrane matrix indicates that neither ER expression nor myoepithelial cells are strictly required for this phenomenon to occur in culture and that the EHS matrix and the characteristics of cultured luminal breast cells are compensating for the other cell types that exist in vivo (Petersen et al., 1992; Rønnov-Jessen et al., 1996).
Analogous to the rodent model (Medina et al., 1987; Schmidhauser et al., 1990; Lin and Bissell, 1993), the effect of the ECM on human breast epithelial differentiation was not restricted to freshly explanted primary cultures with a finite life span. Rather, two nonmalignant, but immortal, cell lines (MCF-10A and HMT-3522) from benign breast lesions responded essentially in the same manner, that is with acinus-like formation and growth arrest. Although our assay was performed in a serum-free medium to obtain normal behavior, others have reported similar data using MCF-10A, even with serum in the medium (Basolo et al., 1996). A related assay, also based on three-dimensional culturing, but in type I collagen instead of a reconstituted basement membrane, has been used for human breast epithelial cells (Berdichevsky and Taylor-Papadimitriou, 1991; Shearer et al., 1992). In contrast to what has been reported for MDCK cells, however, this assay does not contain sufficient cues to polarize mammary epithelial cells correctly nor for them to deposit an endogenous basement membrane (Howlett et al., 1995; Lu et al., 1995). Thus the basement membrane (EHS) assay appears to be the first assay that allows recapitulation of critical aspects of normal breast in culture.
With the establishment of the EHS assay for human breast cells, the way was paved for the rapid identification of even subtle deviations from a differentiated behavior. Thus, within the same period of 7–12 days, during which “normal” cells organized an acinus, HBL-100 cells formed small, dispersed colonies, and tumor cells from either biopsies or established cell lines formed large irregular clusters of cells that did not growth arrest, deposit a basement membrane, or polarize correctly in response to the EHS (Petersen et al., 1992, 1995; Howlett et al., 1994, 1995). Thus as predicted, the tumor cells failed to sense the ECM properly, which was consistent with our hypothesis that the ability to respond to ECM correctly is a function of a new class of “suppressor genes” that are lost or altered when cells become malignant (Petersen et al., 1992). The reason for this dramatic difference in response to the ECM could lie in defects anywhere from the ECM to integrins to cytoskeleton to nuclear matrix and to the genes themselves (Bissell et al., 1982) and need not depend on the same genetic defects in different tumor cells. Our initial observations on the different behavior of “normal” and “malignant” human breast cancer cells inside EHS were confirmed by others (Shearer et al., 1992; Bergstraesser and Weitzman, 1993). Since, then, it has been observed that the morphogenic effect of EHS on normal and cancer cells makes the use of EHS a more sensitive assay than the soft agar assay for predicting whether cells are tumorigenic in nude mice (Basolo et al., 1996). Moreover, not only does the EHS assay serve to reveal normal behavior, it also supports growth of carcinoma cells better than the soft agar assay (Oridate et al., 1996).
To test the hypothesis that the assay could shed light on possible tumor suppressor genes, we tested the behavior of the highly malignant MDA-MB435 breast carcinoma cells that had been stably transfected with the tumor suppressor gene NM-23H1/NDP-kinase (Leone et al., 1993). Whereas the neotransfected control cells behaved analogous to other breast cancer cells in the EHS assay, the NM-23-transfected cells showed an altered morphology and growth and polarized deposition of a basement membrane (Howlett et al., 1994). In particular, they reached a final size of about 50 µm, as opposed to the neotransfected cells that kept on growing. It would be too naive to attribute these findings to a specific function of this particular tumor suppressor gene, because similar data in the EHS assay were obtained with the same cell line transfected with quite another gene, a novel H-cadherin (Lee, 1996), which may thus also be considered a tumor suppressor by our definition. Rather these data collectively point to the fact that as long as aspects of the phenotype can be compensated for, the exact error leading to the initial loss need not be corrected. The data also indicate that structural reorganization, if successfully imposed on the cells, is a very strong regulator of phenotype that can lead to reestablishment of a basement membrane and polarity, even in the absence of critical epithelial markers such as keratins and cadherins, which are permanently lost in MDA-MB435 cells (Pierceall et al., 1995).
B. HMT-3522 Epithelial Cell Line of Breast Cancer Development
For a number of years, all our understanding of the early malignant lesions has had to rely on experimentally generated malignant transformation as obtained by SV-40 transfection, or benzo[a]pyrene treatment of cultured cells from normal individuals (Chang et al., 1982; Stampfer and Bartley, 1985) (for reviews, see Rønnov-Jessen et al., 1996; Weaver et al., 1996). In general, human cells do not undergo the spontaneous transformation in culture often seen with rodent cells. The artificially generated malignant cells are quite aggressive to begin with and cannot therefore be taken to represent the early stages of cancer. Attempts to overcome the problem of spontaneous transformation of normal human cells have included the possible generation of established cell lines from benign lesions assumed to have initiated and immortalized prior to explantation (Pauley et al., 1993). Success in establishing such “spontaneously” immortal cell lines from the human breast has been experienced in two cases. Originally, one of our laboratories generated the HMT-3522 S1 cell line under chemically defined culture conditions (Briand et al., 1987) from a person with fibrocystic disease. Later, a different isolate was made from a similar source but with serum in the medium (the MCF-10A) (Soule et al., 1990). The two cell lines share similar characteristics of being nonmalignant up to a very high passage number and showing very few chromosomal aberrations in the early passages. Clearly, they form an excellent basis for generating model systems for malignant progression. We have concentrated on the HMT-3522 cell line. The first question to address was whether they would progress spontaneously to a malignant state. The cells were kept under very defined culture conditions for more than 10 years and > 500 passages. It is relevant here to mention that most SV-40-immortalized cells become malignant around passage 100 (Rønnov-Jessen et al., 1996). However, no signs of tumorigenicity were recorded in these non-xenobiotic-exposed cells. Interestingly, the cells have drifted substantially with regard to their karyotype such that later passages are grossly aneuploid. Still, however, they do not become tumorigenic (Vang Nielsen et al., 1994). This correlates with the recent findings that aneuploidy has been found in breast tumors long before they are overtly malignant (Leal et al., 1995; Teixeira et al., 1995). It is also interesting to note that the other spontaneously immortal human breast epithelial cell line, MCF-10A, resembles HMT-3522 in its lack of tumorigenicity even in high passages.
