Abstract
Purpose
Accurate patient risk perception of adverse health events promotes greater autonomy over, and motivation towards, health-related lifestyles. We compared self-perceived fracture risk and 3-year incident fracture rates in postmenopausal women with a range of morbidities in the Global Longitudinal study of Osteoporosis in Women (GLOW).
Methods
GLOW is an international cohort study involving 723 physician practices across 10 countries (Europe, North America, Australasia). 60,393 women aged ≥55 years completed baseline questionnaires detailing medical history and self-perceived fracture risk. Annual follow-up determined self-reported incident fractures.
Results
In total 2,945/43,832 (6.8%) sustained an incident fracture over 3 years. All morbidities were associated with increased fracture rates, particularly Parkinson’s disease (hazard ratio [HR]; 95% confidence interval [CI], 3.89; 2.78–5.44), multiple sclerosis (2.70; 1.90–3.83), cerebrovascular events (2.02; 1.67–2.46), and rheumatoid arthritis (2.15; 1.53–3.04) (all p<0.001). Most individuals perceived their fracture risk as similar to (46%) or lower than (36%) women of the same age.
While increased self-perceived fracture risk was strongly associated with incident fracture rates, only 29% experiencing a fracture perceived their risk as increased. Under-appreciation of fracture risk occurred for all morbidities, including neurological disease, where women with low self-perceived fracture risk had a fracture HR 2.39 (CI 1.74–3.29) compared with women without morbidities.
Conclusions
Postmenopausal women with morbidities tend to under-appreciate their risk, including in the context of neurological diseases, where fracture rates were highest in this cohort. This has important implications for health education, particularly among women with Parkinson’s disease, multiple sclerosis, or cerebrovascular disease.
Keywords: Fracture risk, Self-perception, Postmenopausal, Parkinson’s disease, Multiple sclerosis, Cerebrovascular event
Introduction
Non-communicable diseases have recently been highlighted as a major international health priority [1]. Ischemic heart disease, chronic obstructive pulmonary disease (COPD), and osteoarthritis are highly prevalent, and are major contributors to the global burden of non-communicable diseases [2]. Approximately 1/2 of women and 1/5 of men aged >50 years will fracture their hip, spine or forearm during their lifetime [3]. Fractures present a significant burden to society, being expensive both in terms of direct medical costs and their social sequelae. An estimated nine million osteoporosis-related fractures occurred globally in 2000, costing around $20 billion in the USA and €30 billion in the European Union [4]. As populations age, costs are expected to escalate.
A number of common non-communicable diseases have been associated with increased fracture risk, including ischemic heart disease [5], cerebrovascular events [6], type 1 diabetes mellitus [7, 8], COPD [9], and cancer [10]. Parkinson’s disease and multiple sclerosis are less prevalent conditions, but are associated with some of the highest fracture risks, conveying a similar risk as that of prior history of fracture [11, 12].
Health education aimed at empowering patients through improving disease understanding and motivating adoption of a healthier lifestyle are key approaches to tackling disease [1]. Furthermore, increased self-awareness leads to greater healthcare engagement and treatment [13, 14], and patient’s beliefs and perceptions are associated with medication adherence [15-17]. It is therefore important to determine whether those individuals with the highest fracture risks have an appropriate appreciation of their risk since under-appreciation of fracture risk by individuals with key morbidities has important health policy and prevention implications. If certain disease groups have a better understanding of actual fracture risk, it may be possible to draw lessons from these models of care to improve awareness within those in whom fracture risk is underappreciated. Such information is timely as analyses have previously suggested that women with increased fracture risk, determined by FRAX assessment, fail to appreciate their actual risk [18].
Hence, we aimed to determine whether the patient’s fracture risk perception agreed with prospective incident 3-year fracture rates in postmenopausal women with a range of morbidities, sampled in a large, multinational, cohort study. Our goal was to establish (i) how likely women who fractured were to have perceived themselves at high risk before they fractured, and (ii) whether self-perception of fracture risk varied according to specific diseases, the implication being that patients in some disease groups might particularly benefit from targeted educational intervention.
Methods
Study design
GLOW is an observational cohort study conducted through physician’s practices in 17 centers across 10 countries (Australia, Belgium, Canada, France, Germany, Italy, Netherlands, Spain, UK, and USA). Clinical investigators at the sites constitute the GLOW Scientific Advisory Board with responsibility for study management. The study design and methods have previously been described [19]. In brief, typical regional practices were recruited through primary care networks. Each centre obtained local ethics committee approval.
Participant sampling
Each practice listed all women aged ≥55 years who had consulted their primary care physician within the past 24 months. Random sampling was age-stratified at each practice to ensure two thirds were aged ≥65 years. Patients were excluded if they were unable to complete the study survey due to cognitive impairment, language barriers, institutionalization, or were too ill. The median response rate among the 17 study sites was 62% (range 15-75) [19].
Questionnaires
Self-administered questionnaires covered domains including: patient characteristics and risk factors; medical diagnoses (asked if a doctor or health provider had ever told them if they had any of the listed morbidities), healthcare use, and access; medications; physical activity; and physical and emotional health status. The validated SF-36 instrument was used to measure overall health status, physical function, and vitality [20]. Self-reported personal risk factors included: current weight and height; parental hip fracture; number of falls in the past 12 months (none/one/≥2); current use of cortisone/prednisone; rheumatoid arthritis; personal history of fracture since age 45 years; current cigarette smoking; and alcohol consumption ≥3 units/day. Rheumatoid arthritis was believed to be present if disease-modifying therapy (methotrexate and/or biologic therapies) was also reported. Women rated their self-perceived fracture risk compared with women of the same age using a five-point scale ranging from “much lower” to “much higher”. Bone mineral density testing was not part of the GLOW protocol.
Follow-up questionnaires were sent at 1, 2, and 3 years enquiring about incident fractures, fracture site, and hospital treatment. Questionnaires were translated into five languages (French, Spanish, German, Italian, and Dutch). All data were collected by patient self-report. Data entry, verification, and management were uniform across all study sites. Completed questionnaires were sent to the central coordinating center; twice-yearly meetings with study site coordinators reviewed survey administration and ensured uniformity. For study sites using telephone follow-up in addition to mail, a standard telephone script was used and reviewed with each site to ensure consistency.
Statistical analysis
Women who had baseline data with continuous survey follow-up until either year 2 or 3 were analyzed if they had complete morbidity data, to avoid misclassification. Incident fractures occurring between baseline and 2 or 3 years of follow-up were analyzed. Self-perception of fracture risk is presented in three categories; “much or a little lower”, “about the same” and “much or a little higher” than women of the same age.
Baseline lifestyle and demographic characteristics (age [categorized], body mass index [BMI] [categorized], menopause before age 45 years, fracture after age 45 years, current smoking, alcohol ≥3 units/day, fall within the last year, parental hip fracture, current glucocorticoid use, hospitalization within the last year, anxiety/depression, a previous diagnosis of osteoporosis) are presented as numbers and percentages. Skewed continuous descriptive data (FRAX 10-year fracture probability, SF-36 indices) are presented as medians with inter-quartile ranges (IQRs).
