Abstract
Although oral bisphosphonates (BP) are highly effective in preventing fractures, some patients will fracture while on treatment. We identified predictors of such fractures in a population-based cohort of incident users of oral BP.
We screened the SIDIAP database to identify new users of oral BP in 2006-2007. SIDIAP includes pharmacy invoice data and primary care electronic medical records for a representative 5 million people in Catalonia (Spain). Exclusion criteria were: Paget disease, <40 years of age, and any anti-osteoporosis treatment in the previous year.
A priori defined risk factors included age, gender, body mass index, vitamin D deficiency, smoking, alcohol drinking, pre-existing comorbidities, and medications.
Fractures were considered if they appeared after at least 6 months after treatment initiation. Fractures while on treatment were defined as those occurring among participants persisting for at least 6 months and with an overall high compliance (medication possession ratio ≥ 80%). Fine and Gray survival models accounting for competing risk with therapy discontinuation were fitted to identify key predictors.
Results
Only 7,449/21,385 (34.8%) participants completed >6 months of therapy. Incidence of “fracture while on treatment” was 3.4/100 person-years [95%CI 3.1-3.7]. Predictors of these among patients persisting and adhering to treatment included: older age (sub-hazard ratio (SHR) for 60 to <80 years 2.18 [1.70-2.80]; for ≥80years 2.5 [1.82-3.43]), previous fracture (SHR 1.75 [1.39-2.20] and 2.49 [1.98-3.13] in the last 6 months and longer respectively), underweight (SHR 2.11 [1.14-3.92]), inflammatory arthritis (SHR 1.46 [1.02-2.10]), use of proton pump inhibitors (PPI) (SHR 1.22 [1.02-1.46]) and vitamin D deficiency (SHR 2.69 [1.27-5.72].
Conclusion(s)
Even among high compliers, 3.4% of oral BP users will fracture every year. Older age, underweight, vitamin D deficiency, PPI use, previous fracture and inflammatory arthritides increase risk. Monitoring strategies and/or alternative therapies should be considered for these patients.
BACKGROUND
A number of guidelines propose oral bisphosphonates as first-line therapies to prevent fragility fractures in osteoporotic patients(1), and data from clinical trials suggest that they can reduce the risk of fractures by 50%(2-4). In order to achieve such benefits, patients need to persist with treatment for 5 years, which rarely happens in actual practice, where approximately half of the patients discontinue therapy in the first 3-6 months(5). Poor compliance is hence recognised as a risk factor for fractures(6), and makes it difficult to disentangle whether “fracture while on treatment” is due to patient characteristics or to low compliance in cohort studies where data on anti-osteoporosis medications is usually scarce and often self-reported. In addition, fractures occur while on treatment even among high adherers in strictly controlled conditions such as randomized controlled trials settings(3,4,7,8), but data on predictors of such fractures are lacking. Investigators of the Observational Study of Severe Osteoporosis (OSSO) study proposed a clinical definition of “non-responders” based on the appearance of incident fragility fractures while on oral bisphosphonate therapy(9).
Poor response to bisphosphonate therapy in high-risk patients, either due to low compliance or to patient characteristics, have consequences both for the patient, who remains at increased risk of fracture, and for healthcare providers, as this phenomenon reduces cost-efficacy of treatment(10).
We used data from computerized primary care medical records linked to pharmacy invoice data for >80% of the population of Catalonia (Spain) to identify key predictors of “fracture while on treatment” in: 1.a population-based cohort of all incident users of oral bisphosphonate users who started therapy in the years 2006 and 2007, and 2.participants who persisted with therapy and had a high therapy compliance.
METHODS
Study population and source of data
General practitioners (GPs) are responsible for primary health-care, long-term prescriptions and specialist and hospital referrals in Spain. The Spanish public health-care system covers practically the totality of the population. The data in this study were obtained from the SIDIAP (Sistema d’Informació per al Desenvolupament de l’Investigació en Atenció Primària) Database. SIDIAP comprises of primary care electronic medical records of a sample of patients in Catalonia (North-East Spain), covering a population of about 5 million patients (80% of the total population for the region) from 274 primary care practices and a total of 3,414 participating GPs.
SIDIAP comprises the clinical and referral events registered by primary care health professionals (GPs and nurses) and administrative staff in e-records, comprehensive demographic information, prescription and corresponding pharmacy invoicing data, specialist referrals, primary care laboratory test results, hospital admissions, and their major outcomes(11). Health professionals encode this information using ICD-10 codes, and structured forms designed for the collection of variables relevant for primary care clinical management, such as height, weight, body mass index, smoking and drinking status, blood pressure measurements, blood and urine test results, etc. Only GPs who achieve quality control standards can contribute to the SIDIAP database (12). Encoding personal and clinic identifiers ensures the confidentiality of the information in the SIDIAP Database.
