Abstract
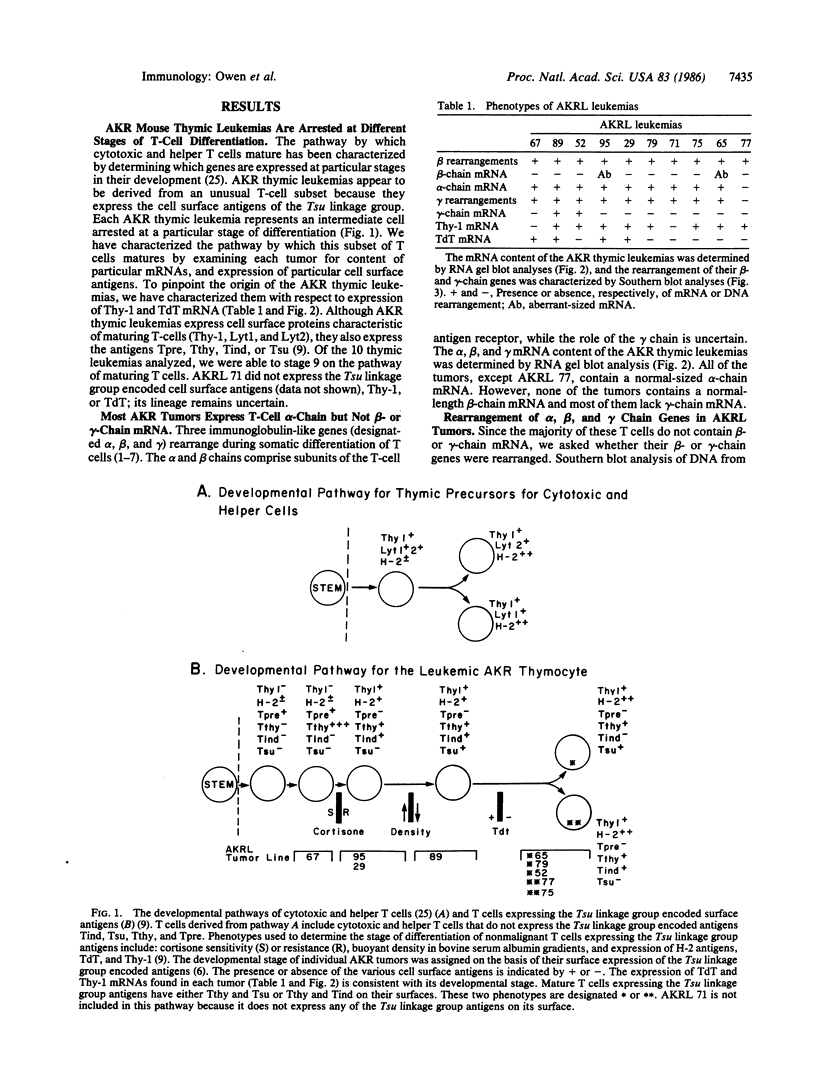
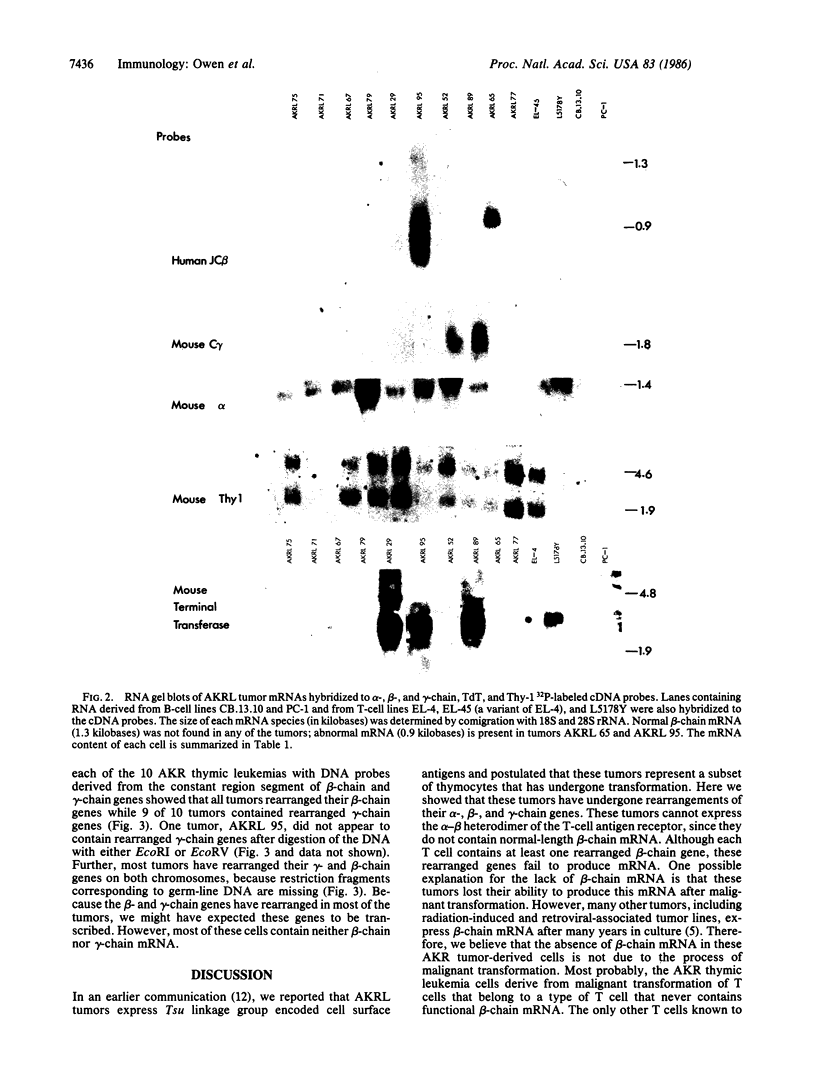
Characterization of tumors that arise spontaneously in the AKR mouse indicates that they are derived from cells of a distinct T-cell lineage. Cells in this subclass bear surface antigens, designated Tpre, Tthy, Tind, and Tsu, which are encoded by genes in the Tsu linkage group on murine chromosome 12. We have examined the rearrangement and expression of genes encoding the T-cell alpha, beta, and gamma chains in these tumors. Although these cells contain alpha-chain mRNA, they do not produce a normal-sized beta-chain mRNA. Most of them also lack gamma-chain mRNA. Each thymic leukemia was derived from a cell arrested at a different stage of development as defined by their expression of terminal deoxynucleotidyl transferase and Thy-1 mRNA. The data presented here are consistent with a model in which thymocytes expressing Tpre, Tthy, Tind, or Tsu undergo somatic development parallel to the development of other T cells. However, these thymocytes do not appear to differentiate into cells bearing alpha-beta heterodimers of the T-cell antigen receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Duby A. D., Klein K. A., Murre C., Seidman J. G. A novel mechanism of somatic rearrangement predicted by a human T-cell antigen receptor beta-chain complementary DNA. Science. 1985 Jun 7;228(4704):1204–1206. doi: 10.1126/science.3839095. [DOI] [PubMed] [Google Scholar]
- Haars R., Kronenberg M., Gallatin W. M., Weissman I. L., Owen F. L., Hood L. Rearrangement and expression of T cell antigen receptor and gamma genes during thymic development. J Exp Med. 1986 Jul 1;164(1):1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Germain R. N., Bevan M. J., Dorf M., Engel I., Fink P., Gascoigne N., Heber-Katz E., Kapp J., Kaufmann Y. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985 Jan;82(2):531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshima H., Hiai H., Fujiki H., Iijima S., Sugimura T., Nishizuka Y. Teleocidin-induced modulation of growth and cell interaction in microenvironment-dependent mouse leukemias. Leuk Res. 1983;7(2):287–293. doi: 10.1016/0145-2126(83)90019-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G. Monoclonal cytotoxic T lymphocyte hybridomas capable of specific killing activity, antigenic responsiveness, and inducible interleukin secretion. J Immunol. 1983 Jul;131(1):50–56. [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Disteche C. M., Swisshelm K., Pravtcheva D., Ruddle F. H., Eisen H. N., Tonegawa S. Chromosomal locations of the murine T-cell receptor alpha-chain gene and the T-cell gamma gene. Science. 1985 Feb 22;227(4689):941–945. doi: 10.1126/science.3918347. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Landau N. R., St John T. P., Weissman I. L., Wolf S. C., Silverstone A. E., Baltimore D. Cloning of terminal transferase cDNA by antibody screening. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5836–5840. doi: 10.1073/pnas.81.18.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Miyatani S., Hiramatsu K., Nakajima P. B., Owen F. L., Tada T. Structural analysis of antigen-specific Ia-bearing regulatory T-cell factors: gel electrophoretic analysis of the antigen-specific augmenting T -cell factor. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6336–6340. doi: 10.1073/pnas.80.20.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., Waldmann R. A., Morton C. C., Bongiovanni K. F., Waldmann T. A., Shows T. B., Seidman J. G. Human gamma-chain genes are rearranged in leukaemic T cells and map to the short arm of chromosome 7. Nature. 1985 Aug 8;316(6028):549–552. doi: 10.1038/316549a0. [DOI] [PubMed] [Google Scholar]
- Nakajima P. B., Ochi A., Owen F. L., Tada T. Presence of IgT-C and I-A subregion-encoded determinants on distinct chains of monoclonal antigen-specific augmenting factor derived from a T cell hybridoma. J Exp Med. 1983 Jun 1;157(6):2110–2120. doi: 10.1084/jem.157.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Peterman G. M. Neoplastic model for the differentiation of a subpopulation of lymphocytes bearing IgH-1-linked gene products. Immunol Rev. 1984 Dec;82:29–46. doi: 10.1111/j.1600-065x.1984.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Owen F. L. Products of the IgT-C region of chromosome 12 are maturational markers for T cells. Sequence of appearance in immunocompetent T cells parallels ontogenetic appearance of Tthyd, Tindd, and Tsud. J Exp Med. 1982 Sep 1;156(3):703–718. doi: 10.1084/jem.156.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L. T cell alloantigens encoded by the IgT-C region of chromosome 12 in the mouse. Adv Immunol. 1983;34:1–38. doi: 10.1016/s0065-2776(08)60375-2. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Bell G. I., Rutter W. J., Brown J. A., Shows T. B. The insulin gene is located on the short arm of chromosome 11 in humans. Diabetes. 1981 Mar;30(3):267–270. doi: 10.2337/diab.30.3.267. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Bonyhadi M., Herberman R. B., Young H. A., Hedrick S. M. Lack of gene rearrangement and mRNA expression of the beta chain of the T cell receptor in spontaneous rat large granular lymphocyte leukemia lines. J Exp Med. 1985 May 1;161(5):1249–1254. doi: 10.1084/jem.161.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Caccia N., Kronenberg M., Chin B., Roder J., Rohel D., Kiyohara T., Lauzon R., Toyonaga B., Rosenthal K. Gene rearrangement in cells with natural killer activity and expression of the beta-chain of the T-cell antigen receptor. Nature. 1985 Apr 18;314(6012):631–633. doi: 10.1038/314631a0. [DOI] [PubMed] [Google Scholar]