Incident fracture rates are presented for each baseline morbidity; ischemic heart disease, COPD, osteoarthritis, Parkinson’s disease, multiple sclerosis, rheumatoid arthritis, cerebrovascular event, ulcerative colitis or Crohn’s disease (inflammatory bowel disease), celiac disease, cancer, type 1 diabetes mellitus, hypertension, and hypercholesterolemia. A cumulative morbidity index was generated by summing reported morbidities to quantify overlap in morbidities. Morbidities were also grouped by systems.
Unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) are reported from fitting Cox regression models assessing the outcome (incident fracture over 3 years) according to; (i) each morbidity, (ii) the morbidity index, and (iii) grouped morbidities compared with morbidity-free women. Cox models were ordered by age, as fracture risk increases with age and self-perceived fracture risk is judged by participants in relation to women of the same age. Contributions to incident fracture risk were censored at fracture, loss-to-follow-up or termination of follow-up (3 years) in those free from fracture. No evidence of departure from proportionality was detected for any variable. The sensitivity and specificity of fracture risk perception were calculated for 3-year incident fracture. Kaplan-Meier estimates, unadjusted, and age-adjusted HRs for 3-year incident fracture rates were then stratified by the three categories of fracture risk perception. Interaction testing assessed whether the effect of each morbidity on incident fracture rate was modified by a woman’s self-perceived fracture risk, i.e. testing whether there are some morbidities, associated with an overall increased fracture rate, in whom women with lower perceived risk have lower fracture rates, and women with higher perceived risk have higher fracture rates. All analyses were performed using SAS version 9.2 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics
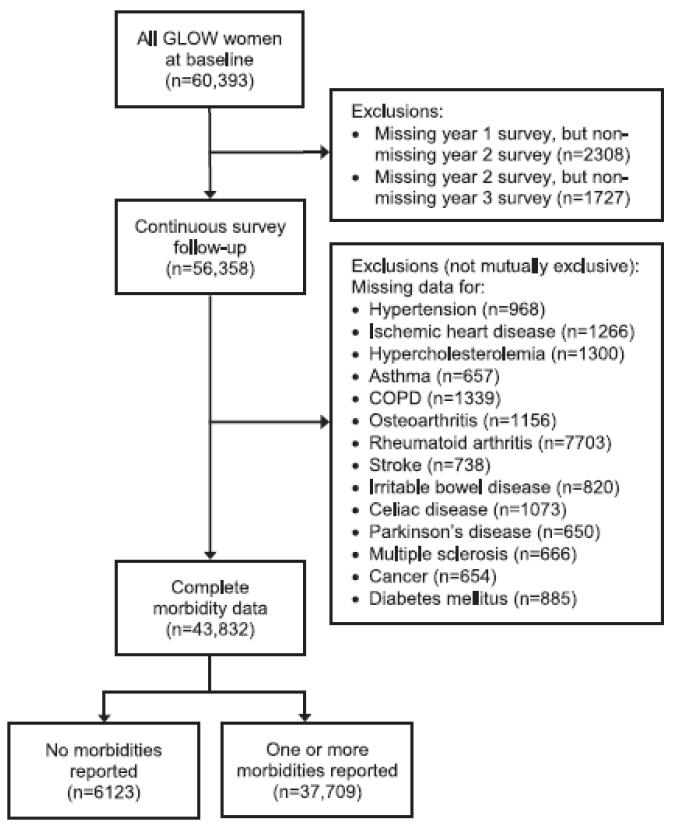
A total of 60,393 women from 723 physician’s practices were enrolled between October 2006 and February 2008. Approximately 25,000 came from eight sites and 274 practices in Europe; 28,000 from 255 practices in the USA, and almost 7,000 from 86 practices in Canada and Australia. At baseline, 43,832 (73%) had complete morbidity data (Fig. 1). Fracture outcomes were available in 43,699 (99.7%) at 2-year follow-up and 43,606 (99.5%) at 3-year follow-up.
Fig. 1.
Study flow diagram. COPD, chronic obstructive pulmonary disease.
Baseline characteristics are shown in Table 1. Over three-quarters (n=33,689; 77%) of the women were aged 55–74 years and 57% (n=23,956) were overweight/obese (BMI ≥25 kg/m2). Nearly one quarter (n=9,759; 23%) reported a prior fracture since age 45 years, with 8,742 (21%) reporting an established diagnosis of osteoporosis. Only 215 (0.5%) reported consuming ≥3 alcoholic drinks a day, and 8.4% (n=3,683) admitted to currently smoking; 37% reported a fall and 15% reported overnight hospitalization within the previous year.
Table 1.
Characteristics of postmenopausal women with and without morbidities at baselinea
| No morbidity (n=6,123) |
≥1 morbidity (n=37,709) |
p value | ||
|---|---|---|---|---|
| Age (years), n (%) | 55–64 | 3,485 (57) | 14,393 (38) | <0.001 |
| 65–74 | 1,818 (30) | 13,993 (37) | ||
| 75–84 | 666 (11) | 7,753 (21) | ||
| ≥85 | 154 (2.5) | 1,570 (4.1) | ||
| BMI (kg/m2), n (%) | <18.5 | 142 (2.4) | 598 (1.7) | <0.001 |
| 18.5 to <25 | 3,345 (57) | 14,147 (39) | ||
| 25 to <30 | 1,734 (29) | 12,547 (35) | ||
| >30 | 681 (12) | 8,994 (25) | ||
| Other characteristics, n (%) | Any fractureb since age 45 years | 1,030 (17) | 8,729 (23) | <0.001 |
| Fall in the last 12 months | 1,797 (29) | 14,337 (38) | <0.001 | |
| Menopause before age 45 years | 621 (10) | 5,555 (15) | <0.001 | |
| Current smoker | 560 (9.2) | 3,123 (8.3) | 0.02 | |
| Alcohol ≥3 drinks/day | 27 (0.4) | 188 (0.5) | 0.55 | |
| Current steroid use | 53 (0.9) | 1,148 (3.1) | <0.001 | |
| Anxiety/depressionc | 1,975 (33) | 16,205 (43) | <0.001 | |
| Hospitalized overnightd | 391 (6.4) | 6,164 (16) | <0.001 | |
| Osteoporosis diagnosis | 763 (13) | 7,979 (22) | <0.001 | |
| Maternal hip fracture | 767 (13) | 4,845 (14) | 0.36 | |
| Paternal hip fracture | 226 (4.0) | 1,340 (3.9) | 0.78 | |
| FRAX 10-year probability of major fracture, median (IQR) | 9 (6–14) | 11 (7–19) | <0.001 | |
| SF-36 physical function index, median (IQR) | 95 (85–100) | 80 (60–95) | <0.001 | |
| SF-36 vitality index, median (IQR) | 75 (63–81) | 63 (50–75) | <0.001 | |
BMI body mass index; IQR interquartile range; SF-36 short form health survey with 36 questions
Percentages may not exactly match numbers due to missing data (<10% for each category)
Includes: clavicle, upper arm, distal forearm, spine, rib, hip, pelvis, upper leg, lower leg, and ankle
Self-reported moderate or extreme anxiety/depression
During the last 12 months
Morbidities and incident fracture rates
Morbidities were common: 37,709 women (86%) reported ≥1 morbidity (Fig. 1), most commonly hypercholesterolemia (50%) and hypertension (49%) (Table 2). Overall, 31,369 (72%) reported cardiovascular disease or a risk factor for ischemic heart disease, whereas only 223 (0.5%), 272 (0.6%), and 273 (0.6%) reported Parkinson’s disease, multiple sclerosis, and celiac disease, respectively. In total, 32% had ≥3 morbidities.
