Abstract
Background
Postpartum haemorrhage is a leading cause of maternal morbidity and mortality worldwide. Identifying risk indicators for postpartum haemorrhage is crucial to predict this life threatening condition. Another major contributor to maternal morbidity and mortality is pre-eclampsia. Previous studies show conflicting results in the association between pre-eclampsia and postpartum haemorrhage. The primary objective of this study was to investigate the association between pre-eclampsia and postpartum haemorrhage. Our secondary objective was to identify other risk indicators for postpartum haemorrhage in the Netherlands.
Methods
A nationwide cohort was used, containing prospectively collected data of women giving birth after 19 completed weeks of gestation from January 2000 until January 2008 (n = 1 457 576). Data were extracted from the Netherlands Perinatal Registry, covering 96% of all deliveries in the Netherlands. The main outcome measure, postpartum haemorrhage, was defined as blood loss of ≥1000 ml in the 24 hours following delivery. The association between pre-eclampsia and postpartum haemorrhage was investigated with uni- and multivariable logistic regression analyses.
Results
Overall prevalence of postpartum haemorrhage was 4.3% and of pre-eclampsia 2.2%. From the 31 560 women with pre-eclampsia 2 347 (7.4%) developed postpartum haemorrhage, compared to 60 517 (4.2%) from the 1 426 016 women without pre-eclampsia (odds ratio 1.81; 95% CI 1.74 to 1.89). Risk of postpartum haemorrhage in women with pre-eclampsia remained increased after adjusting for confounders (adjusted odds ratio 1.53; 95% CI 1.46 to 1.60).
Conclusion
Women with pre-eclampsia have a 1.53 fold increased risk for postpartum haemorrhage. Clinicians should be aware of this and use this knowledge in the management of pre-eclampsia and the third stage of labour in order to reach the fifth Millenium Developmental Goal of reducing maternal mortality ratios with 75% by 2015.
Introduction
Postpartum haemorrhage (PPH) accounts for 25–35% of all maternal deaths worldwide. [1], [2] Unfortunately, varying definitions exist. The World Health Organization defines PPH as blood loss ≥500 ml in the first 24 hours after delivery, although in high resources settings a definition of ≥1000 ml blood loss seems more suitable. [3]–[6] Main causes of PPH are uterine atony, retained placenta, maternal soft tissue trauma and coagulopathy. [7]–[9]
Even though in high income countries the maternal mortality ratio is much lower than in low income countries, still 13% results from PPH. [2] Because of low maternal mortality ratios severe acute maternal morbidity has gained interest as a new quality indicator of obstetric care. In a nationwide cohort study in the Netherlands the incidence of major obstetric haemorrhage was 4.5 per 1000 deliveries and the case fatality rate was 1:201 (0.5%). [10] Prevention and management of PPH is crucial in order to reach the fifth Millennium Developmental Goal to reduce maternal mortality ratios with 75% by 2015. [1] This emphasises the importance of identifying specific risk indicators, such as previous PPH, multiple pregnancies, macrosomia, induction of labour, prolonged labour, operative vaginal deliveries and caesarean section. [8], [11]–[18] Another major contributor to maternal death and morbidity is pre-eclampsia (PE), complicating 2–8% of pregnancies worldwide and 2–5% in high resource countries. [2], [19], [20] While multiple studies investigated PE as one of many risk indicators for PPH [8], [11], [12], [15], [16], [21], [22] only one study focused primarily on the association between PE and PPH. [21] This is conceivable considering its multifactorial pathogenesis, where angiogenic factors, endothelial dysfunction and impaired uteroplacental blood flow result in hypertension and coagulation abnormalities. [23]
Clarification of the association between the two most important causes of maternal mortality and morbidity will help improve their prevention and management. Therefore, our objective was to investigate the association between PE and PPH. Our secondary objective was to determine the prevalence and risk indicators for PPH among pregnant women in the Netherlands.
Materials and Methods
Database
We used data from the Netherlands Perinatal Registry, a linked nationwide registry that includes maternal, obstetric, postpartum and neonatal information of each delivery from January 2000 until January 2008. [24]–[26] Approximately 96% of all births in the Netherlands are entered into this registry.
Definitions
Main outcome measure, PPH, was defined as blood loss of ≥1000 ml in the 24 hours following delivery. PE was defined as a minimum diastolic blood pressure of 90 mmHg with the presence of proteinuria after 20 weeks of gestation, according to the recommendation of the International Society for the Study of Hypertension in Pregnancy. [27] The registration form contains a field for maximum diastolic blood pressure and an option for proteinuria "yes" or "no" with a field for the amount of proteinuria. The definition of PE was based on the presence of proteinuria rather than ≥0.3 g/day, since 24.0% of women with proteinuria had a missing field for "amount of proteinuria". Women in primary care are referred to secondary care if proteinuria is present or diastolic blood pressure is over 95 mmHg or if the combination of more than 90 mmHg with complaints exists. In primary care all women with a diastolic blood pressure of 90 mmHg are checked more frequently and controlled for proteinuria according to existing guidelines. [28] In addition we defined severe PE as eclampsia or PE with blood pressure ≥110 mmHg or proteinuria ≥5 grams or gestational age of less than 32 weeks.
Characteristics
We evaluated maternal, pregnancy, labour and postpartum characteristics. Maternal characteristics included age at the time of delivery, categorised into six groups: <20, 20–24, 25–29, 30–34, 35–39 or ≥40 years. Parity was grouped as either nulliparous, parous (para 1–4), or grand multiparous (para ≥5). Ethnicity included the categories European descent and non-European descent, in which the options Dutch and other European represented the European descent group. Socioeconomic status was categorised into high, middle or low by using mean household income levels of a neighborhood, which was determined with the first four digits of the postal code, using data from the Netherlands Institute for Social Research. [29]
Pregnancy characteristics were multiple pregnancy (defined as "yes" or "no"), and gestational age, categorised as early preterm (≤31 weeks), late preterm (32–36 weeks), term (37–41 weeks) or post term (≥42 weeks) birth.
Labour characteristics included fetal presentation, defined as vertex, breech or other (transverse, face and other presentations). Onset of labour was defined as whether or not induction or elective caesarean section was performed. Furthermore, duration of ruptured membranes was grouped as < or ≥12 hours. We defined augmented labour as "yes" or "no" and prolonged expulsive phase of labour as < or ≥60 minutes. Mode of delivery was categorised as spontaneous delivery, assisted vaginal delivery (vacuum, forcipal or breech extraction), elective (planned) or emergency (unplanned) caesarean section. In the Netherlands, caesarean sections are recorded differently (planned/unplanned) compared to other countries (elective/emergency). Our system is based on an intention to treat mechanism, implying that a woman is identified as planned (elective) if she was intended to deliver by caesarean, even if she presents in labour. [30] Genital tract injury was defined as perineal tear, episiotomy or both. First degree perineal tears were not classified as genital tract injury. We classified the use of anaesthetics hierarchically, considering the possibility of receiving different types during delivery. Options were: no or primary care medication (non-opioid analgesics and sedatives), opioids, epidural during labour, epidural or spinal anaesthesia at caesarean section or general anaesthesia. If a woman had been given multiple options she was assigned to the highest category.