We screened the SIDIAP Database to identify incident users of oral bisphosphonates (excluding presentations of high dose bisphosphonates) in the period 1/1/2006 to 31/12/2007. Eligible participants aged below 40 years, those with a diagnosis of Paget disease, and previous users of any anti-osteoporosis drug in the year prior to the first prescription of oral bisphosphonates were excluded.
Ascertainment of “fracture while on treatment”
Fractures registered in the period 2006-2011 in the SIDIAP database were identified using ICD-10 codes for any osteoporotic fracture (of any site but fingers, toes, and skull). We also looked separately at hip fractures and major fractures (wrist/forearm, proximal humerus, and clinical spine fracture) as a group. Fracture coding has been validated in the SIDIAP database, and shown to be highly specific (>95% for all fracture sites studied)(13).
Treatment discontinuation was defined as a refill gap of at least 6 months without medication, and the date of discontinuation was therefore the date when the last prescription before the gap was purchased. As a sensitivity analysis, a refill gap of at least 12 months was used instead, to take into account the long off-effect of bisphosphonates on bone.
For these analysis two populations of patients were considered:
all incident bisphosphonate users (Intention-to-Treat-like approach): this cohort included all patients starting oral bisphosphonates in the period 2006-2007 with no previous use of any anti-osteoporosis medications in the previous year. Here, fractures were considered if they appeared after at least 6 months of oral bisphosphonate therapy, independently of compliance or persistence with oral bisphosphonates;
persistent and adherent to therapy: this subcohort included only those amongst the previously defined population who had high compliance to oral bisphosphonate therapy, defined as a medication possession ratio (MPR) ≥80%. MPR is calculated as the proportion of days covered with therapy between the first and the last prescription of bisphosphonates (total number of DDDs purchased / number of days between first and last prescription date). Fractures while on treatment were defined as occurring after having persisted for a minimum of 6 months and only whilst continuing BP therapy.
Potential predictors of “fracture while on treatment”
Potential risk factors for fracture in oral BP users were defined a priori based on previous literature, and assessed at the time of first oral BP prescription (therapy initiation). These included the following: age, gender, body mass index(14) (WHO categories: <18.5 underweight, 18.5 to <25 normal weight, 25 to <30 overweight, 30 to <35 grade 1 obesity, and ≥35 grade 2 obesity), smoking, alcohol drinking, country of origin (national vs foreign), vitamin D deficiency (as coded in primary care records), diagnosis of Osteoporosis, falls, history of fracture, estimated glomerular filtration rate (MDRD-4), pre-existing co-morbid conditions (dementia, inflammatory bowel disease, malabsorption syndrome, Parkinson disease, type 2 Diabetes Mellitus, Chronic Obstructive Pulmonary Disease [COPD], Osteoarthritis, Cardiovascular disease, and Inflammatory Arthritis including rheumatoid arthritis, ankylosing spondylitis, and systemic lupus eritematous), and a list of concomitant medications (proton pump inhibitors, oral glucocorticoids, oral anticoagulants, levotiroxin, aromatase inhibitors, anticonvulsants, benzodiazepines, antidepressants, hormone replacement therapy, calcium+/−vitamin D supplements), and previous compliance to other long-term medications for asymptomatic conditions (statins and antihypertensives).
Statistical analyses
Among all incident bisphosphonate users standard multivariable Cox Regression methods were used to identify independent predictors of fracture at least 6 months after treatment initiation, independently of persistence and compliance.
Secondly, independent risk factors of time to first “fracture while on treatment” were assessed using multivariable Fine and Gray regression for survival analyses in competing risk scenarios(15) where, by definition, therapy discontinuation precluded “fracture while on treatment” events (i.e. we only considered fractures when they occurred before therapy discontinuation date). As a sensitivity analysis, competing risk for mortality was also accounted for using these same methods. All these models were then fitted separately for hip fracture and major fractures (wrist/forearm, proximal humerus and clinical spine fracture).
In both cases, backwards-stepwise selection was used to identify key risk factors (p-entry 0.049; p-exit 0.10) for incident fracture.
In order to establish the percentage of fractures in the first year that could be prevented were the reversible risk factors (vitamin D deficiency and PPI prescription) were modified, the baseline survival curve and linear predictor obtained from the competing risks model were used to calculate the probability of fracturing the first year (following six months treatment) for each person. These probabilities were then recalculated assuming that no person had vitamin D deficiency or PPI prescription. Comparing these two sets of probabilities, the number and percentage of fractures that could have been prevented by modifying these two risk factors was calculated.