Table 2.
Unadjusted incident fracture rate estimates by morbidities and self-perceived fracture risk
| Women (n=43,832) n (%) |
Annual fracture incidence, (%/year) |
Unadjusted HR (95% CI) for fracture |
p value | ||
|---|---|---|---|---|---|
| Individual morbidities |
None | 6,123 (14) | 1.9 | 1.00 | – |
| Osteoarthritis | 16,979 (39) | 3.1 | 1.63 (1.44, 1.85) | <0.001 | |
| Rheumatoid arthritis | 341 (0.8) | 3.9 | 2.15 (1.53–3.04) | <0.001 | |
| Parkinson’s disease | 223 (0.5) | 7.4 | 3.89 (2.78–5.44) | <0.001 | |
| Multiple sclerosis | 272 (0.6) | 5.1 | 2.70 (1.90–3.83) | <0.001 | |
| Cerebrovascular event | 1,569 (3.6) | 3.9 | 2.02 (1.67–2.46) | <0.001 | |
| Inflammatory bowel disease | 788 (1.8) | 3.8 | 2.00 (1.56–2.57) | <0.001 | |
| Celiac disease | 273 (0.6) | 3.8 | 2.11 (1.43–3.11) | <0.001 | |
| Type 1 diabetes mellitus | 1,390 (3.2) | 3.5 | 1.85 (1.50–2.28) | <0.001 | |
| Hypertension | 21,581 (49) | 2.6 | 1.39 (1.23–1.57) | <0.001 | |
| Hypercholesterolemia | 21,953 (50) | 2.5 | 1.35 (1.19–1.52) | <0.001 | |
| Ischemic heart disease | 5,986 (14) | 3.5 | 1.86 (1.62–2.14) | <0.001 | |
| COPD | 3,409 (7.8) | 3.6 | 1.95 (1.67–2.28) | <0.001 | |
| Asthma | 4,868 (11) | 3.0 | 1.58 (1.36–1.84) | <0.001 | |
| Cancer | 6,174 (14) | 3.1 | 1.61 (1.40–1.86) | <0.001 | |
|
| |||||
| Cumulative morbidity index |
0 | 6,123 (14) | 1.9 | 1.00 | – |
| 1 | 11,674 (27) | 2.1 | 1.13 (0.98–1.29) | 0.08 | |
| 2 | 12,239 (28) | 2.5 | 1.33 (1.17–1.51) | <0.001 | |
| 3 | 8,152 (19) | 2.8 | 1.46 (1.27–1.67) | <0.001 | |
| ≥4 | 5,644 (13) | 3.7 | 2.01 (1.75–2.31) | <0.001 | |
|
| |||||
| Grouped morbidities |
None | 6,123 (14) | 1.9 | 1.00 | – |
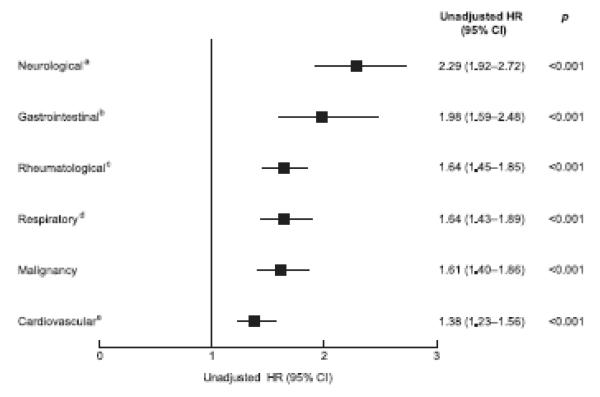
| Rheumatologicala | 17,130 (39) | 3.1 | 1.64 (1.45–1.85) | <0.001 | |
| Neurologicalb | 2,012 (4.6) | 4.4 | 2.29 (1.92–2.72) | <0.001 | |
| Gastrointestinalc | 1,042 (2.4) | 3.7 | 1.98 (1.59–2.48) | <0.001 | |
| Cardiovasculard | 31,369 (72) | 2.6 | 1.38 (1.23–1.56) | <0.001 | |
| Respiratorye | 6,877 (16) | 3.1 | 1.64 (1.43–1.89) | <0.001 | |
| Malignancy | 6,174 (14) | 3.1 | 1.61 (1.40–1.86) | <0.001 | |
|
| |||||
| Self-perceived fracture risk |
Much or a little lower | 15414 (35) | 2.0 | 1.00 | – |
| About the same | 19915 (45) | 2.3 | 1.20 (1.10, 1.31) | <0.001 | |
| Much or a little higher | 7582 (17) | 4.4 | 2.27 (2.06, 2.50) | <0.001 | |
CI confidence interval; COPD chronic obstructive pulmonary disease; HR hazard ratio
Osteoarthritis, rheumatoid arthritis
Parkinson’s disease, multiple sclerosis, cerebrovascular event
Inflammatory bowel disease, celiac disease
Type 1 diabetes mellitus, hypertension, hypercholesterolemia, ischemic heart disease
COPD, asthma
Of the 43,832 women with morbidity data, 2,945 (6.8%) sustained an incident fracture over 3 years. All morbidities were strongly associated with increased fracture rates (Table 2). The highest fracture rate was observed in women with Parkinson’s disease, with an almost four-fold increased fracture risk, followed by those women with multiple sclerosis. Cerebrovascular events approximately doubled the incident fracture rate, as did rheumatoid arthritis, celiac disease, inflammatory bowel disease, and COPD (Table 2). Even hypercholesterolemia and hypertension were associated with an approximately 40% increased 3-year incident fracture rate. Cumulative morbidities conferred a sequentially increasing incident fracture rate, with ≥4 morbidities associated with a doubling of fracture risk (Table 2). After system grouping of morbidities, as shown in Table 2, neurological diseases were associated with the highest fracture rates (Fig. 2). Age-adjustment moderately attenuated most HRs (Table S1), although all morbidities continued to confer increase fracture rates independent of age.
Fig. 2.
Unadjusted Kaplan-Meier 3-year incident fracture rate estimates by grouped morbidities. a Parkinson’s disease, multiple sclerosis, cerebrovascular event; b Inflammatory bowel disease, celiac disease; c Osteoarthritis, rheumatoid arthritis; d COPD, asthma; e Type 1 diabetes mellitus, hypertension, hypercholesterolemia, ischemic heart disease.