Postpartum characteristics consisted of manual placenta removal ("yes" or "no") and birth weight, categorised into ≤999, 1000–1999, 2000–2999, 3000–3999 or ≥4000 gram.
Statistical analysis
Contingency tables were created to assess frequencies and percentages of the characteristics and the main outcome measure among women with and without PE. Differences were tested with χ2-statistics. The characteristics of the excluded women with missing values on PE or PPH were investigated. Univariable logistic regression analysis was performed on all characteristics to identify risk indicators for PPH. The reference category of each variable represented the largest number of women in the population.
The association between (severe) PE and PPH was studied with uni- and multivariable logistic regression, adjusting for maternal age, parity, ethnicity, socioeconomic status, multiple pregnancy, and gestational age. All these indicators have a pathofysiologic explanation for confounding the association of PE with PPH. [31], [32] We also assessed presence of effect modification between multiple pregnancy and PE using the likelihood-ratio test.
Even though mode of delivery and induction of labour are important variables in the association of PE and PPH, they are intermediate factors in the causal pathway of the association and were therefore not considered as confounders. [31], [32] For that reason we performed subgroup analysis on women with non-induced spontaneous delivery and investigated the other subgroups for the association between PE and PPH [33].
Seeing an increasing trend of PPH over time the International Postpartum Hemorrhage Collaborative Group recommends countries to publish its yearly prevalence. [6], [17], [18], [34] Also the prevalence of PE has been shown to increase, therefore we analysed yearly prevalences of both PE and PPH. [20] We performed Cochran-Armitage and Jonckheere-Terpstra tests to evaluate possible trends.
Statistical analysis was performed using SAS (version 9.3; SAS Institute, Cary, NC).
Ethics statement
The presented data are anonymised and cannot be related to individual women. The privacy committee of the Netherlands Perinatal Registry approved this study. Further consent and ethical approval is not needed in the Netherlands for this type of study.
Results
During our study period 1 621 053 women delivered above 19 completed weeks of gestation. After excluding missing values our study population comprised 1 457 576 women (excluded were 108 478 missing PE, 47 885missing PPH and 7 114 missing PE and PPH; respectively 6.7%, 2.9% and 0.4% of the total population). Tables 1, 2, and 3 show the prevalence of maternal, pregnancy, labour and postpartum characteristics among all women and women with and without PE or PPH, together with corresponding odds ratios (ORs) for PPH.
Table 1. Maternal and pregnancy characteristics of the study population (n = 1.457.576) and the association between these characteristcs and postpartum haemorrhage.
| Pre-eclampsia | Postpartum haemorrhage | Unadjusted odds | |||||||||||
| Yes* | No* | Yes* | No* | Total* | ratio for postpartum haemorrhage | ||||||||
| Characteristic | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | OR | (95% CI) | |
| Pregnant women | 31560 | (2.2) | 1426016 | (97.8) | 62864 | (4.3) | 1394712 | (95.7) | 1457576 | (100.0) | |||
| Maternal characteristics | |||||||||||||
| Maternal age, years† | |||||||||||||
| ≤19 | 535 | (1.7) | 23340 | (1.6) | 770 | (1.2) | 23105 | (1.7) | 23875 | (1.6) | 0.70 | (0.66 to 0.75) | |
| 20–24 | 3519 | (11.2) | 143912 | (10.1) | 5149 | (8.2) | 142282 | (10.2) | 147431 | (10.1) | 0.76 | (0.74 to 0.78) | |
| 25–29 | 9841 | (31.2) | 411110 | (28.8) | 17163 | (27.3) | 403788 | (29.0) | 420951 | (28.9) | 0.90 | (0.88 to 0.92) | |
| 30–34 | 11332 | (35.9) | 563918 | (39.6) | 25903 | (41.2) | 549347 | (39.4) | 575250 | (39.5) | 1.00 | reference | |
| 35–39 | 5342 | (16.9) | 248610 | (17.4) | 12091 | (19.2) | 241861 | (17.3) | 253952 | (17.4) | 1.07 | (1.04 to 1.09) | |
| ≥40 | 989 | (3.1) | 34907 | (2.4) | 1780 | (2.8) | 34116 | (2.4) | 35896 | (2.5) | 1.13 | (1.08 to 1.19) | |
| Missing | 2 | 219 | 8 | 213 | 221 | ||||||||
| Parity† | |||||||||||||
| 0 | 22006 | (69.7) | 643195 | (45.1) | 32592 | (51.9) | 632609 | (45.4) | 665201 | (45.6) | 1.27 | (1.25 to 1.29) | |
| 1–4 | 9338 | (29.6) | 770926 | (54.1) | 29783 | (47.4) | 750481 | (53.8) | 780264 | (53.5) | 1.00 | reference | |
| ≥5 | 214 | (0.7) | 11762 | (0.8) | 481 | (0.8) | 11495 | (0.8) | 11976 | (0.8) | 1.07 | (0.98 to 1.16) | |
| Missing | 2 | 133 | 8 | 127 | 135 | ||||||||
| Ethnicity (European descent)‡ | |||||||||||||
| No | 5002 | (15.9) | 216407 | (15.3) | 8914 | (14.2) | 212495 | (15.3) | 221409 | (15.3) | 0.90 | (0.88 to 0.92) | |
| Yes | 26475 | (84.1) | 1200916 | (84.7) | 53712 | (85.8) | 1173679 | (84.7) | 1227391 | (84.7) | 1.00 | reference | |
| Missing | 83 | 8693 | 238 | 8538 | 8776 | ||||||||
| Socioeconomic status† | |||||||||||||
| High | 6812 | (21.9) | 333703 | (23.7) | 15720 | (25.4) | 324795 | (23.6) | 340515 | (23.7) | 1.09 | (1.07 to 1.11) | |
| Medium | 14069 | (45.2) | 647662 | (46.0) | 28165 | (45.5) | 633566 | (46.0) | 661731 | (46.0) | 1.00 | reference | |
| Low | 10258 | (32.9) | 426359 | (30.3) | 18071 | (29.2) | 418546 | (30.4) | 436617 | (30.3) | 0.97 | (0.95 to 0.98) | |
| Missing | 421 | 18292 | 908 | 17805 | 18713 | ||||||||
| Pregnancy characteristics | |||||||||||||
| Multiple pregnancy† | |||||||||||||
| No | 29446 | (93.3) | 1400362 | (98.2) | 59492 | (94.6) | 1370316 | (98.3) | 1429808 | (98.1) | 1.00 | reference | |
| Yes | 2114 | (6.7) | 25654 | (1.8) | 3372 | (5.4) | 24396 | (1.7) | 27768 | (1.9) | 3.07 | (2.96 to 3.17) | |
| Gestational age, weeks† | |||||||||||||
| ≤31+6 | 2277 | (7.3) | 14846 | (1.0) | 1360 | (2.2) | 15763 | (1.1) | 17123 | (1.2) | 2.01 | (1.91 to 2.11) | |
| 32+0 – 36+6 | 8086 | (25.8) | 70628 | (5.0) | 4435 | (7.1) | 74279 | (5.4) | 78714 | (5.4) | 1.37 | (1.33 to 1.41) | |
| 37+0 – 41+6 | 20680 | (65.9) | 1262219 | (89.0) | 52339 | (83.7) | 1230560 | (88.7) | 1282899 | (88.5) | 1.00 | reference | |
| ≥42+0 | 342 | (1.1) | 70116 | (4.9) | 4409 | (7.0) | 66049 | (4.8) | 70458 | (4.9) | 1.54 | (1.49 to 1.59) | |
| Missing | 175 | 8207 | 321 | 8061 | 8382 | ||||||||
Missing values of postpartum haemorrhage and pre-eclampsia are exluded.