It is a widely held notion that growth autonomy is a well-defined premalignant step in the multistep process of cancer development. In the absence of spontaneous transformation within a reasonable time frame, the HMT-3522 cells were forced to become autonomous with respect to EGF/amphiregulin by omitting this cytokine from the chemically defined medium in passage 120 (Madsen et al., 1992). With this treatment, the cells remained relatively unaffected on the culture flask, but refrained from further growth for a few weeks (Briand et al., 1996). After this latency period, growth commenced in a collective fashion from a slow to a more usual rate within one or two passages. Apart from being EGF autonomous, there were no discernible signs of further transformation of these lines (referred to as S2) grown on tissue culture plastic, because they remained nontumorigenic in nude mice. However, after exactly 118 passages in EGF-free medium, the cells suddenly gave rise to tumors, and the cells explanted from one of these tumors was named HMT-3522 T4-2. This spontaneous shift in phenotype coincided with the acquisition of an extra short arm of chromosome 7 (trisomy 7p) as the only obvious detectable karyotypic change. Other karyotypic changes, however, have more recently been identified using comparative genomic hybridization (collaborative studies with the Joe Gray and Dan Pinkel laboratories; Weaver et al., 1998). The transformation was reproducible in passage 238 as revealed by thawing frozen stocks of earlier passages and closely following them through the critical passages by inoculations in nude mice (Briand et al., 1996). In other words, we have now succeeded in making a controlled progression series within the same cell line and without imposing any additional destabilizing exogenous transformation steps. Other attempts to make a progression series within a single cell line have included at least one additional measure to facilitate transformation (Pauley et al., 1993). The HMT-3522 series has given us a unique opportunity to focus on the transformation-associated phenotype in the absence of the usual “noise” arising from tremendous dissimilarities among the normal and malignant cell lines.
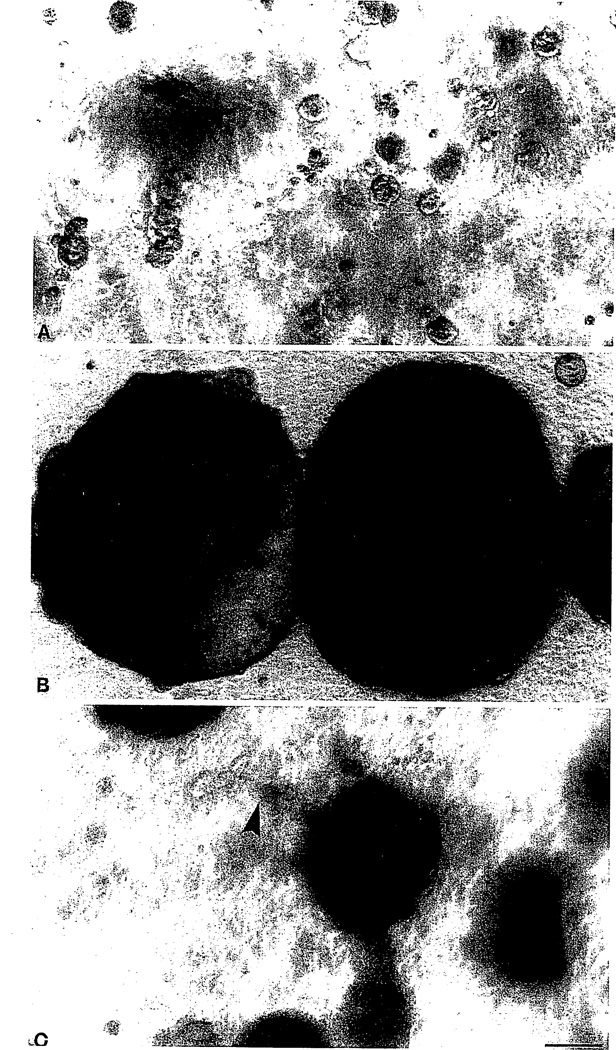
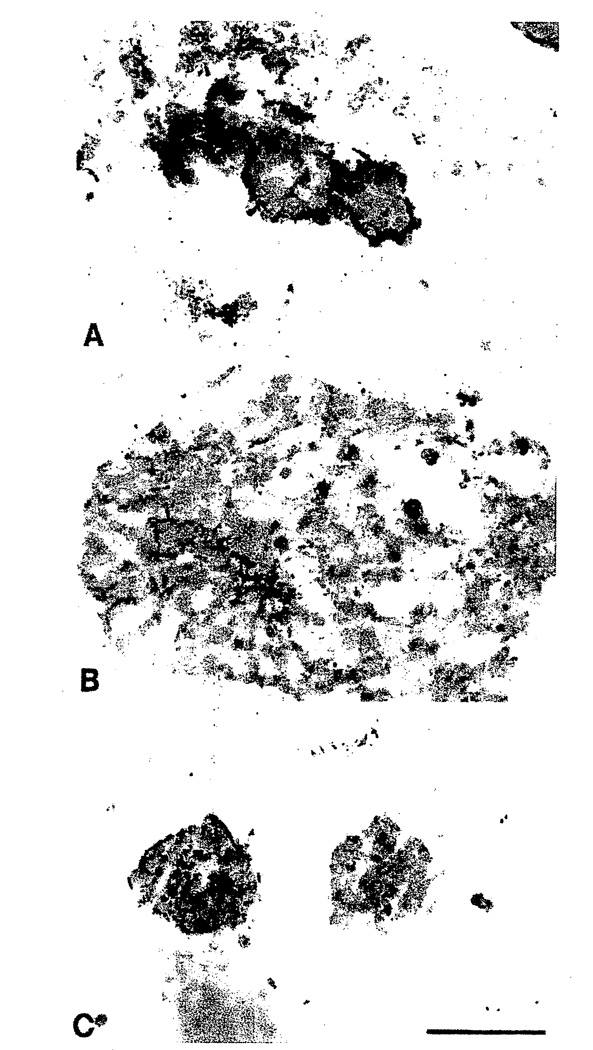
An initial question that needed immediate clarification was whether the series from S1, EGF dependent, through S2, EGF independent, to tumorigenic T4-2 could be sufficiently resolved in our differentiation assay inside the reconstituted basement membrane. Indeed, all S1 passages behaved normally as described above by forming small acinus-like structures (Petersen et al., 1992; Weaver et al., 1998). More interestingly, however, not only T4-2 but also S2 diverged from such behavior and exhibited their own characteristic