RESULTS
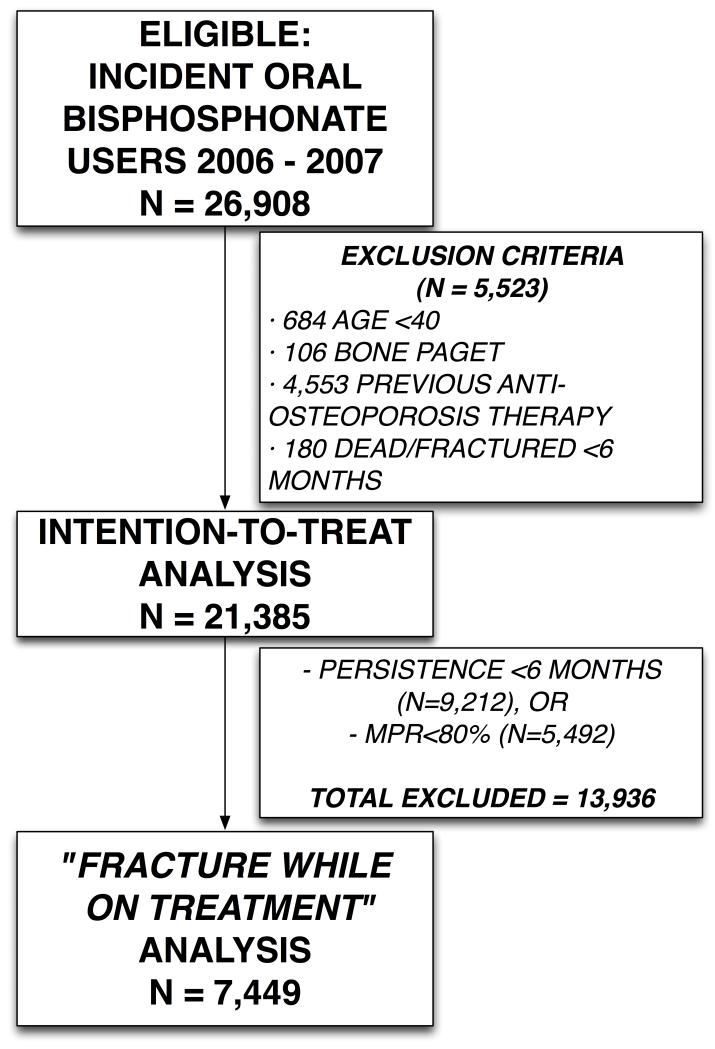
The total number of incident oral bisphosphonate users in SIDIAP in the years 2006-2007 was 26,908. After excluding 5,523 patients that did not meet the inclusion criteria, leaving 21,385 participants incident users for analysis. Of these patients only 7,449 (34.8%) completed 6 months of therapy and had overall high compliance to therapy (MPR≥80%) and thus were included for the study of predictors of “fracture while on treatment” [Figure 1].
FIGURE 1.
Population flow-chart
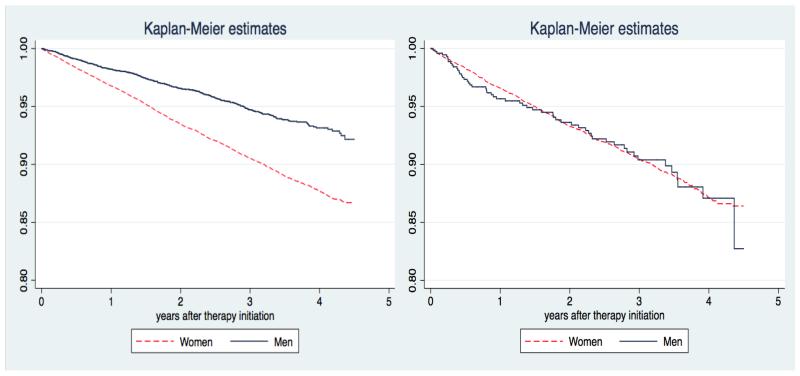
A total of 2,026 (9.5%) out of 21,385 incident users of oral bisphosphonates suffered at least one fracture in the study period. Similarly, 617 (8.3%) fractured while on treatment among the 7,449 patients who adhered to therapy. Unadjusted incidence rate of “fracture while on treatment” was 3.4/100 person-years [95%CI 3.1-3.7]. Baseline characteristics for patients who completed at least 6 months of treatment and for all new incident users of bisphosphonates are reported in Table 1. Figure 2 shows Kaplan-Meier estimates of the cumulative probability of fracture in both populations, stratified by gender: these show an excess risk of fracture for women only in the ITT-like approach cohort, but not in the “fracture while on treatment” analysis.