2test between pre-eclampsia and no pre-eclampsia, P ≤ 0.0001. X
2test between pre-eclampsia and no pre-eclampsia,, P = 0.01. X
Table 2. Labour characteristics of the study population (n = 1.457.576) and the association between these characteristcs and postpartum haemorrhage.
| Pre-eclampsia | Postpartum haemorrhage | Unadjusted odds ratio for | |||||||||||
| Yes* | No* | Yes* | No* | Total* | postpartum haemorrhage | ||||||||
| Characteristic | n | (%) | N | (%) | n | (%) | n | (%) | n | (%) | OR | (95% CI) | |
| Pregnant women | 31560 | (2.2) | 1426016 | (97.8) | 62864 | (4.3) | 1394712 | (95.7) | 1457576 | (100.0) | |||
| Presentation† | |||||||||||||
| Vertex | 28405 | (90.1) | 1346629 | (94.5) | 59701 | (95.0) | 1315333 | (94.3) | 1375034 | (94.4) | 1.00 | reference | |
| Breech | 2742 | (8.7) | 69527 | (4.9) | 2496 | (4.0) | 69773 | (5.0) | 72269 | (5.0) | 0.80 | (0.77 to 0.83) | |
| Other | 382 | (1.2) | 9327 | (0.7) | 618 | (1.0) | 9091 | (0.7) | 9709 | (0.7) | 1.54 | (1.42 to 1.66) | |
| Missing | 31 | 533 | 49 | 515 | 564 | ||||||||
| Onset of labour† | |||||||||||||
| Non-induced labour | 6700 | (21.3) | 1146966 | (80.5) | 46120 | (73.6) | 1107546 | (79.5) | 1153666 | (79.2) | 1.00 | reference | |
| Induced labour | 17438 | (55.4) | 193983 | (13.6) | 12945 | (20.6) | 198476 | (14.2) | 211421 | (14.5) | 1.52 | (1.49 to 1.55) | |
| Elective caesarean | 7334 | (23.3) | 83639 | (5.9) | 3624 | (5.8) | 87349 | (6.3) | 90973 | (6.2) | 1.02 | (0.99 to 1.06) | |
| Missing | 88 | 1428 | 175 | 1341 | 1516 | ||||||||
| Ruptured membranes, hours† | |||||||||||||
| <12 | 25617 | (86.1) | 1134348 | (82.9) | 47867 | (78.5) | 1112098 | (83.2) | 1159965 | (83.0) | 1.00 | reference | |
| ≥12 | 4132 | (13.9) | 233257 | (17.1) | 13117 | (21.5) | 224272 | (16.8) | 237389 | (17.0) | 1.35 | (1.32 to 1.37) | |
| Missing | 1811 | 58411 | 1880 | 58342 | 60222 | ||||||||
| Augmentation of labour† | |||||||||||||
| No | 22931 | (72.7) | 1141004 | (80.0) | 45180 | (71.9) | 1118755 | (80.2) | 1163935 | (79.9) | 1.00 | reference | |
| Yes | 8629 | (27.3) | 285012 | (20.0) | 17684 | (28.1) | 275957 | (19.8) | 293641 | (20.1) | 1.52 | (1.50 to 1.55) | |
| Expulsive phase, minutes† | |||||||||||||
| <60 | 27553 | (88.0) | 1186426 | (84.0) | 48349 | (77.8) | 1165630 | (84.4) | 1213979 | (84.1) | 1.00 | reference | |
| ≥60 | 3767 | (12.0) | 225148 | (16.0) | 13780 | (22.2) | 215135 | (15.6) | 228915 | (15.9) | 1.52 | (1.49 to 1.55) | |
| Missing | 240 | 14442 | 735 | 13947 | 14682 | ||||||||
| Mode of delivery† | |||||||||||||
| Spontaneous delivery | 14784 | (46.9) | 1088924 | (76.4) | 46376 | (74.0) | 1057332 | (75.9) | 1103708 | (75.8) | 1.00 | reference | |
| Assisted delivery | 3959 | (12.6) | 144854 | (10.2) | 9383 | (15.0) | 139430 | (10.0) | 148813 | (10.2) | 1.49 | (1.46 to 1.52) | |
| Elective caesarean | 7334 | (23.3) | 83639 | (5.9) | 3624 | (5.8) | 87349 | (6.3) | 90973 | (6.2) | 0.98 | (0.95 to 1.08) | |
| Emergency caesarean | 5453 | (17.3) | 107304 | (7.5) | 3275 | (5.2) | 109482 | (7.9) | 112757 | (7.7) | 0.72 | (0.70 to 0.74) | |
| Missing | 30 | 1295 | 206 | 1119 | 1325 | ||||||||
| Genital tract injury† | |||||||||||||
| Episiotomy (no)/perineal tear (no) | 23376 | (74.1) | 1024303 | (71.9) | 37681 | (60.0) | 1009998 | (72.4) | 1047679 | (71.9) | 1.00 | reference | |
| Episiotomy (no)/perineal tear (yes) | 364 | (1.2) | 23953 | (1.7) | 1679 | (2.7) | 22638 | (1.6) | 24317 | (1.7) | 1.92 | (1.83 to 2.01) | |
| Episiotomy (yes)/perineal tear (no) | 7714 | (24.5) | 371807 | (26.1) | 22933 | (36.5) | 356588 | (25.6) | 379521 | (26.0) | 1.69 | (1.66 to 1.71) | |
| Episiotomy (yes)/perineal tear (yes) | 84 | (0.3) | 5473 | (0.4) | 522 | (0.8) | 5035 | (0.4) | 5557 | (0.4) | 2.68 | (2.46 to 2.92) | |
| Missing | 22 | 480 | 49 | 453 | 502 | ||||||||
| Anaesthesia† | |||||||||||||
| No/primary care medication | 13797 | (43.7) | 1075885 | (75.4) | 45774 | (72.8) | 1043908 | (74.8) | 1089682 | (74.8) | 1.00 | reference | |
| Opioid analgesics | 3449 | (10.9) | 104489 | (7.3) | 5939 | (9.4) | 101999 | (7.3) | 107938 | (7.4) | 1.28 | (1.24 to 1.31) | |
| Epidural during labour | 2892 | (9.2) | 77721 | (5.5) | 5219 | (8.3) | 75394 | (5.4) | 80613 | (5.5) | 1.27 | (1.24 to 1.31) | |
| Spinal or epidural at caesarean | 8795 | (27.9) | 151714 | (10.6) | 4970 | (7.9) | 155539 | (11.2) | 160509 | (11.0) | 0.75 | (0.73 to 0.77) | |
| General anaesthesia | 2627 | (8.3) | 16207 | (1.1) | 962 | (1.5) | 17872 | (1.3) | 18834 | (1.3) | 1.32 | (1.24 to 1.40) | |
Missing values of postpartum haemorrhage and pre-eclampsia are excluded
2test between pre-eclampsia and no pre-eclampsia, P ≤ 0.0001 X
Table 3. Postpartum characteristics of the study population (n = 1.457.576) and the association between these characteristcs and postpartum haemorrhage.