abnormal phenotype. As seen in Fig. 2, at least one population within S2 formed very large balls of cells many times larger than those formed by S1 cells. T4-2, on the other hand, formed intermediate-sized clusters with irregular boundaries and dissemination into the matrix (Fig. 2). Also, on sectioning of the gels and staining for endogenous basement membrane, the S1 cells stained as expected with a continuous line at the cell–ECM junction, S2 cells were almost negative, and T4-2 clusters in general were either not polarized or inversely polarized, but stained for type IV collagen (Fig. 3).
Fig. 2.
Morphological characterization of the HMT-3522 series in the EHS assay. Phase-contrast micrographs of S1 cells (A), S2 cells (B), and T4-2 cells (C) plated inside EHS and cultured for 12 days in serum-free conditions. Whereas the “normal” S1 cells form typical acinus-like spheres, a subpopulation of the “benign” S2 cells form megaclusters reminiscent of hyperplasia, and the T4-2 cells form smaller irregular colonies with some penetration of cells into the EHS, similar to invasion (arrowhead in C). Magnification, ×90. Bar = 100 µm.
Fig. 3.
Characterization of basement membrane deposition of the HMT-3522 series. Light micrographs of cryostat sections of S1 cells (A), S2 cells (B), and T4-2 cells (C) immunoperoxidase stained for human type IV collagen and counterstained with hematoxylin. Note that although the S1 cells deposit an almost continuous basement membrane, this is lost in the S2 cells. The T4-2 cells deposited a disorganized and apolar basement membrane. Magnification, ×150. Bar = 100 µm.
The fact that S2 cells apparently do not form their own basement membrane but proliferate continuously is compatible with their classification as benign hyperplasia. Thus, S2 cells resemble keratin K19-negative benign hyperplastic epithelium (Rønnov-Jessen et al., 1996). That T4-2 cells apparently reexpress type IV collagen, albeit in an uncoordinated manner, has precedent for breast carcinomas in vivo, as mentioned previously. Thus, some invasive carcinomas in fact do stain for basement membrane (BM) components either in the cytoplasm or as an abortive attempt to form a BM. Staining for BM in breast cancer is considered a differentiated trait and correlates with better prognosis (Albrechtsen et al., 1981; Natali et al., 1992). Also, BM staining is a consistent feature of early in situ cancers and it is therefore not unreasonable to classify the derived T4-2 cell line as either an early cancer cell line or a differentiated cancer. Accordingly, in contrast to the existing established breast carcinoma cell lines, T4-2 is likely to be more subject to regulatory microenvironmental cues, and as such an excellent model for further exploration of possible differentiation therapy or reversion studies.