Morbidities and self-perceived fracture risk
Overall, 36% of women perceived their fracture risk as being “much or a little lower” than that of women of the same age, 46% rated their risk “about the same,” and 18% as “much or a little higher”. Amongst morbidity-free women, 43% considered their risk to be “much or a little lower”, and only 12% thought their risk to be “much or a little higher” than women of the same age, All morbidities were strongly associated with increased self-perceived fracture risk (Table 3). Women with multiple sclerosis, rheumatoid arthritis and celiac disease had the largest proportion of women with higher self-perceived risk.
Table 3.
Self-perceived fracture risk according to reported morbidities at study baseline
|
n
(42832) |
Self-perceived fracture risk, n (%) |
|||
|---|---|---|---|---|
| Much or a little lower (n=15414) |
About the same (n=19915) |
Much or a little higher (n=7582) |
||
| No morbidities | 5973 | 2572 (43) | 2709 (45) | 692 (12) |
| Osteoarthritis | 16671 | 5223 (31) | 7527 (45) | 3921 (24) |
| Rheumatoid arthritis | 336 | 54 (16) | 155 (46) | 127 (38) |
| Parkinson’s disease | 220 | 56 (25) | 95 (43) | 69 (31) |
| Multiple sclerosis | 267 | 64 (24) | 94 (35) | 109 (41) |
| Cerebrovascular event | 1531 | 450 (29) | 674 (44) | 407 (27) |
| Inflammatory bowel disease | 769 | 223 (29) | 338 (44) | 208 (27) |
| Celiac disease | 270 | 62 (23) | 108 (40) | 100 (37) |
| Type 1 diabetes mellitus | 1353 | 414 (31) | 635 (47) | 304 (22) |
| Hypertension | 21131 | 7423 (35) | 9986 (47) | 3722 (18) |
| Hypercholesterolemia | 21496 | 7429 (35) | 10112 (47) | 3955 (18) |
| Ischemic heart disease | 5845 | 1804 (31) | 2675 (46) | 1366 (23) |
| COPD | 3326 | 954 (29) | 1525 (46) | 847 (25) |
| Asthma | 4790 | 1500 (31) | 2212 (46) | 1078 (23) |
| Cancer | 6057 | 2014 (33) | 2754 (45) | 1289 (21) |
Row percentages shown
COPD: chronic obstructive pulmonary disease
Self-perceived fracture risk and incident fracture rates
As self-perceived fracture risk increased, incident fracture rates also increased. Of note, women who perceived their fracture risk to be “about the same” as other women of the same age had a significant 20% increased risk of fracture compared with those who perceived their risk to be “much or a little lower” (unadjusted HR 1.20 [95% CI 1.10, 1.31] p<0.001) (Table 2), which was unattenuated by age-adjustment (Table S1), so that the age-adjusted fracture rate amongst women who perceived their risk to be ‘about the same’ as women of the same age was HR 1.24 [1.13, 1.35], and was 2.27 [2.06, 2.50] amongst women who perceived their risk to be ‘much or a little higher’ than women of the same age (Table S1).
Only 29% of women who reported an incident fracture perceived their fracture risk to be “much or a little higher” than average (sensitivity=844/2,887=29%). Also, 83% of women who were fracture-free at 3 years perceived their fracture risk to be “much or a little lower” or “about the same” as average (specificity=33,132/39,807=83%).
Incident fracture rates by morbidity stratified by self-perceived fracture risk
In general, incident fracture rates were greater in all morbidity groups, compared with morbidity-free women when stratified by fracture risk perception; the exceptions being rheumatoid arthritis, Parkinson’s disease and multiple sclerosis where samples sizes were small following stratification (Table 4). For all morbidities women who perceived their fracture risk to be “much or a little lower” than average actually had increased rates of incident fracture compared with morbidity free women. This was particularly apparent in Parkinson’s disease (HR 3.8 [1.9, 7.5]), multiple sclerosis (HR 2.7 [1.3, 5.7]) and women with prior cerebrovascular events (HR 2.2 [1.5, 3.1]). The exception were women with celiac disease in whom women with low self-perceived fracture risk showed a trend towards reduced rate of incident fracture (HR 0.7 [0.2, 3.0]). When testing formally for interaction we found no evidence to support an interaction between any morbidity and self-perceived fracture risk, i.e. the increased fracture rate associated with each morbidity, did not vary according to a woman’s self-perceived risk. Only for celiac disease was there weak evidence (interaction p=0.07) that women with low perceived fracture risk had reduced incident fracture rates, and women with high perceived risk had increased incident fracture rates, compared with women with no morbidities.
Table 4.
Unadjusted Kaplan-Meier 3-year incident fracture rate estimates according to morbidity, stratified by self-perceived fracture risk
| Individual morbidities | KM estimates of 3-year incident fracture rates |
p value a | |||||
|---|---|---|---|---|---|---|---|
| Much or a little lower (n=15,414) | About the same (n=19,915) | Much or a little higher (n=7,582) | |||||
| n (%) | HR (95% CI) | n (%) | HR (95% CI) | n (%) | HR (95% CI) | ||
| None | 116 (4.9) | 1.00 | 140 (5.7) | 1.00 | 54 (8.6) | 1.00 | 0.002 |
| Osteoarthritis | 342 (7.2) | 1.47 (1.19–1.81) | 524 (7.6) | 1.37 (1.14–1.65) | 494 (14) | 1.69 (1.28–2.24) | <0.001 |
| Rheumatoid arthritis | 5 (10) | 2.16 (0.88–5.29) | 12 (8.6) | 1.53 (0.85–2.76) | 19 (16) | 2.02 (1.20–3.41) | 0.14 |
| Parkinson’s disease | 9 (20) | 3.79 (1.92–7.47) | 17 (20) | 3.73 (2.23–6.26) | 12 (20) | 2.82 (1.51–5.28) | 0.77 |
| Multiple sclerosis | 7 (12) | 2.67 (1.25–5.73) | 12 (14) | 2.58 (1.43–4.65) | 15 (17) | 1.87 (1.06–3.32) | 0.87 |
| Cerebrovascular event | 41 (11) | 2.17 (1.52–3.10) | 49 (8.2) | 1.51 (1.09–2.09) | 58 (17) | 2.05 (1.41–2.97) | <0.001 |
| Inflammatory bowel disease | 14 (7.0) | 1.44 (0.83–2.51) | 24 (8.0) | 1.41 (0.92–2.18) | 37 (20) | 2.48 (1.63–3.76) | <0.001 |
| Celiac disease | 2 (3.7) | 0.73 (0.18–2.96) | 8 (7.8) | 1.47 (0.72–3.00) | 17 (18) | 2.36 (1.37–4.06) | 0.02 |
| Type 1 diabetes mellitus | 33 (9.5) | 1.90 (1.29–2.80) | 41 (7.2) | 1.35 (0.95–1.91) | 46 (19) | 2.23 (1.51–3.31) | <0.001 |
| Hypertension | 381 (5.7) | 1.15 (0.94–1.42) | 654 (7.1) | 1.30 (1.08–1.56) | 434 (13) | 1.57 (1.18–2.08) | <0.001 |
| Hypercholesterolemia | 382 (5.7) | 1.15 (0.93–1.42) | 614 (6.6) | 1.19 (0.99–1.43) | 462 (13) | 1.56 (1.18–2.07) | <0.001 |
| Ischemic heart disease | 120 (7.6) | 1.54 (1.19–1.98) | 213 (8.8) | 1.61 (1.30–2.00) | 195 (17) | 2.02 (1.49–2.73) | <0.001 |
| COPD | 70 (8.3) | 1.73 (1.28–2.32) | 126 (9.2) | 1.72 (1.35–2.19) | 113 (15) | 1.85 (1.34–2.57) | <0.001 |
| Asthma | 88 (6.7) | 1.33 (1.01–1.76) | 147 (7.3) | 1.32 (1.05–1.67) | 139 (15) | 1.76 (1.28–2.40) | <0.001 |
| Cancer | 115 (6.3) | 1.29 (1.00–1.67) | 196 (7.8) | 1.42 (1.14–1.77) | 171 (16) | 1.79 (1.32–2.43) | <0.001 |
CI confidence interval; COPD chronic obstructive pulmonary disease; HR hazard ratio; KM Kaplan-Meier
Wald chi-square p-value from a Cox regression for perceived fracture risk predicting fracture in women with a given morbidity compared with women without morbidity.