TABLE 1. Baseline characteristics.
| Persisting and adhering to treatment N = 7,449 |
All Incident bisphosphonate users N = 21,385 |
||||
|---|---|---|---|---|---|
| Sex | Male | 750 | 10% | 5119 | 24% |
| Age | <=60 years | 1939 | 26% | 6181 | 29% |
| 60-80 years | 4706 | 63% | 12573 | 59% | |
| >80 years | 804 | 11% | 2631 | 12% | |
| BMI | Missing | 1043 | 14% | 3654 | 17% |
| <18.5 | 49 | 1% | 126 | 1% | |
| 18.5-25 | 1576 | 21% | 4130 | 19% | |
| 25-30 | 2698 | 36% | 7524 | 35% | |
| 30-35 | 1513 | 20% | 4335 | 20% | |
| >35 | 570 | 8% | 1616 | 8% | |
| Nationality | Spain | 7354 | 99% | 21027 | 98% |
| Others | 95 | 1% | 358 | 2% | |
| Smoking | Missing | 714 | 10% | 2404 | 11% |
| Never | 5852 | 79% | 15219 | 71% | |
| Ex | 395 | 5% | 1636 | 8% | |
| Current | 488 | 7% | 2126 | 10% | |
| Drinking | Missing | 1407 | 19% | 4861 | 23% |
| Never | 4805 | 65% | 12502 | 58% | |
| Moderate | 1171 | 16% | 3777 | 18% | |
| Severe | 66 | 1% | 245 | 1% | |
| Previous Fracture | No | 6088 | 82% | 18308 | 86% |
| >6m ago | 752 | 10% | 1783 | 8% | |
| <6m ago | 609 | 8% | 1294 | 6% | |
| Vitamin D deficiency | 41 | 1% | 81 | 0% | |
| PPI user | 4546 | 61% | 12804 | 60% | |
| Calcium/D supplement user | 2322 | 31% | 5193 | 24% | |
| Oral corticosteroid user | 1245 | 17% | 3266 | 15% | |
| HRT user | 249 | 3% | 567 | 3% | |
| Number of Co-morbidities | 0 | 5602 | 75% | 15250 | 71% |
| 1 | 1585 | 21% | 5070 | 24% | |
| 2 | 242 | 3% | 962 | 4% | |
| ≥ 3 | 20 | 0% | 103 | 0% | |
| Osteoporosis | 1928 | 26% | 4090 | 19% | |
| Inflammatory arthritis | 253 | 3% | 570 | 3% | |
FIGURE 2.
Kaplan-Meier estimates for probability of fracture amongst incident users of oral bisphosphonates (ITT analysis) [Left] and of fracture while on treatment [Right].
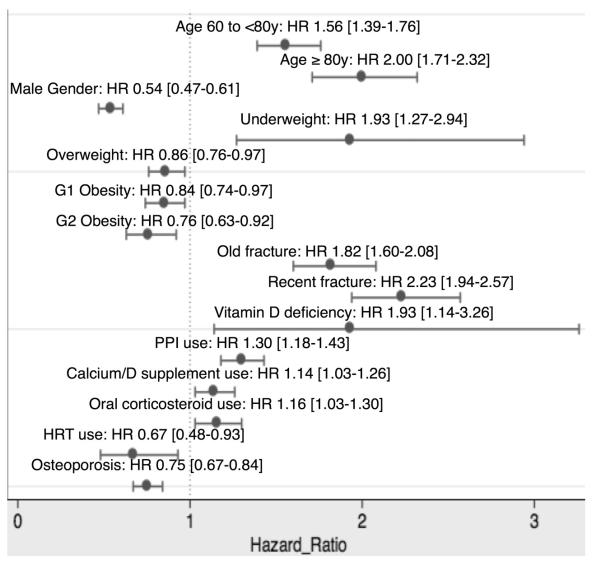
Predictors and protective factors for fracture in all incident users are shown in Figure 3. Risk factors included: age, BMI (underweight), previous fracture, vitamin D deficiency, and previous use of proton pump inhibitors, oral glucocorticoids, and calcium/vitamin D supplements. Conversely, male gender, overweight, obesity, a diagnosis of osteoporosis, and previous hormone replacement therapy were associated with lower fracture risk. In this population, reversible predictors (vitamin D deficiency and use of proton pump inhibitors) accounted for approximately 16% of the fractures observed early after therapy initiation.
FIGURE 3.
Predictors of incident fracture in new bisphosphonate users (ITT-like analysis).