C. Reversion of Malignant HMT-3522-T4 Phenotype
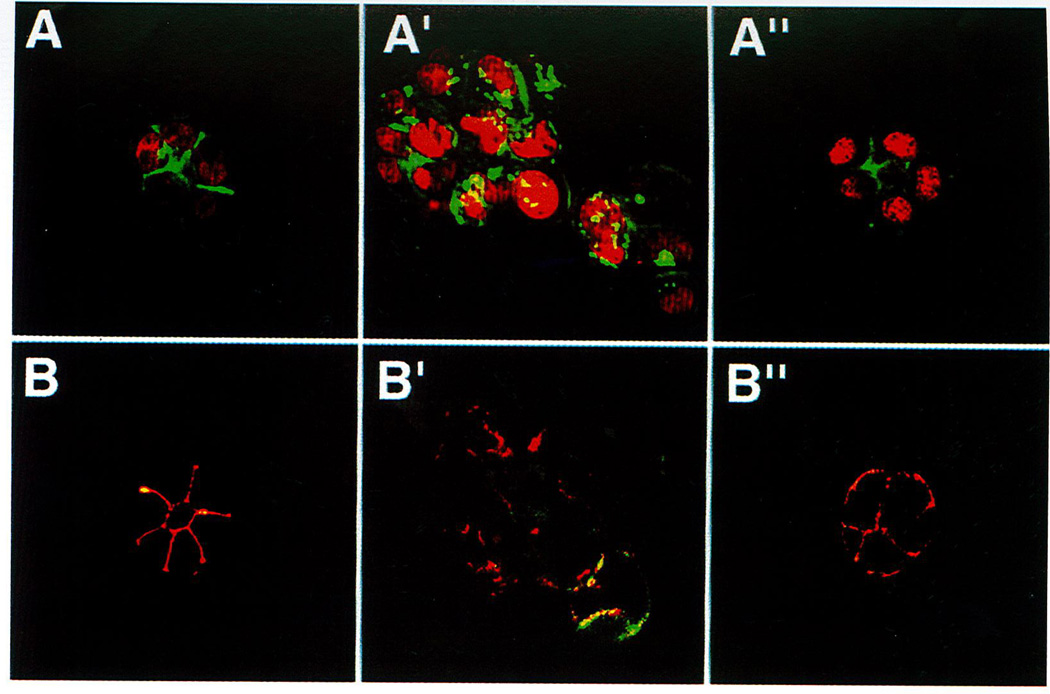
One remarkable feature that clearly discriminated the T4-2 cell line from our previously tested established breast cancer cell lines was the distinct expression of β1-integrin on the cell surface of T4-2 cells [compare Howlett et al. (1995) and Weaver et al. (1997)]. We therefore used this new cell line in the previously described assay aimed at establishing the role of β1-integrin in morphogenesis of normal versus tumor cells. This could not be tested in the tumor cell lines that were tested previously because they did not contain much β1 (Howlett et al., 1995). The T4-2 line responded to a β1-integrin-inhibitory antibody in a surprising manner by forming regular spheres with an occasional central lumen (Weaver et al., 1997): They exhibited growth arrest within the experimental period, similar to what we had recorded with the nonmalignant S1 line without the antibody (Petersen et al., 1992; Howlett et al., 1995; Weaver, et al., 1997). A quantification of the number of acini showed that almost all cells were in fact reverted by the treatment, leaving essentially no irregular clusters of cells after 10–12 days of cultivation. The growth arrest was also genuine as evidenced by thymidine incorporation and cyclin D1 expression. Moreover, sectioning and staining for endogenous type IV collagen showed a distinct organized and polarized BM at the cell–ECM junction similar to our observation in the S1 cells. The morphological, proliferative, and polarized reversion was also associated with a reorganization of the cytoskeleton. Thus, whereas the untreated or mock-treated T4-2 cells showed a diffuse staining for actin, E-cadherin, and β-catenin, the β1-integrin antibody-treated cells polarized these molecules to the cell–cell junction (Fig. 4; see color plate) (Weaver et al., 1997). That the S1 cells and the β1-integrin-treated T4-2 cells were in fact different was evident only from the fact that the latter stained for β-catenin and E-cadherin at the cell–ECM junction in addition to the staining at the cell–cell junction (Fig. 4). In addition, in contrast to S1 cells, very few lumina were observed at the apical surface. Finally, we established a link between our culture observations and traditional tumorigenicity in nude mice in that β1-integrin-treated T4-2 cells gave rise to significantly fewer and smaller tumors than did the mock-treated control cells (Weaver et al., 1997).
Fig. 4.
β1-Inhibitory antibody treatment of tumor cells leads to the formation of reverted acini. (A–A″) Confocal fluoresence microscopy images of F-actin. Both the S1 (A) and T4-β1 reverted acini (A″) showed basally localized nuclei (propidium iodide) and organized filamentous F-actin (FITC), whereas T4-2 mock-treated colonies (T4-2 IgG) had disorganized, hatched bundles of actin and pleiomorphic nuclei (A′). (B–B″) Confocal immunofluorescence microscopy images of E–cadherin (FITC) and β-catenin (Texas red): In S1 (B) and T4-β1 reverted acini (B″), E-cadherin and β-catenins were colocalized and superimposed at the cell–cell junctions. Reproduced from Weaver et al., (1997). J. Cell Biol. 137, 231–245, by permission of The Rockefeller University Press.
Because the behavior of T4-2 cells could be almost completely corrected by an inhibitory antibody to β1-integrin, it was tempting to assign conversion to the malignant phenotype in this system to an overexpression of β1-integrin at the cell surface as compared to S1 cells. The expression level of β1-integrin was therefore measured on immunoblots of total proteins and of biotin-labeled surface proteins. In both cases, the level of β1-integrin was higher in T4-2 cells versus S1 cells. Thus, if the action of the β1-integrin antibody could be truly attributed to specific blocking of the signaling pathway and cells were not selected for, then we had conclusive evidence for the role of this integrin in the dramatic reversion.