In further analyses (interaction p values not shown in the table), we found no evidence to support an interaction between each morbidity and self-perceived fracture risk, i.e. fracture rate (associated with any of the morbidities) does not differ by self-perception. Only for Celiac disease was there weak evidence (interaction p=0.07) that women with low perceived fracture risk had reduced fracture rates, and women with high perceived risk had increased fracture rates, compared with women with no morbidities
When morbidities were grouped by system, the highest HR for incident fracture amongst women with low self-perceived fracture risk was seen in those with neurological diseases (HR 2.4 [1.7, 3.3]) (Table S2), this persisted after age-adjustment (HR 1.9 [1.4, 2.6]). Overall age-adjustment partially attenuated HRs, but similar patterns persisted (Table S3 & S4).
Potential explanations for self-perceived fracture risk
Having detected such under-appreciation of incident fracture risk, we assessed associations between self-perceived fracture risk and key baseline characteristics. A greater proportion of women reporting a fall had perceived their fracture risk as “much or a little higher” than women without falls (49% vs. 18%) (Table S5). However, 32% of women who had fallen perceived their fracture risk to be “much or a little lower” than women of the same age, as did a surprising 18% of women reporting a diagnosis of osteoporosis, 20% reporting a bisphosphonate prescription and 22% who had previously fractured (Table S5). As expected, early menopause, hospitalization and severe anxiety/depression were associated with a higher self-perceived fracture risk, as shown by the higher percentages of these women with higher perceived fracture risk compared with all women (Table S5).
Discussion
In our large, international, observational study, although increased self-perceived fracture risk was strongly associated with incident fracture rate, only 29% of women experiencing a fracture had perceived their risk as “much or a little higher” than average at baseline. All morbidities assessed were associated with an increased rate of self-reported 3-year incident fracture. Accumulation of morbidities conveyed an incrementally increasing fracture risk. The highest 3-year fracture rate occurred in women reporting Parkinson’s disease (21%), followed by multiple sclerosis (15%) and rheumatoid arthritis, cerebrovascular events, inflammatory bowel disease, and celiac disease (all 11%).
For all morbidities apart from celiac disease, women who perceived their fracture risk to be “much or a little lower” than average actually had increased rates of incident fracture; this was particularly evident amongst women with the neurological diseases Parkinson’s disease, multiple sclerosis and previous stroke, both before and after age-adjustment. Overall, the increased fracture rates associated with each of the 14 morbidities, did not differ according to self-perceived fracture risk; meaning that none of the diseases studied were characterised by women with lower perceived risk having lower fracture rates, and women with higher perceived risk having higher fracture rates. The only possible exception being women with celiac disease in whom self-perceived fracture risk appeared to follow the 3-year incident fracture rates, although numbers were small. The explanation remains unclear, but may relate to the detailed education necessary to aid self-management of a gluten-free diet.
Self-perceived osteoporosis risk has been under-appreciated in Australian and US postmenopausal women [21, 22]; and Canadian postmenopausal women have shown poor understanding of future osteoporosis and fracture risks, despite previous fragility fracture [23]. However, studies assessing self-perceived fracture risk are sparse. To the best of our knowledge, our study is the first to assess this according to morbidities, each associated with specific fracture rates. Our results extend earlier findings from GLOW obtained after 2-year follow-up [12]. The increased power achieved by 3-year follow-up likely aided identification of positive associations between hypertension, hypercholesterolemia, and cancer with fracture risk, not seen in longitudinal analyses of shorter duration [12].
Our findings of high incident fractures rates among women with neurological disease are consistent with previous studies [6, 11, 24], in which reduced physical activity, frequent falls, decreased sunlight exposure, lower dietary calcium and vitamin D, and iatrogenic consequences of polypharmacy may all contribute to increased fracture risk [25, 26]. While all three neurological conditions are highlighted as risk factors for fracture in the US Surgeon General’s report 2004 [27], currently, the American Academy of Neurology clinical practice guidelines for the management of Parkinson’s disease, multiple sclerosis, stroke (and falls) do not mention fracture/osteoporosis risk assessment [28-30]. This is also the case for the 2012 Canadian Parkinson’s disease guidelines [31]; and the English National Institute for Health and Clinical Excellent (NICE) stroke pathway [32] does not include assessment of bone health. NICE Parkinson’s disease guidance acknowledges fracture as a potential non-motor complication and includes an appendix reference to NICE falls guidance with mention of optional osteoporosis risk assessment [33]. While NICE and Australian multiple sclerosis guidelines mention increased osteoporosis risk, no management suggestions are provided [34, 35]. The high incident fracture rates with low self-awareness observed in women with neurological diseases may in part be explained by the apparent absence of bone health assessment from key national guidelines.
Aside from neurological diseases, rheumatoid arthritis, inflammatory bowel disease, celiac disease, and COPD conferred some of the higher incident fracture rates. By comparison, the need for regular osteoporosis assessment and management in these conditions is well established [36-43]. However, responsibility for managing patients with chronic diseases lies with both primary and secondary care physicians, and it is possible to fall between the two [44, 45]. Under-appreciation of fracture risk by women with rheumatoid arthritis may be explained by rheumatologists, attentive towards bone health, giving false reassurance. Disturbingly, 32% of women who reported falls, 22% prior fracture and 20% reporting a bisphosphonate prescription, perceived their fracture risk as below average for their age. Understanding of the influences governing self-perceived fracture risk (and osteoporosis risk) is limited; however, it is possible prescription of osteoporosis medications may give false reassurance In qualitative telephone interviews from 127 Canadians, mean age 68 years, communication from healthcare providers appeared to be the dominant influence [46]. However, the information provided and its interpretation was highly variable. Misperceptions, e.g. by “being careful” fracture risk is negated, were also evident. Perception of risk is a subjective judgment. Risk as a concept is a measure of uncertainty, which may derive from lack of knowledge; on this premise patient education should improve fracture risk perception. Unfortunately there is no evidence-based target for ‘appropriate’ fracture risk perception; however, reporting findings from a large real-life cohort, increases awareness, and ultimately may educate and empower patients.