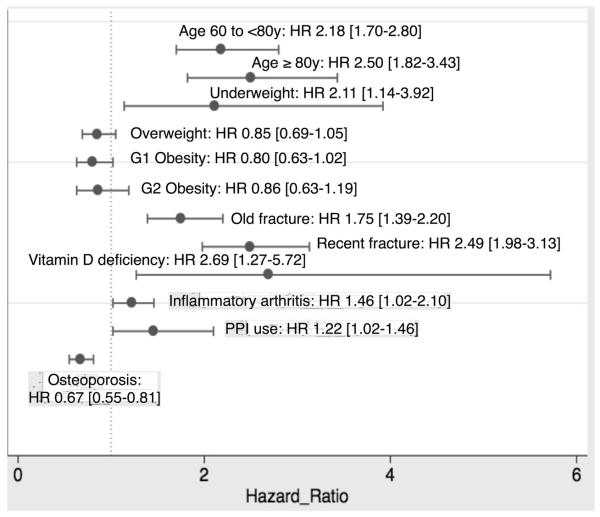
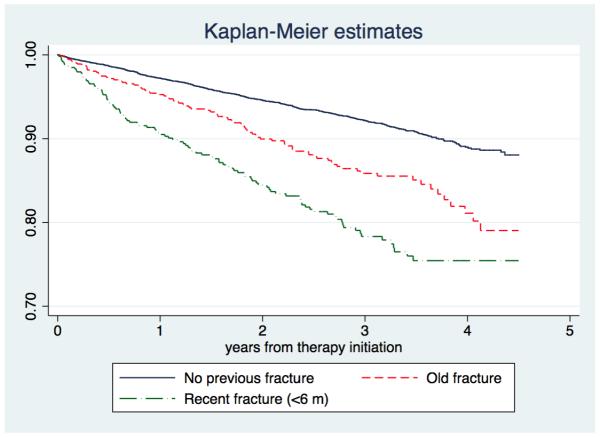
Risk factors of hip and major fractures are shown in Tables 2 and 3 respectively. In the analysis of “fracture while on treatment” (for patients adhering and persisting to therapy), a diagnosis of osteoporosis was the only protective factor, whilst older age, underweight, fracture history, inflammatory arthritis, vitamin D deficiency and use of proton pump inhibitors were related to a higher risk of fracture [Figure 4]. Probability of “fracture while on treatment” was higher in patients with a recent fracture (in the last 6 months before starting oral bisphosphonates) than in those who had either no fracture history or a fracture more than 6 months prior to therapy initiation [Figure 5]. Amongst these, reversible predictors (vitamin D deficiency and use of proton pump inhibitors) accounted for approximately 13% of the fractures observed early after therapy initiation.
Table 2. Predictors of hip fracture.
| Hip fractures (N=256) |
||
|---|---|---|
| SHR [95%CI]; p-val | ||
| Age | <60 years | REFERENCE |
| 60 to <80 years | 4.40 [2.64 – 7.34]; p<0.001 | |
| ≥ 80 years | 13.48 [7.89 – 23.05]; p<0.001 | |
| BMI | Normal weight | REFERENCE |
| Underweight | 2.36 [0.94 – 5.93]; p=0.07 | |
| Overweight | 0.60 [0.43 – 0.83]; p=0.003 | |
| Obese grade I | 0.65 [0.45 – 0.95]; p=0.027 | |
| Obese grade II | 0.42 [0.22 – 0.81]; p=0.009 | |
|
Previous fracture
history |
None | REFERENCE |
| Old fracture | 1.84 [1.26 - 2.70]; p=0.002 | |
| Recent fracture | 2.73 [1.92 – 3.88]; p<0.001 | |
Table 3. Predictors of major fracture.
| Major fractures (N=811) |
||
|---|---|---|
| SHR [95%CI]; p-val | ||
| Age | <60 years | REFERENCE |
| 60 to <80 years | 1.32 [1.10 – 1.57]; p=0.002 | |
| ≥ 80 years | 1.67 [1.32 – 2.11]; p<0.001 | |
|
Vitamin D
deficiency |
2.38 [1.11 – 5.14]; p=0.027 | |
|
User of proton
pump inhibitors |
1.41 [1.22 – 1.65]; p<0.001 | |
|
Previous fracture
history |
None | REFERENCE |
| Old fracture | 1.89 [1.54 - 2.33]; p<0.001 | |
| Recent fracture | 2.01 [1.60 – 2.53]; p<0.001 | |
FIGURE 4.
Predictors of incident fracture while on treatment.
FIGURE 5.
Kaplan-Meier estimates of cumulative incidence of fracture while on treatment among incident users of oral bisphosphonates with: no previous fracture, a history of recent fracture (in the past 6 months), and a history of old fracture (more than 6 months prior to therapy initiation)
Predictors of hip and major “fracture while on treatment” are reported in Tables 4 and 5 respectively.