The specificity of the function blockade was further tested by use of other inhibitory antibodies against β1-integrin and one β1-integrin stimulatory antibody. The latter did not inhibit. Also, Fab fragments were generated to distinguish between receptor clustering and inhibition of signaling. Fab fragments inhibited as well as the intact antibody. Finally, we could exclude selection of possible contaminating S1 cells because repeated cycles of reexplantation into monolayer and reembedding into EHS with anti-β1-integrin-treated and mock-treated cells showed that the “malignant” and “normal” phenotypes were reversible (Weaver et al., 1997).
Neutralizing β1-integrin was previously shown to lead to a less malignant behavior in terms of invasion and tumorigenicity in vivo (Fujita et al., 1992; Schiller and Bittner, 1995). However, the mechanism(s) for this lowered tumorigenicity was neither explored nor understood. Thus, the dramatic differentiating effect of down-regulating β1-integrin in tumor cells is not only a novel finding for β1-integrin action on tumor cells, but it also reveals a very important principle for glandular function. In an effort to explain the finding with the integrin balance theory in mind (see Section II, C), we speculated that the corrected phenotype was not only the result of a diminished β1-integrin signaling per se but also of restoring the signaling of one or more of the other integrins. In other words, the malignant behavior was conveyed by an imbalance of the array of available integrins at the cell surface. Cell surface labeling showed that there was less β4-integrin on T4-2 cells compared to the nonmalignant S1 cells. The ratios between β1 and β4 were 1.85 and 5.18 in S1 and T4-2 cells, respectively. In further support of the imbalance hypothesis were the facts that the level of cell surface β4-integrin in the T4-2 cells was essentially normalized on treatment with the β1-integrin antibody, and that, conversely, treatment of S1 cells with either β4- or α6-integrin function-altering antibodies resulted in a disorganized phenotype somewhat reminiscent of that seen in malignant cells (Weaver et al., 1997).
As discussed in the introduction, the concept that signaling from the microenvironment may revert an otherwise overtly malignant phenotype is not unprecedented, although a mechanism has not been put forward. For example, it has been reported that the information imparted by pure thrombospondin may revert malignant hemangiomas to differentiated nontumorigenic capillary-cord-like cells, and that the expression of thrombospondin inversely correlates with malignant behavior of melanoma, lung, and breast carcinoma cell lines (Zabrenetsky et al., 1994; Sheibani and Frazier, 1995).
What is equally significant about the collective observations we have made with this breast epithelial series inside a three-dimensional reconstituted basement membrane assay is a new integrated view of how normal tissues function in vivo. Our results may point to a hitherto unknown switch that may be pulled, the net effect of which in a three-dimensional assay is to override several genotypic abnormalities associated with malignant behavior. Our data should also be viewed within the context of integrin signaling whereby the apparent redundancy of integrins has been a puzzle to solve. Further understanding may be gained from the concept that multiplicity does not indicate redundancy but serves to maintain an equilibrium of signaling from different pathways responding to a microenvironment in such a way as to maintain adequate homeostasis and growth when necessary.
IV. CONCLUSION
The recent spectacular ability to identify and clone the susceptibility genes for many forms of cancers has led to much excitement and also much expectation for a rapid understanding and cure for breast and other epithelial cancers.
The central thesis of this short review, focused mainly on our current work, is that we will not understand breast cancer unless we understand normal epithelial biology. We argue that a simple genetic model of breast cancer, even if multistep, cannot, and does not, explain much of the literature and our current data. The fact that malignant behavior can be reverted by manipulation of a single ECM signaling pathway (even if so far in a single model system) argues that many of the changes that were responsible for this behavior (unregulated growth, aberrant morphogenesis, lack of a basement membrane, loss of cell–cell interaction, levels of cyclins, etc.) were lost not by mutation, but by changes in their levels or localization as a result of a global disorganization of tissue structures. Because we can shuttle these cells back and forth in dramatic changes in phenotype, and retain the same malignant genotype, it is clear that the tissue phenotype is dominant over the cellular genotype.
Thus, we argue that breast cancer is the result of not just genetic change, developmental regulation, or loss of growth regulation, but an interweaving of all of these factors. This is an insight that should help us to unravel the molecular mechanisms that allow the genes and the microenvironment to come together to create tissues and specificity. To understand how these specialized cues are lost as cells become malignant may require collaboration of oncologists, molecular and cell biologists, but also pathologists, physiologists, computation biologists, and bioengineers.
ACKNOWLEDGMENTS
We thank Tove Marianne Lund for expert technical assistance and Bill Johansen for his administrative and editorial assistance. This work was supported by the Office of Health and Environmental Research of the U.S. Department of Energy (under contract DE-AC03-76SF00098, to MJB), and in part by the National Institutes of Health (CA64786-02, to MJB and OWP), the Breast Cancer Fund of the State of California (BCRP University of California-IFB-0400, to VMW), the Danish Cancer Society, the Danish Medical Research Council, the Novo Nordic Foundation, and the Thaysen Foundation (to OWP and LR-J).