Limitations
Educational and cultural differences are likely to influence fracture risk perception. Unfortunately, ethnicity data were not collected in GLOW, except in the USA and Canada, where 86% were white, non-Hispanic [12]. Education data were collected; however, heterogeneity between educational systems prevented derivation of one combined variable. Insufficient details regarding morbidity severity and symptoms were collected, prohibiting use of established morbidity indices; hence a simple disease count was employed [47]. Although loss-to-follow-up was minimal, we lacked access to vital statistics to determine death (including by fracture) outcomes. Incident fractures were reported, but osteoporotic fractures specifically could not be identified. Characteristics associated with morbidity were as expected, barring smoking and alcohol intake, which are both anticipated to have strong associations with morbidity, but are frequently under-reported in epidemiological studies [48]. Type 1, but not type 2 diabetes mellitus was reported, risking misclassification. Furthermore, self-reported data are subject to recall bias. However, self-report aids efficiency and methodological consistency, facilitating a very large sample collection and maximizing power for a relatively infrequent fracture outcome. Self-reported fractures were not validated radiologically. However, self-report has been shown to be reasonably reliable for fracture [49, 50]. Standardized administration reduces non-comparability and variation in data quality, which would arise if using medical records and public healthcare databases from several different countries. Furthermore, self-report may be preferable to data abstraction from medical records, given inconsistencies in record keeping between physicians, study regions, and countries. Primary care records may also miss specialist-initiated treatments.
GLOW participants may not represent their national populations. Morbidities may be over-represented, as participants had attended their physician within the preceding 24 months. Health-seeking behavior may bias towards participation. Conversely, severe/end-stage morbidities potentially with high fracture risk, may be under-represented, as such women may have been unable to complete the study questionnaire due to cognitive impairment, institutionalization, or if too unwell, were excluded. These selection biases limit generalizability. Our multiple sclerosis prevalence of 0.6% exceeds that in the general population [51], whereas the prevalence of rheumatoid arthritis (0.8%) and Parkinson’s disease (0.5%) are consistent with published US and European estimates [52-55]. Contemporaneous US data (NHANES 2007-08) [56] from non-Hispanic white women aged ≥20 years estimated hypertension prevalence as 28.7% which, in a younger population, is also consistent with our findings.
Conclusions
Taken together, these results, collected across Europe, North America, and Australasia, suggest that women under-appreciate their fracture risk, and having a condition that increases incident fracture risk does not appear to adequately influence their perception. There is a widespread under-appreciation of this fracture risk, even in women reporting neurological disease in whom fracture rates are highest. This has important implications for health education. Our findings support: updating of guidelines, particularly in relation to neurological diseases (Parkinson’s disease, multiple sclerosis, and stroke); prompting fracture risk assessment by non-bone specialists, including primary care physicians; patient education in relation to bone health; and raising awareness among patient societies.
Supplementary Material
Acknowledgments
We thank the physicians, project coordinators, and women participating in GLOW.
Funding Funding/Support and Role of the Sponsor: Financial support for the GLOW study is provided by Warner Chilcott Company, LLC and sanofi-aventis to the Center for Outcomes Research, University of Massachusetts Medical School. The sponsors had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The design, conduct, and interpretation of the GLOW data are undertaken by an independent steering committee. The Medical Research Council and the University of Southampton provided funding infrastructure support for manuscript completion.
Footnotes
Conflicts of interest CLG has no disclosures. EMD has no disclosures. JEC has undertaken paid consultancy work for Servier, Shire, Nycomed, Novartis, Amgen, Procter & Gamble, Wyeth, Pfizer, The Alliance for Better Bone Health, Roche, and GlaxoSmithKline; has been a paid speaker for and received reimbursement, travel and accommodation from Servier, Procter & Gamble, and Lilly; and has received research grants from Servier R&D and Procter & Gamble. SA has received speaker honoraria from Eli-Lilly and Amgen; and consultancy honoraria from MSD, Eli-Lilly, Amgen and Novartis. JDA has been a consultant/speaker for Amgen, Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Servier, Warner Chilcott and Wyeth; and has conducted clinical trials for Amgen, Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, sanofi-aventis, Warner Chilcott, Wyeth, and Bristol-Myers Squibb. FAA has received funding from The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott). SB has received research grants from Amgen, Lilly, Novartis, Pfizer, Procter & Gamble, sanofi-aventis, Roche, and GlaxoSmithKline; and has received honoraria from, served on Speakers’ Bureaus for, and acted as a consultant/Advisory Board member for Amgen, Lilly, Merck, Novartis, Procter & Gamble, sanofi-aventis, and Servier. RC has received funding from the French Ministry of Health, Merck, Servier, Lilly, and Procter & Gamble; has received honoraria from Amgen, Servier, Novartis, Lilly, Roche, and sanofi-aventis; and has acted as a consultant/Advisory Board member for Amgen, Merck, Servier, Nycomed, and Novartis. AD-P has received consulting fees and lectured for Eli Lilly, Amgen, Procter & Gamble, Servier, and Daiichi-Sankyo; has been an expert witness for Merck; and is a consultant/Advisory Board member for Novartis, Eli Lilly, Amgen, and Procter & Gamble; has received honoraria from Novartis, Lilly, Amgen, Procter & Gamble, and Roche; has been an expert witness for Merck; and has acted as a consultant/Advisory Board member for Novartis, Lilly, Amgen, and Procter & Gamble. SLG has acted as a consultant/Advisory Board member for Amgen, Lilly, and Merck; and has received research grants from The Alliance for Better Bone Health (sanofi-aventis and Proctor & Gamble) and Lilly. FHH has received funding from The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott). AZL has received funding from The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott) and is an Advisory Board member for Amgen. JWN has no disclosures. JCN has undertaken paid consultancy work for Roche Diagnostics, Daiichi-Sankyo, Proctor & Gamble, and Nycomed; has been a paid speaker for and received reimbursement, travel and accommodation from Roche Diagnostics, Novartis, Daiichi-Sankyo, and Procter & Gamble; and has received research grants from The Alliance for Better Bone Health and Amgen. JP has received research grants