Table 4. Predictors of hip fracture while on treatment.
| Hip fracture while on treatment (N=63) |
||
|---|---|---|
| SHR [95%CI]; p-val | ||
| Age | <60 years | REFERENCE |
| 60 to <80 years | 3.69 [1.26 – 10.81]; p=0.017 | |
| ≥ 80 years | 7.56 [2.37 – 24.15]; p=0.001 | |
| BMI | Normal weight | REFERENCE |
| Underweight | 5.17 [1.39 - 19.25]; p=0.014 | |
|
Previous fracture
history |
None | REFERENCE |
| Old fracture | 3.06 [1.59 - 5.91]; p=0.001 | |
| Recent fracture | 3.79 [1.97 – 7.29]; p<0.001 | |
|
Recorded
Osteoporosis |
0.40 [0.20 – 0.79]; p=0.008 | |
Table 5. Predictors of major fracture while on treatment.
| Major fracture while on treatment (N=230) |
||
|---|---|---|
| SHR [95%CI]; p-val | ||
| Age | <60 years | REFERENCE |
| 60 to <80 years | 2.00 [1.34 – 2.97]; p=0.001 | |
| ≥ 80 years | 2.24 [1.34 – 3.76]; p=0.002 | |
|
Previous fracture
history |
None | REFERENCE |
| Old fracture | 1.88 [1.31 - 2.69]; p=0.001 | |
| Recent fracture | 2.04 [1.37 – 3.02]; p<0.001 | |
Competing risk with mortality did not change either this list of risk factors and predictors, or their effect size (data not shown).
DISCUSSION
We have shown that incident fractures are not uncommon among patients on oral bisphosphonate therapy: almost 10% of them will fracture in the five years after therapy initiation, and even in the best case scenario (patients with high compliance to treatment), 3.4% will suffer a fracture every year while on treatment.
We report here a number of independent predictors and protective factors for incident fractures among patients who start oral bisphosphonate therapy including age, gender, body mass index, history of fractures, vitamin D deficiency, use of proton pump inhibitors, oral glucocorticoids, calcium and vitamin D supplements, and hormone replacement therapy.
In addition, this is one of the few studies published with accurate data on compliance and persistence, and we have used competing risk survival methods to study predictors of “fracture while on treatment” accounting for the fact that therapy discontinuation precludes such events, as fractures are not considered “while on treatment” if they occur after therapy has been stopped. Using these methods, we demonstrate that older patients, underweight participants, as well as those with a history of previous fracture, inflammatory arthritis, vitamin D deficiency and use of proton pump inhibitors are at increased risk of these fractures even if they persist with and adhere to the therapy as prescribed.
Previous studies have repeatedly shown that low compliance leads to higher fracture risk (6). In parallel, some reports have shown that previous use of HRT is related to better compliance with oral bisphosphonates, whilst previous use of oral glucocorticoids would predict poor compliance (16,17). These factors arise as predictors of fracture among incident users of oral bisphosphonates in our analysis of all incident users, but not in the competing risk analysis of “fracture while on treatment” in those complying with treatment. Hence, their appearance in these analyses might be due to an indirect effect on fracture reduction through their impact on therapy compliance and persistence. Similarly, the gender effect (lower risk of fracture amongst men treated with bisphosphonates) could be associated with poorer compliance, which would explain why this becomes not relevant in the analysis of predictors of “fracture while on treatment”.
Conversely, older age, fracture history, vitamin D deficiency, use of proton pump inhibitors and underweight appear as predictors of fracture both in the incident users and high compliers analyses, suggesting that these might be true predictors of inadequate response to oral bisphosphonate therapy. Older age has been reported in previous studies as a predictor of inadequate response to oral bisphosphonate therapy, with similar effect size and equal direction of effect (18). Similarly, vitamin D deficiency was recently shown to predict inadequate response in a multi-centric study carried out by our group (19). Fracture history emerged as a risk factor for “fracture while on treatment” in all the studies published to date on this topic (18,20-22). It has been speculated that this could translate to a belated initiation of anti-resorptive treatment, and that patients starting oral bisphosphonates after a fragility fracture could have benefited from an earlier therapy (23). A history of inflammatory arthritis appeared as a predictor of inadequate response in the OSSO study (20), but not in a recent study by Abrahamsen et al. Our data support this association.
We have shown that underweight and use of proton pump inhibitors are also predictive of treatment failure. The first is relatively uncommon, and it has been associated with increased risk of hip, pelvis and wrist fractures in the past (24). Conversely, a potential interaction between oral bisphosphonate and proton pump inhibitor treatments might be very relevant in clinical practice: more than 60% of our study participants were taking PPIs at the time they started oral bisphosphonates. Previous reports on the effect of PPIs on fracture risk have obtained conflicting results (25), but few studies to our knowledge have assessed a potential interaction with bisphosphonate anti-fracture efficacy. One recent study by Abrahamsen B et al(26) showed a significant reduction of anti-fracture efficacy of oral alendronate when taken concomitantly with PPIs, and this has been further confirmed in a more recent report by this same author (22).