| Pre-eclampsia | Postpartum haemorrhage | Unadjusted odds ratio for | |||||||||||
| Yes* | No* | Yes* | No* | Total* | postpartum haemorrhage | ||||||||
| Characteristic | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | OR | (95% CI) | |
| Pregnant women | 31560 | (2.2) | 1426016 | (97.8) | 62864 | (4.3) | 1394712 | (95.7) | 1457576 | (100.0) | |||
| Postpartum characteristics | |||||||||||||
| Manual placenta removal† | |||||||||||||
| No | 30348 | (96.3) | 1389321 | (98.1) | 47128 | (75.2) | 1372541 | (99.1) | 1419669 | (98.1) | 1.00 | reference | |
| Yes | 1181 | (3.7) | 26759 | (1.9) | 15525 | (24.8) | 12415 | (0.9) | 27940 | (1.9) | 33.39 | (32.62 to 34.19) | |
| Missing | 31 | 9936 | 211 | 9756 | 9967 | ||||||||
| Birthweight, grams† | |||||||||||||
| ≤999 | 1183 | (3.7) | 9013 | (0.6) | 832 | (1.3) | 9364 | (0.7) | 10196 | (0.7) | 2.08 | (1.96 to 2.23) | |
| 1000 – 1999 | 4639 | (14.7) | 17882 | (1.3) | 1259 | (2.0) | 21262 | (1.5) | 22521 | (1.5) | 1.48 | (1.41 to 1.56) | |
| 2000 – 2999 | 12035 | (38.1) | 230514 | (16.2) | 9663 | (15.4) | 232886 | (16.7) | 242549 | (16.6) | 1.01 | (0.99 to 1.03) | |
| 3000 – 3999 | 11604 | (36.8) | 942823 | (66.1) | 37305 | (59.4) | 917122 | (65.8) | 954427 | (65.5) | 1.00 | reference | |
| ≥4000 | 2097 | (6.6) | 225442 | (15.8) | 13776 | (21.9) | 213763 | (15.3) | 227539 | (15.6) | 1.59 | (1.56 to 1.62) | |
| Missing | 2 | 342 | 29 | 315 | 344 | ||||||||
Missing values of postpartum haemorrhage and pre-eclampsia are excluded.
2test between pre-eclampsia and no pre-eclampsia, P ≤ 0.0001. X
Overall prevalence of PE was 2.2%. Women with PE were more often nulliparous, had more often multiple pregnancies and had lower gestational age at birth (table 1). Furthermore, women with PE more often had elective caesarean section, induction and/or augmentation of labour, a shorter expulsive phase and less often delivered spontaneously (table 2). The groups also differed significantly in genital tract injury, anaesthetic use and manual placenta removal (tables 2 and 3).
In our study population 4.3% of the women suffered from PPH (table 1). From the 31 560 women with PE 2 347 (7.4%) developed PPH, compared to 60 517 (4.2%) from the 1 426 016 women without PE (OR 1.81; 95% CI 1.74 to 1.89, p<0.001).
The group women with missing values for PE contained more PPH (6.6% compared with 4.3% in the total population), while missing values for PPH showed slightly more PE (2.5% compared with 2.2% in the total population). Investigating the missing values showed no clear tendency for either PPH or PE. Therefore missing data is not expected to be adherent to either the exposure or the outcome measure; the missing data is assumed to be randomly distributed [35]
Table 4 shows the adjusted association between PE and PPH in all women and in a subgroup of women with non-induced spontaneous delivery. Univariably, PE increased the risk of PPH 1.81 fold (95% CI 1.74 to 1.89). After adjustment for confounders we observed a slightly lower but still statistically significant increased risk for PPH (OR 1.53, 95% CI 1.46 to 1.60). In this model non-European descent ethnicity and low socioeconomic status lost their statistical significance. In the subgroup analysis of women with non-induced spontaneous delivery PE increased the risk for PPH (adjusted OR 1.91, 95% CI 1.71 to 2.13; other subgroup analyses are shown in footnotes table 4). Distinguishing the severity of PE showed women with mild PE have a higher increased risk for PPH (adjusted OR 1.67; 95% CI 1.58 to 1.77) than women with severe PE (adjusted OR 1.32; 95% CI 1.23 to 1.42), while the subgroup of non-induced spontaneous deliveries showed reversed results (mild PE, adjusted OR 1.76, 95% CI 1.55 to 2.01; severe PE, adjusted OR 2.36; 95% CI 1.92 to 2.88).
Table 4. Multivariate analysis on the association between pre-eclampsia and postpartum haemorrhage and subgroup of women with non-induced spontaneous delivery.
| Total cohort | Subgroup non-induced spontaneous delivery† | ||||
| Adjusted odds ratio for postpartum haemorrhage* | Adjusted odds ratio for postpartum haemorrhage* | ||||
| Risk indicator | OR | (95% CI) | OR | (95% CI) | |
| Pre-eclampsia | |||||
| No | 1.00 | reference | 1.00 | reference | |
| Yes | 1.53 | (1.46 to 1.60) | 1.91 | (1.71 to 2.13) | |
| Maternal age, years | |||||
| ≤19 | 0.62 | (0.58 to 0.67) | 0.63 | (0.57 to 0.68) | |
| 20–24 | 0.71 | (0.69 to 0.73) | 0.71 | (0.68 to 0.74) | |
| 25–29 | 0.86 | (0.84 to 0.88) | 0.85 | (0.83 to 0.88) | |
| 30–34 | 1.00 | reference | 1.00 | reference | |
| 35–39 | 1.09 | (1.07 to 1.11) | 1.08 | (1.05 to 1.12) | |
| ≥40 | 1.15 | (1.09 to 1.21) | 1.13 | (1.05 to 1.21) | |
| Parity | |||||
| 0 | 1.36 | (1.34 to 1.39) | 1.48 | (1.45 to 1.51) | |
| 1–4 | 1.00 | reference | 1.00 | reference | |
| ≥5 | 0.95 | (0.87 to 1.05) | 0.85 | (0.75 to 0.97) | |
| Ethnicity | |||||
| European descent | 1.00 | reference | 1.00 | reference | |
| Non-European descent | 1.02 | (0.99 to 1.04) | 1.04 | (1.01 to 1.07) | |
| Socioeconomic status | |||||
| High | 1.07 | (1.05 to 1.09) | 1.08 | (1.05 to 1.11) | |
| Medium | 1.00 | reference | 1.00 | reference | |
| Low | 1.00 | (0.98 to 1.02) | 1.01 | (0.99 to 1.04) | |
| Multiple pregnancy | |||||
| No | 1.00 | reference | 1.00 | reference | |
| Yes | 2.88 | (2.77 to 3.00) | 2.36 | (2.18 to 2.54) | |
| Gestational age, weeks | |||||
| ≤31+6 | 1.53 | (1.44 to 1.62) | 2.15 | (1.98 to 2.34) | |
| 32+0 – 36+6 | 1.05 | (1.02 to 1.09) | 1.16 | (1.11 to 1.22) | |
| 37+0 – 41+6 | 1.00 | reference | 1.00 | reference | |
| ≥42+0 | 1.56 | (1.51 to 1.61) | 1.68 | (1.58 to 1.79) | |
Adjusted for pre-eclampsia, maternal age, parity, ethnicity, socioeconomic status, multiple pregnancy and gestational age.