from Amgen, Kyphon, Novartis, and Roche; has received other research support (equipment) from GE Lunar; has served on Speakers’ Bureaus for Amgen, sanofi-aventis, GlaxoSmithKline, Roche, Lilly Deutschland, Orion Pharma, Merck, Merckle, Nycomed, and Procter & Gamble; and has acted as an Advisory Board member for Novartis, Roche, Procter & Gamble, and Teva. MR is on the Speaker’ Bureau for Roche. CR has received honoraria from and acted as a consultant/Advisory Board member for Alliance, Amgen, Lilly, Merck, Novartis, Nycomed, Roche, GlaxoSmithKline, Servier, and Wyeth. KGS received consulting fees or other remuneration from Eli Lilly & Co, Merck, Novartis, and Amgen; and has conducted paid research for Eli Lilly & Co, Merck, Novartis, and Amgen. CR has received honoraria from and acted as a consultant/Advisory Board member for Alliance, Amgen, Lilly, Merck, Novartis, Nycomed, Roche, GlaxoSmithKline, Servier, and Wyeth. KGS received consulting fees or other remuneration from Eli Lilly & Co, Merck, Novartis, and Amgen; and has conducted paid research for Eli Lilly & Co, Merck, Novartis, and Amgen. KGS received consulting fees or other remuneration from Eli Lilly & Co, Merck, Novartis, and Amgen; and has conducted paid research for Eli Lilly & Co, Merck, Novartis, and Amgen. SS has received research grants from Wyeth, Lilly, Novartis, and Alliance; has served on Speakers’ Bureaus for Lilly, Novartis, Pfizer, and Procter & Gamble; has received honoraria from Procter & Gamble; and has acted as a consultant/Advisory Board member for Lilly, Argen, Wyeth, Merck, Roche, and Novartis. ESS has acted as a consultant for Amgen, Lilly, Novartis, and The Alliance for Better Bone Health; and has served on Speakers’ Bureaus in the past year for Amgen, and Lilly. NBW has received honoraria for lectures in the past year from Amgen, Novartis, and Warner Chilcott; has acted as a consultant in the past year for Amgen, Arena, Baxter, InteKrin, Johnson & Johnson, Lilly, Medpace, Merck, NPS, Orexigen, Pfizer/Wyeth, Takeda, Vivus, Warner Chilcott; has received research support (through University) from Amgen, Merck, and NPS; and co-founded, has stock options and is a director of OsteoDynamics. AW has no disclosures. CC has received consulting fees from and lectured for Amgen, The Alliance for Better Bone Health (sanofi-aventis and Warner Chilcott), Lilly, Merck, Servier, Novartis, and Roche-GSK.
Contributor Information
C. L. Gregson, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, SO16 6YD, UK; Musculoskeletal Research Unit, University of Bristol, Avon Orthopaedic Centre, Southmead Hospital, Bristol, UK
E. M. Dennison, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, SO16 6YD, UK
J. E. Compston, School of Clinical Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK
S. Adami, Department of Rheumatology, University of Verona, Ospedale, Verona, Valeggio, Italy
J. D. Adachi, St. Joseph’s Hospital, McMaster University, Hamilton, Ontario, Canada
F. A. Anderson, Jr, Center for Outcomes Research, UMASS Medical School, Worcester, MA, USA.
S. Boonen, Leuven University Center for Metabolic Bone Diseases, Division of Geriatric Medicine, Katholieke Universiteit Leuven, Leuven
R. Chapurlat, INSERM U831, Université de Lyon, Division of Rheumatology, Hôpital E. Herriot, Lyon, France
A. Díez-Pérez, Hospital del Mar-IMIM-Autonomous, University of Barcelona, Barcelona, Spain
S. L. Greenspan, University of Pittsburgh, Pittsburgh, PA, USA
F. H. Hooven, Center for Outcomes Research, UMASS Medical School, Worcester, MA, USA
A. Z. LaCroix, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
J. W. Nieves, Helen Hayes Hospital and Columbia University, West Haverstraw, NY, USA
J. C. Netelenbos, Department of Endocrinology, VU University Medical Center, Amsterdam, The Netherlands
J. Pfeilschifter, Alfried Krupp Krankenhaus, Department of Internal Medicine III, Essen, Germany
M. Rossini, Department of Rheumatology, University of Verona, Verona, Italy
C. Roux, Paris Descartes University, Cochin Hospital, Paris, France
K. G. Saag, University of Alabama-Birmingham, Birmingham, AL, USA
S. Silverman, Department of Rheumatology, Cedars-Sinai/UCLA, Los Angeles, CA, USA
E. S. Siris, Department of Medicine, Columbia University Medical Center, New York, NY, USA
N. B. Watts, Bone Health and Osteoporosis Center, University of Cincinnati, Cincinnati, OH, USA
A. Wyman, Center for Outcomes Research, University of Massachusetts Medical School, Worcester, MA, USA
C. Cooper, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, SO16 6YD, UK; Institute of Musculoskeletal Sciences, University of Oxford, Oxford, UK
Reference list
- 1.Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization [Accessed 15 February 2013];The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 3.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen JS, Hogan C, Lyubomirsky G, Sambrook PN. Women with cardiovascular disease have increased risk of osteoporotic fracture. Calcif Tissue Int. 2011;88:9–15. doi: 10.1007/s00223-010-9431-7. [DOI] [PubMed] [Google Scholar]
- 6.Drake MT, Murad MH, Mauck KF, et al. Clinical review. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:1861–1870. doi: 10.1210/jc.2011-3058. [DOI] [PubMed] [Google Scholar]
- 7.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 9.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 10.David C, Confavreux CB, Mehsen N, Paccou J, Leboime A, Legrand E. Severity of osteoporosis: what is the impact of co-morbidities? Joint Bone Spine. 2010;77(Suppl 2):S103–106. doi: 10.1016/S1297-319X(10)70003-8. [DOI] [PubMed] [Google Scholar]
- 11.Bazelier MT, van Staa TP, Uitdehaag BM, Cooper C, Leufkens HG, Vestergaard P, Herings RM, de Vries F. Risk of fractures in patients with multiple sclerosis: a population-based cohort study. Neurology. 2012;78:1967–1973. doi: 10.1212/WNL.0b013e318259e0ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennison EM, Compston JE, Flahive J, et al. Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Bone. 2012;50:1288–1293. doi: 10.1016/j.bone.2012.02.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cline RR, Farley JF, Hansen RA, Schommer JC. Osteoporosis beliefs and antiresorptive medication use. Maturitas. 2005;50:196–208. doi: 10.1016/j.maturitas.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Gerend MA, Aiken LS, West SG, Erchull MJ. Beyond medical risk: investigating the psychological factors underlying women’s perceptions of susceptibility to breast cancer, heart disease, and osteoporosis. Health Psychol. 2004;23:247–258. doi: 10.1037/0278-6133.23.3.247. [DOI] [PubMed] [Google Scholar]
- 15.Cooper V, Moyle GJ, Fisher M, Reilly G, Ewan J, Liu HC, Horne R. Beliefs about antiretroviral therapy, treatment adherence and quality of life in a 48-week randomised study of continuation of zidovudine/lamivudine or switch to tenofovir DF/emtricitabine, each with efavirenz. AIDS Care. 2011;23:705–713. doi: 10.1080/09540121.2010.534433. [DOI] [PubMed] [Google Scholar]
- 16.Horne R, Clatworthy J, Hankins M. High adherence and concordance within a clinical trial of antihypertensives. Chronic Illn. 2010;6:243–251. doi: 10.1177/1742395310369018. [DOI] [PubMed] [Google Scholar]