REFERENCES
- Adams JC, Watt FM. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Aggeler J, Ward J, Blackie L, Barcellos-Hoff MH, Streuli CH, Bissell MJ. J. Cell Biol. 1991;99:407–477. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Cancer Res. 1981;41:5076–5081. [PubMed] [Google Scholar]
- Bani D, Riva A, Bigazzi M, Sacchi BT. Br. J. Cancer. 1994;70:900–904. doi: 10.1038/bjc.1994.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbareschi M, Caffo O, Doglioni C, Fina P, Marchetti A, Buttitta F, Leek R, Morelli L, Leonardi E, Bevilacqua G, Dalla Palma P, Harris AL. Br. J. Cancer. 1996;74:208–215. doi: 10.1038/bjc.1996.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo F, Fiore L, Calvo S, Falcone V, Conaldi PG, Fontanini G, Caligo AM, Merlo G, Gluzman Y, Toniolo A. Br. J. Cancer. 1996;73:1356–1361. doi: 10.1038/bjc.1996.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby S, Anderson TJ. Vircbow’s Arch. A Pathol. Anat. 1988;413:189–196. doi: 10.1007/BF00718610. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Taylor-Papadimitriou J. Exp. Cell Res. 1991;194:267–274. doi: 10.1016/0014-4827(91)90364-z. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J. J. Cell Sci. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Bergstraesser LM, Weitzman SA. Cancer Res. 1993;53:2644–2654. [PubMed] [Google Scholar]
- Bergstraesser LM, Weitzman SA. Int. J. Oncol. 1994;4:915–930. doi: 10.3892/ijo.4.4.915. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Biochem. Biophys. Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bishop JM. Science. 1987;235:305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bishop JM. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. Int. Rev. Cytology. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. In: Tilly JL, Strauss JF III, Tenniswood M, editors. Cell death reproductive physiology; Serono Symposia USA; 1997. pp. 125–140. [Google Scholar]
- Bissell MJ, Barcellos-Hoff MH. J. Cell Sci. Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Werb Z. Semin. Cancer Biol. 1995;6:117–118. doi: 10.1006/scbi.1995.0024. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ. In: Extracellular Matrix, Molecular Components and Interactions. Comper WD, editor. Vol. 2. Amsterdam, The Netherlands: Overseas Publishers Association, Harwood Academic; 1996. pp. 246–261. [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracke ME, van Roy FM, Marell MM. Curr. Topics Microbiol. Immunol. 1996;213:123–161. doi: 10.1007/978-3-642-61107-0_9. [DOI] [PubMed] [Google Scholar]
- Briand P, Petersen OW, Van Deurs B. In Vitro Cell. Dev. Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- Briand P, Nielsen KV, Madsen MW, Petersen OW. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- Bussolati G, Cassoni P, Ghisolfi G, Negro F, Sapino A. Am. J. Pathol. 1996;148:1895–1903. [PMC free article] [PubMed] [Google Scholar]
- Campiglio M, Tagliabue E, Srinivas U, Pellegrini R, Martignone S, Ménard S, Colnaghi MI, Lombardi L, Marchisio PC. J. Cell Biochem. 1994;55:409–418. doi: 10.1002/jcb.240550402. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. J. Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Papotti M, Bussolati G. Int. J. Cancer. 1996;66:817–820. doi: 10.1002/(SICI)1097-0215(19960611)66:6<817::AID-IJC18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chang SE, Keen J, Lane EB, Taylor-Papadimitriou J. Cancer Res. 1982;42:2040–2053. [PubMed] [Google Scholar]
- Clarke AS, Lotz MM, Chao C, Mercurio AM. J. Biol. Chem. 1995;270:22673–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- Cress AE, Rabinovitz I, Zhu W, Nagle RB. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- Deugner MA, Moiseyeva E, Thiery J-P, Glukhova M. Dev. Dyn. 1995;204:107–117. doi: 10.1002/aja.1002040202. [DOI] [PubMed] [Google Scholar]
- Dowling JQ, Yu Q-C, Fuchs E. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R, Henahan M, Armstrong B. Proc. Natl. Acad. Sci. U.S.A. 1982;79:7346–7350. doi: 10.1073/pnas.79.23.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Simons K. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Thurfjell E, Hsieh CC, Trichopoulos D, Adami HO. Int. J. Cancer. 1995;61:177–180. doi: 10.1002/ijc.2910610206. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Ferguson DJP, Anderson TJ. Virchow–s Arch. [Pathol. Anat.] 1983;401:163–175. doi: 10.1007/BF00692642. [DOI] [PubMed] [Google Scholar]
- Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tony T. J. Natl. Cancer Inst. 1993;85:892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Nature (London) 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Friedrichs K, Ruiz P, Franke F, Gille I, Terpe H-J, Imhof BA. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- Fujita S, Suzuki H, Kinoshita M, Hirohashi S. Jpn. J. Cancer Res. 1992;83:1317–1326. doi: 10.1111/j.1349-7006.1992.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Mainiero F. Biochem. Biophys. Acta. 1994;1198:47–64. doi: 10.1016/0304-419x(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Glukhova M, Koteliansky V, Sastre X, Thiery J-P. Am. J. Pathol. 1995;146:706–716. [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Nature (London) 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Harris CC. Cancer Res. 1987;47:1–10. [PubMed] [Google Scholar]