The finding of a diagnosis of Osteoporosis related to a reduction in fracture risk is counter-intuitive, and has not been explored in the past. Although the lack of data on bone mineral density or other biomarkers in SIDIAP did not allow us to explore this further, we are confident that this is likely to be a coding artefact: a primary care diagnosis of osteoporosis might only reveal a higher awareness of disease, leading to health-seeking behaviour and healthy adherer effects.
When our study was designed and the analysis plan specified, no definition of treatment failure was available (23). Therefore we used the criterion of at least one “fracture while on treatment” and our results do not necessarily translate to those patients suffering two or more incident fractures while on treatment. This is why we do not use treatment failure as the outcome of our analysis but a more comprehensive term: “fracture while on treatment”.
Non-adherers have not been analyzed in our study because the question addressed was the limiting factors in the response to treatment when the exposure to the drug has reached enough levels of efficacy. Several publications have demonstrated the increased fracture risk in poor or non-adherers(6). In our results too, a rough estimate of this issue may be the higher proportion of fractures while on treatment in the whole cohort (9.5 %) vs. the adherers (8.3%).
A possible explanation for our findings might be a continuous bone remodeling with negative balance and structural decay. Unfortunately, the nature of our study does not permit the analysis of bone turnover markers or bone mineral density decline. However, some data could be invoked here. One is the strong suppressive effect of bisphosphonates on remodeling rate, superior to 90% in terms of activation frequency for alendronate(27). The second is previous data (ref) on the lack of association between bone turnover markers and inadequate response to antiresorptives. Therefore, other factors might also play a role, basically a too advanced degree of structure damage (ref) that makes the effect of antiresorptives not powerful enough to restore bone mechanical competence. In other words, the antiresorptives are prescribed “too late” in the natural history of bone deterioration.
The main limitation of our study is the lack of individual validation of the fractures observed. However, coding of clinical fractures has been recently validated in the SIDIAP database and shown to be highly specific when compared to prospective cohort studies and to the official national hospital admission database (13). In addition, the analysis of predictors of “fracture while on treatment” in a cohort of patients who adhere to therapy is potentially prone to conditional confounding. However, the analysis of predictors of fracture amongst all incident bisphosphonate users overcomes this issue.
Strengths of our study are the high sample size, and the representativeness of the data used: SIDIAP covers a sample of more than 80% of the total population of Catalonia, and the information is coded in actual practice conditions. In addition, the information gathered on dispensed bisphosphonates is detailed, and likely to be more reliable than patient reports or GP prescriptions.
Conclusions
A number of clinical variables at the time of oral bisphosphonate therapy initiation are associated with higher risk of fractures. In addition, some patient characteristics including age, body mass index, history of previous fractures and inflammatory arthritides, vitamin D status, and use of proton pump inhibitors will predict the occurrence of fractures while on treatment, independently of compliance and persistence to therapy. Information on all these variables is readily available in actual practice conditions both in primary care and in specialized settings, and should be taken into consideration: interventions to improve compliance to therapy and to further reduce fracture risk could be considered for high risk patients.
Acknowledgements
Funding for this study was obtained from the Spanish Department of Health and Innovation (Independent Pharmaco-Epidemiological Studies Grant – DGTATX/ISCI2011).
The Internal Medicine Department and the URFOA IMIM receive support from the RETICEF (Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad, Instituto Carlos III, Government of Spain).
DPA, MKJ, AJ, NKA and CC receive partial support from the NIHR Biomedical Research Unit.
We want to thank all the health professionals involved in registering data in computerized medical records for SIDIAP.