- 17.Kucukarslan SN. A review of published studies of patients’ illness perceptions and medication adherence: lessons learned and future directions. Res Social Adm Pharm. 2012;8:371–382. doi: 10.1016/j.sapharm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Siris ES, Gehlbach S, Adachi JD, et al. Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW) Osteoporos Int. 2011;22:27–35. doi: 10.1007/s00198-010-1211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooven FH, Adachi JD, Adami S, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int. 2009;20:1107–1116. doi: 10.1007/s00198-009-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. Quality Metric, Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 21.Gerend MA, Erchull MJ, Aiken LS, Maner JK. Reasons and risk: factors underlying women’s perceptions of susceptibility to osteoporosis. Maturitas. 2006;55:227–237. doi: 10.1016/j.maturitas.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Phillipov G, Phillips PJ, Leach G, Taylor AW. Public perceptions and self-reported prevalence of osteoporosis in South Australia. Osteoporos Int. 1998;8:552–556. doi: 10.1007/s001980050098. [DOI] [PubMed] [Google Scholar]
- 23.Giangregorio L, Papaioannou A, Thabane L, DeBeer J, Cranney A, Dolovich L, Adili A, Adachi JD. Do patients perceive a link between a fragility fracture and osteoporosis? BMC Musculoskelet Disord. 2008;9:38. doi: 10.1186/1471-2474-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004;52:1479–1486. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 25.Gibson JC, Summers GD. Bone health in multiple sclerosis. Osteoporos Int. 2011;22:2935–2949. doi: 10.1007/s00198-011-1644-8. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi M, Carda S, Viscontini GS, Cisari C. Osteoporosis in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:339–346. doi: 10.1016/j.parkreldis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services . Bone Health and Osteoporosis: A Report of the Surgeon General (2004) Office of the Surgeon General; Rockville, USA: 2004. p http://www.surgeongeneral.gov/library/bonehealth/content.html. [Google Scholar]
- 28.Goodin DS, Frohman EM, Garmany GP, Jr., Halper J, Likosky WH, Lublin FD, Silberberg DH, Stuart WH, van den Noort S. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58:169–178. doi: 10.1212/wnl.58.2.169. [DOI] [PubMed] [Google Scholar]
- 29.Thurman DJ, Stevens JA, Rao JK. Practice parameter: Assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:473–479. doi: 10.1212/01.wnl.0000299085.18976.20. [DOI] [PubMed] [Google Scholar]
- 30.Zesiewicz TA, Sullivan KL, Arnulf I, Chaudhuri KR, Morgan JC, Gronseth GS, Miyasaki J, Iverson DJ, Weiner WJ. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924–931. doi: 10.1212/WNL.0b013e3181d55f24. [DOI] [PubMed] [Google Scholar]
- 31.Grimes D, Gordon J, Snelgrove B, et al. Canadian Guidelines on Parkinson’s Disease. Can J Neurol Sci. 2012;39:S1–30. doi: 10.1017/s031716710001516x. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Clinical Excellence NICE [Accessed 15 February 2013];Pathways: Stroke overview. http://pathways.nice.org.uk/pathways/stroke.
- 33.National Institute for Health and Clinical Excellence [Accessed 15 February 2013];Parkinson’s Disease: National clinical guideline for diagnosis and management in primary and secondary care. http://www.nice.org.uk/nicemedia/live/10984/30087/30087.pdf.
- 34. [Accessed 15 February 2013];MS Australia Practice for health professionals: Balance for people with multiple sclerosis (MS) http://www.msaustralia.org.au/documents/MS-Practice/balance.pdf.
- 35.National Institute for Health and Clinical Excellence [Accessed 15 February 2013];Multiple sclerosis: National clinical guideline for diagnosis and management in primary and secondary care. http://www.nice.org.uk/nicemedia/live/10930/46699/46699.pdf.
- 36.Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 37.American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:791–794. doi: 10.1053/gast.2003.50107. [DOI] [PubMed] [Google Scholar]
- 38.American Thoracic Society / European Respiratory Society [Accessed 15 February 2013];Task Force Standards for the Diagnosis and Management of Patients with COPD. http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf.
- 39.British Columbia Ministry of Health [Accessed 15 February 2013];Rheumatoid Arthritis: Diagnosis, Management and Monitoring. http://www.bcguidelines.ca/pdf/rheumatoid_arthritis.pdf.
- 40.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 41.National Clinical Guideline Centre [Accessed 15 February 2013];Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. http://www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf.
- 42.National Collaborating Centre for Chronic Conditions [Accessed 15 February 2013];Rheumatoid arthritis: national clinical guideline for management and treatment in adults. http://www.ncbi.nlm.nih.gov/books/NBK51812/pdf/TOC.pdf.
- 43.The Royal Australian College of General Practitioners [Accessed 15 February 2013];Clinical guideline for the diagnosis and management of early rheumatoid arthritis. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/cp118-early-rheum-arthritis.pdf.
- 44.Kasje WN, Denig P, De Graeff PA, Haaijer-Ruskamp FM. Physicians’ views on joint treatment guidelines for primary and secondary care. Int J Qual Health Care. 2004;16:229–236. doi: 10.1093/intqhc/mzh038. [DOI] [PubMed] [Google Scholar]
- 45.Kvamme OJ, Olesen F, Samuelson M. Improving the interface between primary and secondary care: a statement from the European Working Party on Quality in Family Practice (EQuiP) Qual Health Care. 2001;10:33–39. doi: 10.1136/qhc.10.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giangregorio L, Dolovich L, Cranney A, Adili A, Debeer J, Papaioannou A, Thabane L, Adachi JD. Osteoporosis risk perceptions among patients who have sustained a fragility fracture. Patient Educ Couns. 2009;74:213–220. doi: 10.1016/j.pec.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 48.Stockwell T, Donath S, Cooper-Stanbury M, Chikritzhs T, Catalano P, Mateo C. Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction. 2004;99:1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 49.Hundrup YA, Høidrup S, Obel EB, Rasmussen NK. The validity of self-reported fractures among Danish female nurses: comparison with fractures registered in the Danish National Hospital Register. Scandinavian Journal of Public Health. 2004;32:136–143. doi: 10.1080/14034940310017490. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 51.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001;22:117–139. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 52.Carmona L, Villaverde V, Hernandez-Garcia C, Ballina J, Gabriel R, Laffon A. The prevalence of rheumatoid arthritis in the general population of Spain. Rheumatology (Oxford) 2002;41:88–95. doi: 10.1093/rheumatology/41.1.88. [DOI] [PubMed] [Google Scholar]
- 53.Cimmino MA, Parisi M, Moggiana G, Mela GS, Accardo S. Prevalence of rheumatoid arthritis in Italy: the Chiavari Study. Ann Rheum Dis. 1998;57:315–318. doi: 10.1136/ard.57.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 55.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 56.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.