- Hart IR, Easty D. Semin. Cancer Biol. 1991;2:87–95. [PubMed] [Google Scholar]
- Haslam SZ, Nummy KA. J. Steroid Biochem. Mol. Biol. 1992;42:589–595. doi: 10.1016/0960-0760(92)90449-s. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Shyamala G. Endocrinology. 1981;108:825–830. doi: 10.1210/endo-108-3-825. [DOI] [PubMed] [Google Scholar]
- Hay ED. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Petersen OW, Steeg PS, Bissell MJ. J. Natl. Cancer Inst. 1994;86:1838–1844. doi: 10.1093/jnci/86.24.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. J. Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Semin. Cancer Biol. 1992;3:57–63. [PubMed] [Google Scholar]
- Jones JL, Critchley DR, Walker RA. J. Pathol. 1992;167:399–406. doi: 10.1002/path.1711670409. [DOI] [PubMed] [Google Scholar]
- Jones J, Sugiyama M, Speight PM, Watt FM. Oncogene. 1996;12:119–126. [PubMed] [Google Scholar]
- Joslyn SA, Gesme DH, Lynch CF. Breast J. 1996;2:187–196. [Google Scholar]
- Kelsey JL, Berkowitz GS. Cancer Res. 1988;48:5615–5623. [PubMed] [Google Scholar]
- Khan SA. Breast J. 1995;1:251–261. [Google Scholar]
- Klein G, Klein E. Nature (London) 1985;315:190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Koukoulis GK, Virtanen I, Korhonen M, Laitinen L, Quarante V, Gould VE. Am. J. Pathol. 1991;139:787–799. [PMC free article] [PubMed] [Google Scholar]
- Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE. Submicrosc. Cytol. Pathol. 1993;25:285–295. [PubMed] [Google Scholar]
- Leal CB, Schmitt FC, Bento MJ, Maia NC, Lopes CS. Cancer. 1995;75:2123–2131. doi: 10.1002/1097-0142(19950415)75:8<2123::aid-cncr2820750815>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lee SW. Nature Med. 1996;2:776–782. doi: 10.1038/nm0796-776. [DOI] [PubMed] [Google Scholar]
- Leone A, Flatow U, van Houtte K, Steeg PS. Oncogene. 1993;8:2325–2333. [PubMed] [Google Scholar]
- Levenson AS, Jordon VC. J. Steroid Biochem. Mol. Biol. 1994;51:229–239. doi: 10.1016/0960-0760(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L. Cancer Res. 1996;56:1155–1163. [PubMed] [Google Scholar]
- Lopez-Conejo T, Olmo N, Turnay J, Navarro J, Lizarbe A. Int. J. Cancer. 1996;67:668–675. doi: 10.1002/(SICI)1097-0215(19960904)67:5<668::AID-IJC13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Lotan R. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Lu QL, Rughetti A, Taylor-Papadimitriou J. J. Cell Biol. 1995;129:1363–1378. doi: 10.1083/jcb.129.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholt BK, Madsen MW, Lykkesfeldt AE, Petersen OW, Briand P. Mol. Cell. Endocrinol. 1996;119:47–59. doi: 10.1016/0303-7207(96)03793-8. [DOI] [PubMed] [Google Scholar]
- Lynch RG. Proc. Natl. Acad. Sci. U.S.A. 1995;92:647–648. doi: 10.1073/pnas.92.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MW, Lykkesfeldt AE, Laursen I, Nielsen KV, Briand P. Cancer Res. 1992;52:1210–1217. [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. J. Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui T, Wakefield LM, Lechner JF, LaVeck MA, Sporn MB, Harris CC. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2438–2442. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer G, Munk M, Barth T, Koretz K, Möller P. Virchow’s Arch. A Pathol. Anat. 1993;422:203–210. doi: 10.1007/BF01621803. [DOI] [PubMed] [Google Scholar]
- Medina D, Li ML, Oborn CJ, Bissell MJ. Exp. Cell Res. 1987;172:192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Botti C, Mottolese M, Bigotti A, Segatto O. Br. J. Cancer. 1992;66:318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettesheim P, Barrett JC. Carcinogenesis; Comprehensive Surv. 1985;9:283–291. [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T. Development. 1995;121:2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- Noda M. FASEB J. 1993;7:834–840. doi: 10.1096/fasebj.7.10.8344483. [DOI] [PubMed] [Google Scholar]
- Oda K, Itoh H, Utsunomiya H, Itoh J, Osamura RY, Tokuda Y, Kubota M, Tajima T. Pathol. Int. 1994;44:435–441. doi: 10.1111/j.1440-1827.1994.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Oridate N, Lotan D, Lotan R. In Vitro Cell. Dev. Biol.-Animal. 1996;32:192–193. doi: 10.1007/BF02722944. [DOI] [PubMed] [Google Scholar]
- Pauley RJ, Soule HD, Tait L, Miller FR, Wolman SR, Dawson PJ, Heppner GH. Eur. J. Cancer Prev. 1993;2:67–76. [PubMed] [Google Scholar]
- Petersen OW, Høyer PE, van Deurs B. Cancer Res. 1987;47:5748–5751. [PubMed] [Google Scholar]
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Rønnov-Jessen L, Bissell MJ. Breast, J. 1995;1:22–35. [Google Scholar]
- Petrangeli E, Lubrano C, Ortolani F, Ravenna L, Vacca A, Sciacchitano S, Frati L, Gulino A. J. Steroid Biochem. Mol. Biol. 1994;49:327–331. doi: 10.1016/0960-0760(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Pierce GB, Speers WC. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- Pierceall WE, Woodard AS, Morrow JS, Rimm D, Fearson ER. Oncogene. 1995;11:1319–1326. [PubMed] [Google Scholar]
- Previtali S, Quattrini A, Nemini R, Truci G, Ducati A, Wrabetz L, Canal N. J. Neuropathol. Exp. Neurol. 1996;55:456–465. doi: 10.1097/00005072-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson NSB, Gazet J-C, Nolan C, Coombes RC. Cancer Res. 1991;51:1817–1822. [PubMed] [Google Scholar]