REFERENCES
- 1.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, FIT Research Group Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85(11):4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 4.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD, Vertebral Efficacy With Risedronate Therapy (VERT) Study Group Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 5.Sheehy O, Kindundu CM, Barbeau M, LeLorier J. Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int. 2009;20(8):1369–76. doi: 10.1007/s00198-008-0795-8. [DOI] [PubMed] [Google Scholar]
- 6.Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15(12):1003–8. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 7.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 8.Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakob F, Marin F, Martin-Mola E, Torgerson D, Fardellone P, Adami S, Thalassinos NC, Sykes D, Melo-Gomes J, Chinn C, Nicholson T, Cooper C. Characterization of patients with an inadequate clinical outcome from osteoporosis therapy: the Observational Study of Severe Osteoporosis (OSSO) QJM. 2006;99(8):531–43. doi: 10.1093/qjmed/hcl073. [DOI] [PubMed] [Google Scholar]
- 10.Landfeldt E, Lundkvist J, Strom O. The societal burden of poor persistence to treatment of osteoporosis in Sweden. Bone. 2011;48(2):380–8. doi: 10.1016/j.bone.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Ramos R, Balló E, Marrugat J, Elosúa R, Sala J, Grau M, Vila J, Bolibar B, Garcia-Gil M, Martí R, Fina F, Hermosilla E, Rossell M, Muñoz M, Prieto-Alhambra D, Quesada M. Validity of the SIDIAP database for the study of cardio-vascular disease: the EMMA Study. Rev Esp Cardiol. 2011 doi: 10.1016/j.recesp.2011.07.017. in press. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Gil M, Hermosilla E, Prieto-Alhambra D, Fina F, Rossell M, Ramos R, Rodriguez J, Williams T, Van Staa T, Bolibar B. Construction and Validation of a Scoring System for Selction of High Quality Data in a Spanish Population Primary Care Database (SIDIAP) Informatics in Primary Care. 2011 doi: 10.14236/jhi.v19i3.806. Submitted. [DOI] [PubMed] [Google Scholar]
- 13.Pages-Castella A, Carbonell-Abella C, Aviles FF, Alzamora M, Baena-Diez JM, Laguna DM, Nogues X, Diez-Perez A, Prieto-Alhambra D. Burden of osteoporotic fractures in primary health care in Catalonia (Spain): a population-based study. BMC Musculoskelet Disord. 2012;13:79. doi: 10.1186/1471-2474-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premaor MO, Compston JE, Aviles FF, Pages-Castella A, Nogues X, Diez-Perez A, Prieto-Alhambra D. The association between fracture site and obesity in men: A population-based cohort study. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1878. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 16.Lo JC, Pressman AR, Omar MA, Ettinger B. Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int. 2006;17(6):922–8. doi: 10.1007/s00198-006-0085-2. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JR, Xi J, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47(3):334–41. doi: 10.1097/MLR.0b013e31818afa1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adami S, Isaia G, Luisetto G, Minisola S, Sinigaglia L, Silvestri S, Agnusdei D, Gentilella R, Nuti R. Osteoporosis treatment and fracture incidence: the ICARO longitudinal study. Osteoporos Int. 2008;19(8):1219–23. doi: 10.1007/s00198-008-0566-6. [DOI] [PubMed] [Google Scholar]
- 19.Diez-Perez A, Olmos JM, Nogues X, Sosa M, Diaz-Curiel M, Perez-Castrillon JL, Perez-Cano R, Munoz-Torres M, Torrijos A, Jodar E, Del Rio L, Caeiro-Rey JR, Farrerons J, Vila J, Arnaud C, Gonzalez-Macias J. Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res. 2012;27(4):817–24. doi: 10.1002/jbmr.1496. [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Jakob F, Chinn C, Martin-Mola E, Fardellone P, Adami S, Thalassinos NC, Melo-Gomes J, Torgerson D, Gibson A, Marin F. Fracture incidence and changes in quality of life in women with an inadequate clinical outcome from osteoporosis therapy: the Observational Study of Severe Osteoporosis (OSSO) Osteoporos Int. 2008;19(4):493–501. doi: 10.1007/s00198-007-0488-8. [DOI] [PubMed] [Google Scholar]
- 21.Adami S, Isaia G, Luisetto G, Minisola S, Sinigaglia L, Gentilella R, Agnusdei D, Iori N, Nuti R. Fracture incidence and characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res. 2006;21(10):1565–70. doi: 10.1359/jbmr.060715. [DOI] [PubMed] [Google Scholar]
- 22.Abrahamsen B, Rubin KH, Eiken PA, Eastell R. Characteristics of patients who suffer major osteoporotic fractures despite adhering to alendronate treatment: a National Prescription registry study. Osteoporos Int. 2012;24(1):321–8. doi: 10.1007/s00198-012-2184-6. [DOI] [PubMed] [Google Scholar]
- 23.Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C. Treatment failure in osteoporosis. Osteoporos Int. 2012;23(12):2769–74. doi: 10.1007/s00198-012-2093-8. [DOI] [PubMed] [Google Scholar]
- 24.Prieto-Alhambra D, Premaor MO, Fina Aviles F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, Nogues X, Compston JE, Diez-Perez A. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 25.de Vries F. Inverse association between duration of use of acid-suppressive medications and fracture risk. Arch Intern Med. 2011;171(11):1044. doi: 10.1001/archinternmed.2011.226. author reply 1044. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171(11):998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- 27.Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20(7):1244–53. doi: 10.1359/JBMR.050309. [DOI] [PubMed] [Google Scholar]