% CI; 1.68 to 2.44). Subgroup non-induced emergency caesarean section OR 1.55 (95% CI; 1.16 to 2.06). Subgroup induced spontaneous delivery OR 1.46 (95% CI; 1.36 to 1.58). Subgroup induced assisted delivery OR 1.61 (95% CI; 1.41 to 1.84). Subgroup induced emergency caesarean section OR 1.40 (95% CI; 1.17 to 1.68). Subgroup elective caesarean section OR 0.98 (95% CI; 0.86 to 1.12). Adjusted risk of pre-eclampsia for postpartum haemorrhage in other subgroups: Subgroup non-induced assisted delivery OR 2.02 (95
We found no evidence of effect modification between PE and multiple pregnancy.
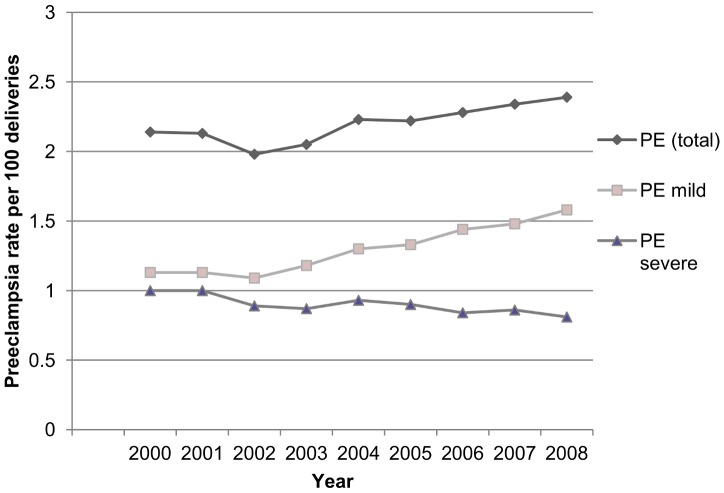
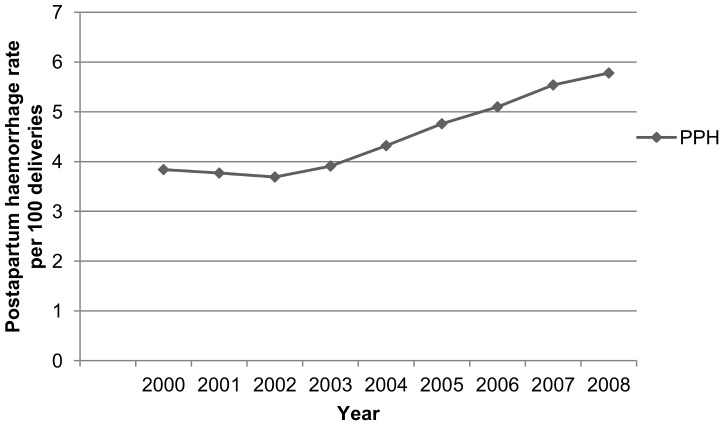
Figure 1 and 2 show the yearly percentages of PE (total, mild and severe) and PPH. There is a statistically significant increase of PE (P<0.0001) and PPH (P<0.0001) over time. We observed a rising trend of mild PE, while the proportion of severe PE gradually declined (P<0.0001).
Figure 1. Annual rates of pre-eclampsia (PE), 2000–2008.
Figure 2. Annual rates of postpartum haemorrhage (PPH), 2000–2008.
Discussion
Principal findings
Our study shows an adjusted 1.53 fold increased risk (95% CI 1.46 to 1.60) for developing PPH in women with PE.
Women with PE were more often older, nulliparous, had multiple pregnancies, a lower gestational age and a lower socioeconomic status. These indicators are also associated with increased risk for PPH, which could explain a slight decrease in risk for PPH after adjustment for confounders. [8], [11]–[15], [22], [36]–[39] Remarkably non-induced women with PE who delivered vaginally revealed an even stronger adjusted association with PPH (adjusted OR 2.29, 95% CI 2.06 to 2.55). Except for women undergoing elective caesarean section all other subgroups showed women with PE had an increased risk for PPH, suggesting the association is independent for onset of labour or mode of delivery. The observed shift in risk when assessing the severity of PE could indicate a positive side-effect in the management of PE; induction possibly reduces the risk for PPH in women with severe PE.
Our nationwide study population had a prevalence of 2.2% for PE and 4.3% for PPH, similar rates are seen internationally (United States [20], Canada [17], [40]–[42], Scandinavia [43]–[45], United Kingdom [46], Australia [47], [48]). A worrying finding is the rising trend of both PE and PPH. The increase in PE could be explained by a rising prevalence of risk indicators for PE, e.g. high body mass index, hypertension, diabetes mellitus, high maternal age. [17], [18], [20], [34], [49] The decreasing trend of severe PE may be attributed to a more aggressive management of PE over the years. The increase in PPH has been studied in many other high resource countries. [6], [17], [18], [34] They state this finding could be explained by an increase in risk indicators (e.g. maternal age, multiple pregnancies, induction of labour, caesarean section). However adjustment for these indicators did not explain the observed trend. [17], [18], [34] Another possibility could be an increasing awareness for PPH with subsequently better recognition and registration.
Strengths and weaknesses of the study
A strength of this study is the large population based database with a nationwide and near complete inclusion of all deliveries with detailed information on maternal, pregnancy, labour and postpartum characteristics. Despite the retrospective design data were prospectively collected. To our knowledge this is the largest cohort study on the relation between PE and PPH.