- Robertson JFR. Br. J. Cancer. 1996;73:5–12. doi: 10.1038/bjc.1996.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L. Breast J. 1996;2:320–339. [Google Scholar]
- Rønnov-Jessen L, Petersen OW, Kotelainsky VE, Bissell MJ. J. Clin. Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L, Petersen OW, Bissell MJ. Physiol. Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Bissell MJ. Biochem. Cell Biol. 1995;73:391–397. doi: 10.1139/o95-046. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Fernig DG, Smith JA. Biomed. Pharmacother. 1995;49:389–399. doi: 10.1016/0753-3322(96)82676-x. [DOI] [PubMed] [Google Scholar]
- Russo J, Tay LK, Russo IH. Breast Cancer Res. Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- Sachs L. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4742–4749. doi: 10.1073/pnas.93.10.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapino A, Macri L, Gugliotta P, Pacchioni D, Liu Y-J, Medina D, Bussolati G. Differentiation. 1993;55:13–18. doi: 10.1111/j.1432-0436.1993.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Bittner G. Cancer Res. 1995;55:6215–6221. [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C-A, Zuk A, Zinki GM, Kendal D, Matlin KS. J. Cell Biol. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Sell S, Pierce GB. Lab. Invest. 1994;70:6–22. [PubMed] [Google Scholar]
- Serini G, Trusolino L, Saggiorato E, Cremona O, De Rossi M, Angeli A, Orlandi E, Marchisio PC. J. Natl. Cancer Inst. 1996;88:442–449. doi: 10.1093/jnci/88.7.442. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Chao C, Wewer UM, Mercurio AM. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- Shearer M, Bartkova J, Bartek J, Berdichevsky F, Barnes D, Millis R, Taylor-Papadimitriou J. Int. J. Cancer. 1992;51:602–612. doi: 10.1002/ijc.2910510417. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Frazier WA. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6788–6792. doi: 10.1073/pnas.92.15.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. BioEssays. 1996;18:655–660. doi: 10.1002/bies.950180809. [DOI] [PubMed] [Google Scholar]
- Sledge GW. Semin. Oncol. 1996;23(Suppl 2):76–81. [PubMed] [Google Scholar]
- Snedeker SM, Diagustine RP. Prog. Clin. Biol. Res. 1996;394:211–253. [PubMed] [Google Scholar]
- Sonnenberg A, Daams H, van der Valk MA, Hilkens J, Hilgers J. J. Histochem. Cytochem. 1986;34:1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Linders CJ, Daams JH, Kennel SJ. J. Cell Sci. 1990;96:207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- Soule HD, McGrath CM. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. J. Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar L, Fagerberg G, Chen HH, Duffy SM, Gad A. Int. J. Cancer. 1996;66:413–419. doi: 10.1002/(SICI)1097-0215(19960516)66:4<413::AID-IJC1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA, Man Y. Breast J. 1995;3:155–162. [Google Scholar]
- Taylor-Papadimitriou J, Alford D. Recent Advances in Breast Cancer; Bristol Meyers Squibb (Oncology) Colloquium—Scandinavian Breast Group; October 11–14; Hindsgavl, Funen, Denmark. 1995. pp. 17–32. [Google Scholar]
- Taylor-Papadimitriou J, Birgitte Lane E, Chang SE. In: Understanding Breast Cancer. Rich MA, Hager JC, Furmanski P, editors. New York: Dekker, Inc.; 1983. pp. 215–246. [Google Scholar]
- Teixeira MR, Pandis N, Bardi G, Andersen JA, Mitelman F, Heim S. Int. J. Cancer. 1995;63:63–68. doi: 10.1002/ijc.2910630113. [DOI] [PubMed] [Google Scholar]
- Umemura S, Osamura RY, Tsutsumi Y. Pathology Int. 1996;46:105–121. doi: 10.1111/j.1440-1827.1996.tb03586.x. [DOI] [PubMed] [Google Scholar]
- Underwood JCE. Diagnostic Histopathol. 1983;6:1–22. [PubMed] [Google Scholar]
- Vang Nielsen K, Madsen MW, Briand P. Cancer Genet. Cytogenet. 1994;78:189–199. doi: 10.1016/0165-4608(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Varner JS, Cheresh DA. Curr. Opin. Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. J. Cell Biol. 1990;95:137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Fischer AH, Petersen OW, Bissell MJ. Biochem. Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, et al. In preparation. 1998 [Google Scholar]
- Weinburg RA. Cancer Res. 1989;49:3713–3721. [PubMed] [Google Scholar]
- Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, McAuley A, Ashkenas J, Bissell MJ. Kidney Int.-Suppl. 1996;54:S68–S74. [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Daniel CW. Dev. Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Collman GW, Barret JC, Huff J. Annu. Rev. Pharmacol. Toxicol. 1996;36:573–596. doi: 10.1146/annurev.pa.36.040196.003041. [DOI] [PubMed] [Google Scholar]
- Zabrenetsky V, Harris CC, Steeg PS, Roberts DD. Int. J. Cancer. 1994;59:191–195. doi: 10.1002/ijc.2910590209. [DOI] [PubMed] [Google Scholar]
- Zajchowski DA, Sager R, Webster L. Cancer Res. 1993;53:5004–5011. [PubMed] [Google Scholar]
- Zutter MM, Mazoujian G, Santoro SA. Am. J. Pathol. 1990;137:863–869. [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Krigman HR, Santoro SA. Am. J. Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Staaz WD, Tsuang YL. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]