Another positive aspect of this study is the fact that our definitions for PE and PPH are not based on International Classification Disease (ICD) codes. Inaccuracies may occur when translating definitions to ICD-codes, since they are not thoroughly defined. ICD-codes neglect to take amount of blood loss, blood pressure or amount of proteïnuria into account, while diseases may be defined differently between countries, regions or even hospitals. [6] Therefore registration is not unified and coding might be subject to clinical interpretation of the clinician. This also presents our first limitation of the study. Even though our definition is derived from obligated objective items, we had to base the definition of PPH on estimation of blood loss by the caregiver, either weighted or visual. Visual estimation has been shown to underestimate blood loss, suggesting a higher prevalence of PPH than the observed 4.3%. [5], [50], [51] Furthermore, our overall definition of PE was based on the presence of proteinuria rather than a measured amount. Since dipstick urinalysis of protein is not 100% accurate, [52] we performed a sensitivity analysis in women with a known amount of proteinuria, now defining PE with the amount of proteinuria instead of the presence (≥0.3 g/day). This showed robustness of our findings (adjusted OR 1.49; 95% CI 1.42 to 1.57). Caution is justified in interpreting our results based on severity of PE. As there is a high percentage of missing values for proteinuria some severe PE cases could have been misclassified as mild. Yet other studies show similar results in the observed trends. [20]
Another limitation is the 6.7% women whose information on PE was missing. Characteristics of these women did not clearly correspond to either the PE or the non-PE group. A biased estimation of the association between PE and PPH is not expected, as complete case analysis with adjustment for covariates covering randomly distributed missing data (MAR), has been shown to estimate unbiased results. [35]
The perinatal registry does not contain valid or complete information on body mass index, previous PPH, previous caesarean section and certain medical disorders (e.g. coagulopathy, diabetes mellitus, cardiovascular diseases). These risk indicators have an increased risk for PE, PPH or both, therefore possibly confounding the observed association. [8], [11], [19], [53] However the role of body mass index on PPH is not clear yet. [54] Another possible and not registered confounder is magnesium sulphate, since it could, hypothetically, increase blood loss by vasodilatation, a tocolytic effect predisposing to uterine atony, prolonged bleeding time through platelet activity inhibition and red cell deformity and it is used in the Netherlands for the management of (pre-) eclampsia (as prevention in severe PE women (defined as blood pressure ≥110 mmHg diastolic or ≥110 mmHg systolic or proteinuria ≥5 grams or the presence of symptoms prognostic for eclampsia) or as treatment in women with eclampsia). Rouse et al determined an increased risk for PPH in hypertensive disorders which disappeared after correction for magnesium sulphate. [55] However a recent published systematic review showed no significantly increased risk for PPH. [56]
Findings in relation to other studies
Association pre-eclampsia and postpartum haemorrhage. Most other studies evaluate multiple risk indicators for PPH, in which PE is one of many. Results are however inconsistent, varying from no significant association after adjustment for confounders to a two to five fold increased risk [8], [11], [12], [14]–[16], [22], [37], [38], [55], [57]. Nevertheless, results are difficult to compare since various definitions for PPH are used in combination with different ways to estimate blood loss values. Only one study focused on the relation of PE and PPH. In a cohort study of 315 085 singleton pregnancies, Eskild et al showed an increased risk for PPH in women with PE (≥500 ml blood loss, OR 1.94 95% CI 1.87 to 2.02 and for ≥1500 ml blood loss OR 2.20, 95% CI 1.99 to 2.45) [21]. Similar results were found in nulliparous women with a vaginal mode of delivery. However they did not adjust for age, ethnicity, gestational age or socioeconomic status. Neither did they evaluate subgroups of women with and without induction of labour.
Other risk indicators for postpartum haemorrhage. Comparing our findings with other studies apart from PE as risk indicator, we see similar increased risks for older women, multiple pregnancy, birth weight ≥4000 grams, prolonged ruptured membranes and prolonged expulsive phase. [8], [11]–[16], [22], [36]–[39], [58]
In contrast with other studies, only great grand multiparas (para ≥10) had an increased risk for PPH (OR 1.57, 95% CI 1.12 to 2.20) when compared with multiparas (para 1–4). Other studies showed similar results, suggesting a possible linear relationship. [8], [59], [60]
An unexpected finding was the decreased risk for PPH after emergency caesarean sections. Other studies showed increased risks, which is more likely since this group often suffers from predisposing indicators for PPH, such as prolonged labour. [8], [15], [37], [57], [61] Possibly, emergency caesarean sections were misclassified as elective due to the intention to treat reporting of caesarean deliveries in the Netherlands. [30] In addition, PPH may be underreported in caesarean sections, [62] which can be expected more often in emergency situations. This could have confounded the risk of PPH in preeclampsia in our total group of women, however, the increased risk persisted in the subgroup-analysis of women who had non-induced spontaneous deliveries.
Meaning of the study
This study indicates a strong relationship between the two most important causes of maternal mortality and morbidity worldwide: PE and PPH. This is an important finding in relation to the alarming notion that there is a rising incidence of both PE and PPH. Even though this study shows an efficient management for PE, the rising overall prevalence of PE and PPH indicates these problems have not been resolved. [17], [18], [20], [34] Optimising prevention and management of these conditions continues being of utmost importance. A higher awareness is indicated during the third stage of labour in a women with PE. Also other preventive and therapeutic measures should be considered, such as easy access to second-line uterotonics, materials for tamponade and packed cells.
Considering the complexity of PE with vascular changes and hemoconcentration in conjunction with the consequences of PPH leading to transfusion, embolisation or surgery, a combination of these two conditions forms a strong indication for a multidisciplinary approach, consisting of obstetric, anaesthetic, radiological and internal support.
Future research
The most important question arising while studying the literature was the variation in risk of indicators for PPH. This can be attributed to the heterogeneity of all studies on PPH, consisting of varying study populations, definitions and statistical analyses performed. A uniform research definition is important to create comparable studies on PPH. With more homogenous studies prediction models for PPH could be formed, from which clinical implications can be drawn. Future research should therefore be on a universally acceptable definition and on prediction models for PPH with internal and external validations.
Further research in the pathogenesis of PE is also important in discerning the association of PE and PPH.
Finally, the observed trends in both PE and PPH should be investigated more detailed to discern whether the Dutch nationwide data can explain these discomforting findings. A study investigating trends for PPH in the Netherlands is currently in production.
Conclusions
In conclusion, our study shows an association between PE and PPH; women with PE have a 1.53 fold increased risk for PPH in the Netherlands. Clinicians should be aware of this increased risk and use this knowledge in the management of PE and the third stage of labour in order to reach the fifth Millennium Developmental Goal.
Acknowledgments
The authors would like to thank the Netherlands Perinatal Registry (PRN) for granting us the opportunity to analyze and publish its data. We would also like to thank Dr Frederike J. de Weger for her help with data analysis.
Funding Statement
The authors have no support or funding to report.
References
- 1. Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, et al. (2010) Countdown to 2015 decade report (2000–2010): taking stock of maternal, newborn and child survival. Lancet 376: 2032–44 10.1016/S0140-6736(10)60678-2 [doi] [DOI] [PubMed] [Google Scholar]
- 2. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF (2006) WHO analysis of causes of maternal death: a systematic review. Lancet 367: 1066–1074 S0140-6736(06)68397-9 [pii]; 10.1016/S0140-6736(06)68397-9 [doi] [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2009) WHO guidelines for the management of postpartum haemorrhage and retained placenta. Geneva: WHO Press. Available: http://apps.who.int/rhl/guidelines/postpartum_haemorrhage/en/. Accessed December 12th 2012. [PubMed]
- 4.RCOG (2011) Prevention and management of postpartum haemorrhage (Green-top 52). London: RCOG. Available: http://www.rcog.org.uk/files/rcog-corp/GT52PostpartumHaemorrhage0411.pdf. Accessed December 12th 2012.
- 5. Stafford I, Dildy GA, Clark SL, Belfort MA (2008) Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol 199: 519–7 S0002-9378(08)00501-2 [pii]; 10.1016/j.ajog.2008.04.049 [doi] [DOI] [PubMed] [Google Scholar]
- 6. Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, et al. (2009) Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth 9: 55 1471-2393-9-55 [pii]; 10.1186/1471-2393-9-55 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doran JR, O'Brien SA Jr, Randall JH (1955) Repeated postpartum hemorrhage. Obstet Gynecol 5: 186–192. [PubMed] [Google Scholar]
- 8. Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B (2008) Prevalence and risk factors of severe obstetric haemorrhage. BJOG 115: 1265–1272 BJO1859 [pii]; 10.1111/j.1471-0528.2008.01859.x [doi] [DOI] [PubMed] [Google Scholar]
- 9. Oyelese Y, Ananth CV (2010) Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol 53: 147–156 10.1097/GRF.0b013e3181cc406d [doi];00003081-201003000-00016 [pii] [DOI] [PubMed] [Google Scholar]
- 10. Zwart JJ, Richters JM, Ory F, Bloemenkamp KW, van Roosmalen J, et al. (2008) Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG 115: 842–850 BJO1713 [pii]; 10.1111/j.1471-0528.2008.01713.x [doi] [DOI] [PubMed] [Google Scholar]
- 11. Combs CA, Murphy EL, Laros RK Jr (1991) Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol 77: 69–76. [PubMed] [Google Scholar]
- 12. Sheiner E, Sarid L, Levy A, Seidman DS, Hallak M (2005) Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med 18: 149–154 N458603137265X73 [pii]; 10.1080/14767050500170088 [doi] [DOI] [PubMed] [Google Scholar]
- 13. Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP (2004) Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. A Dutch population-based cohort study on standard (> or = 500 ml) and severe (> or = 1000 ml) postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol 115: 166–172 10.1016/j.ejogrb.2003.12.008 [doi];S0301211503006390 [pii] [DOI] [PubMed] [Google Scholar]
- 14. Magann EF, Evans S, Hutchinson M, Collins R, Howard BC, et al. (2005) Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J 98: 419–422. [DOI] [PubMed] [Google Scholar]
- 15. Stones RW, Paterson CM, Saunders NJ (1993) Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol 48: 15–18. [DOI] [PubMed] [Google Scholar]
- 16. Naef RW III, Chauhan SP, Chevalier SP, Roberts WE, Meydrech EF, et al. (1994) Prediction of hemorrhage at cesarean delivery. Obstet Gynecol 83: 923–926. [DOI] [PubMed] [Google Scholar]
- 17. Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, et al. (2007) Investigation of an increase in postpartum haemorrhage in Canada. BJOG 114: 751–759 BJO1316 [pii]; 10.1111/j.1471-0528.2007.01316.x [doi] [DOI] [PubMed] [Google Scholar]
- 18. Lutomski JE, Byrne BM, Devane D, Greene RA (2012) Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG 119: 306–314 10.1111/j.1471-0528.2011.03198.x [doi] [DOI] [PubMed] [Google Scholar]
- 19. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R (2010) Pre-eclampsia. Lancet 376: 631–644 S0140-6736(10)60279-6 [pii]; 10.1016/S0140-6736(10)60279-6 [doi] [DOI] [PubMed] [Google Scholar]
- 20. Wallis AB, Saftlas AF, Hsia J, Atrash HK (2008) Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 21: 521–526 ajh200820 [pii]; 10.1038/ajh.2008.20 [doi] [DOI] [PubMed] [Google Scholar]
- 21. Eskild A, Vatten LJ (2009) Abnormal bleeding associated with preeclampsia: a population study of 315,085 pregnancies. Acta Obstet Gynecol Scand 88: 154–158 906894794 [pii]; 10.1080/00016340802613242 [doi] [DOI] [PubMed] [Google Scholar]
- 22. Ohkuchi A, Onagawa T, Usui R, Koike T, Hiratsuka M, et al. (2003) Effect of maternal age on blood loss during parturition: a retrospective multivariate analysis of 10,053 cases. J Perinat Med 31: 209–215 10.1515/JPM.2003.028 [doi] [DOI] [PubMed] [Google Scholar]
- 23. Young BC, Levine RJ, Karumanchi SA (2010) Pathogenesis of preeclampsia. Annu Rev Pathol 5: 173–192 10.1146/annurev-pathol-121808-102149 [doi] [DOI] [PubMed] [Google Scholar]
- 24. Meray N, Reitsma JB, Ravelli AC, Bonsel GJ (2007) Probabilistic record linkage is a valid and transparent tool to combine databases without a patient identification number. J Clin Epidemiol 60: 883–891 S0895-4356(06)00500-2 [pii]; 10.1016/j.jclinepi.2006.11.021 [doi] [DOI] [PubMed] [Google Scholar]
- 25.Stichting Perinatale Registratie Nederland. (2008) Perinatal Care in The Netherlands 2006.
- 26.Tromp M, Ravelli AC, Meray N, Reitsma JB, Bonsel GJ (2008) An efficient validation method of probabilistic record linkage including readmissions and twins. Methods Inf Med 47: : 356–363. 08040356 [pii]. [DOI] [PubMed] [Google Scholar]
- 27.Brown MA, Lindheimer MD, De Swiet M, Van Assche A, Moutquin JM (2001) The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 20: : IX-XIV. http://dx.doi.org/10.1081/PRG-100104165 [doi];100104165 [pii]. [DOI] [PubMed] [Google Scholar]
- 28.KNOV NLC (2003) Verloskundige Indicatielijst. Diemen: Verloskundig Vademecum. Available:http://www.knov.nl/docs/uploads/Verloskundig_Vademecum_2003.pdf. Accessed December 12th 2013.
- 29.The Netherlands Institute for Social Research (SCP) (2006) Status scores.
- 30. van Dillen J, Diesch M, Schutte J, Zwart J, van Roosmalen J, et al. (2009) Comparing grades of urgency for classification of cesarean delivery. Int J Gynaecol Obstet 107: 16–18 S0020-7292(09)00253-7 [pii]; 10.1016/j.ijgo.2009.05.001 [doi] [DOI] [PubMed] [Google Scholar]
- 31. Hernan MA, Clayton D, Keiding N (2011) The Simpson's paradox unraveled. Int J Epidemiol 40: 780–785 dyr041 [pii]; 10.1093/ije/dyr041 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schisterman EF, Cole SR, Platt RW (2009) Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20: 488–495 10.1097/EDE.0b013e3181a819a1 [doi];00001648-200907000-00004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. VanderWeele TJ, Mumford SL, Schisterman EF (2012) Conditioning on intermediates in perinatal epidemiology. Epidemiology 23: 1–9 10.1097/EDE.0b013e31823aca5d [doi];00001648-201201000-00001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callaghan WM, Kuklina EV, Berg CJ (2010) Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 202: 353–356 S0002-9378(10)00022-0 [pii]; 10.1016/j.ajog.2010.01.011 [doi] [DOI] [PubMed] [Google Scholar]
- 35. Groenwold RH, Donders AR, Roes KC, Harrell FE Jr, Moons KG (2012) Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 175: 210–217 kwr302 [pii]; 10.1093/aje/kwr302 [doi] [DOI] [PubMed] [Google Scholar]
- 36. Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, et al. (2005) Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J 98: 681–685. [DOI] [PubMed] [Google Scholar]
- 37. Bateman BT, Berman MF, Riley LE, Leffert LR (2010) The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 110: 1368–1373 ANE.0b013e3181d74898 [pii]; 10.1213/ANE.0b013e3181d74898 [doi] [DOI] [PubMed] [Google Scholar]
- 38. Combs CA, Murphy EL, Laros RK Jr (1991) Factors associated with hemorrhage in cesarean deliveries. Obstet Gynecol 77: 77–82. [PubMed] [Google Scholar]
- 39. Sosa CG, Althabe F, Belizan JM, Buekens P (2009) Risk factors for postpartum hemorrhage in vaginal deliveries in a Latin-American population. Obstet Gynecol 113: 1313–1319 10.1097/AOG.0b013e3181a66b05 [doi];00006250-200906000-00018 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A (2004) The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth 4: 17 10.1186/1471-2393-4-17 [doi];1471-2393-4-17 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.British Columbia Reproductive Care Program (2006) BCRCP Guideline 11 Hypertension in pregnancy. Available: http://www.perinatalservicesbc.ca/Guidelines/Guidelines/maternal/obstetrics/default.htm. Accessed February 20th 2013.
- 42. Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD (2002) Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol 155: 203–209. [DOI] [PubMed] [Google Scholar]
- 43. Dahlstrom BL, Engh ME, Bukholm G, Oian P (2006) Changes in the prevalence of pre-eclampsia in Akershus County and the rest of Norway during the past 35 years. Acta Obstet Gynecol Scand 85: 916–921 748296054 [pii]; 10.1080/00016340500442449 [doi] [DOI] [PubMed] [Google Scholar]
- 44. Hernandez-Diaz S, Toh S, Cnattingius S (2009) Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 338: b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Catov JM, Ness RB, Kip KE, Olsen J (2007) Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol 36: 412–419 dyl271 [pii]; 10.1093/ije/dyl271 [doi] [DOI] [PubMed] [Google Scholar]
- 46.Knight M (2013) The prevalence of pre-eclampsia is 2.9%, coming from the eclampsia study of the UK Obstetric Surveillance System (UKOSS). Oxford: personal communication on February 28th 2013.
- 47. Ford JB, Roberts CL, Simpson JM, Vaughan J, Cameron CA (2007) Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet 98: 237–243 S0020-7292(07)00131-2 [pii]; 10.1016/j.ijgo.2007.03.011 [doi] [DOI] [PubMed] [Google Scholar]
- 48.Le M, Tran B (2010) Perinatal Statistics in Western Australia, 2008: Twenty-sixth Annual Report of the Western Australian Midwives' Notification System. Department of Health, Western Australia. Available: http://www.health.wa.gov.au/publications/subject_index/p/pregnancy.cfm. Accessed February 20th 2013.
- 49.Stichting Perinatale Registratie Nederland (2012) Grote Lijnen 10 jaar Perinatale Registratie Nederland. Available: http://www.perinatreg.nl/verloskunde_in_nederland_grote_lijnen?noCache=5841365175549. Accessed February 20th 2013.
- 50.Prasertcharoensuk W, Swadpanich U, Lumbiganon P (2000) Accuracy of the blood loss estimation in the third stage of labor. Int J Gynaecol Obstet 71: : 69–70. S0020-7292(00)00294-0 [pii]. [DOI] [PubMed] [Google Scholar]
- 51. Bose P, Regan F, Paterson-Brown S (2006) Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG 113: 919–924 BJO1018 [pii]; 10.1111/j.1471-0528.2006.01018.x [doi] [DOI] [PubMed] [Google Scholar]
- 52. Waugh JJ, Clark TJ, Divakaran TG, Khan KS, Kilby MD (2004) Accuracy of urinalysis dipstick techniques in predicting significant proteinuria in pregnancy. Obstet Gynecol 103: 769–777 10.1097/01.AOG.0000118311.18958.63 [doi];103/4/769 [pii] [DOI] [PubMed] [Google Scholar]
- 53. Blomberg M (2011) Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol 118: 561–568 10.1097/AOG.0b013e31822a6c59 [doi];00006250-201109000-00010 [pii] [DOI] [PubMed] [Google Scholar]
- 54. Paglia MJ, Grotegut CA, Johnson LN, Thames B, James AH (2012) Body mass index and severe postpartum hemorrhage. Gynecol Obstet Invest 73: 70–74 000329335 [pii]; 10.1159/000329335 [doi] [DOI] [PubMed] [Google Scholar]
- 55. Rouse DJ, Leindecker S, Landon M, Bloom SL, Varner MW, et al. (2005) The MFMU Cesarean Registry: uterine atony after primary cesarean delivery. Am J Obstet Gynecol 193: 1056–1060 S0002–9378(05)01164-6 [pii]; 10.1016/j.ajog.2005.07.077 [doi] [DOI] [PubMed] [Google Scholar]
- 56.Héman L, Linden P (2011) Does magnesium sulfate increase the incidence of postpartum hemorrhage? A systematic review. Open Journal of Obstetrics and Gynecology, 1, : 168–173. 10.4236/ojog.2011.14032 [doi] [DOI] [Google Scholar]
- 57. Skjeldestad FE, Oian P (2012) Blood loss after cesarean delivery: a registry-based study in Norway, 1999–2008. Am J Obstet Gynecol 206: 76–77 S0002-9378(11)00953-7 [pii]; 10.1016/j.ajog.2011.07.036 [doi] [DOI] [PubMed] [Google Scholar]
- 58. Malabarey O, Almog B, Brown R, Abenhaim HA, Shrim A (2011) Postpartum hemorrhage in low risk population. J Perinat Med 39: 495–498 10.1515/JPM.2011.059 [doi] [DOI] [PubMed] [Google Scholar]
- 59. Shechter Y, Levy A, Wiznitzer A, Zlotnik A, Sheiner E (2010) Obstetric complications in grand and great grand multiparous women. J Matern Fetal Neonatal Med 23: 1211–1217 10.3109/14767051003615459 [doi] [DOI] [PubMed] [Google Scholar]
- 60.Maymon E, Ghezzi F, Shoham-Vardi I, Hershkowitz R, Franchi M, et al.(1998) Peripartum complications in grand multiparous women: para 6-9 versus para > or = 10. Eur J Obstet Gynecol Reprod Biol 81: : 21–25. S0301211598001523 [pii]. [DOI] [PubMed] [Google Scholar]
- 61. Hager RM, Daltveit AK, Hofoss D, Nilsen ST, Kolaas T, et al. (2004) Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol 190: 428–434 10.1016/j.ajog.2003.08.037 [doi];S0002937803010937 [pii] [DOI] [PubMed] [Google Scholar]
- 62. Roberts CL, Ford JB, Thompson JF, Morris JM (2009) Population rates of haemorrhage and transfusions among obstetric patients in NSW: a short communication. Aust N Z J Obstet Gynaecol 49: 296–298 AJO985 [pii]; 10.1111/j.1479-828X.2009.00985.x [doi] [DOI] [PubMed] [Google Scholar]