Abstract
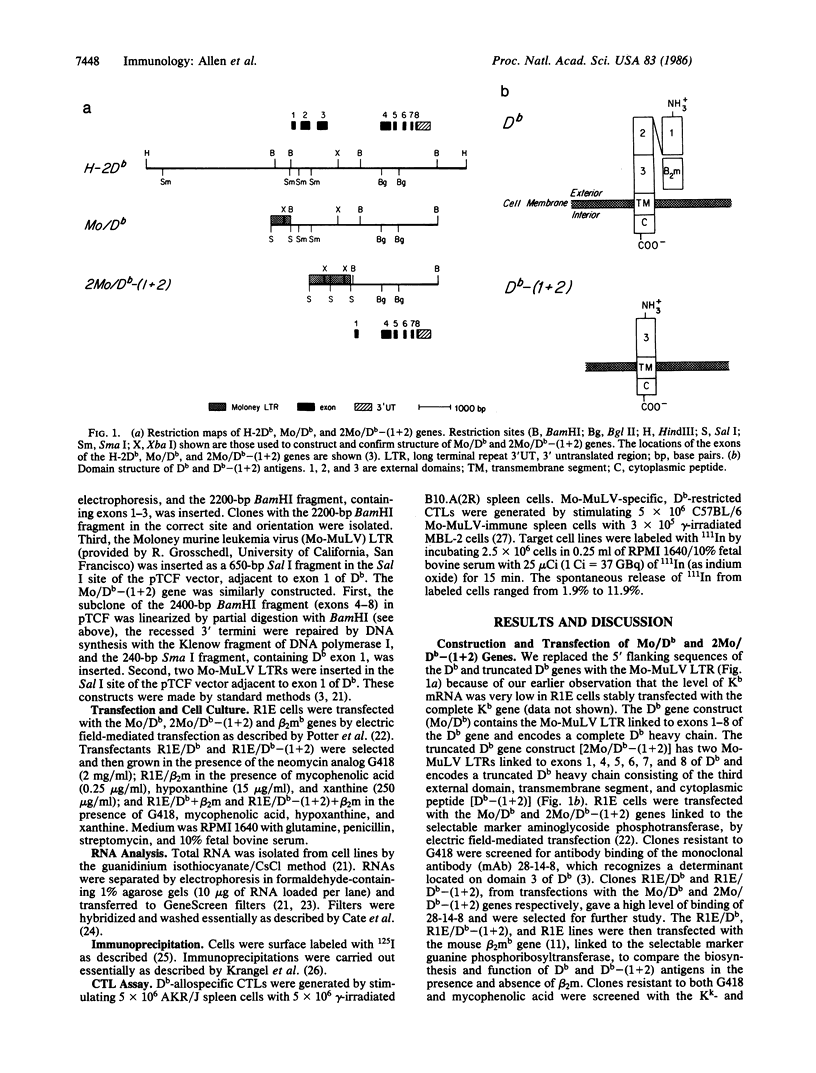
beta 2-Microglobulin (beta 2m) has been thought essential for transport of all major histocompatibility complex class I antigens to the cell surface. Here, we show that the mouse class I antigen H-2Db is expressed at the cell surface even when there is no beta 2m present within the cell. This was established by transfecting the H-2Db gene into the R1E cell line, which lacks beta 2m. The conformation of the Db antigen expressed by the R1E transfectant is very different from that of the native molecule. This Db antigen is not recognized by Db-allospecific and Db-restricted cytotoxic T lymphocytes or by most monoclonal antibodies to the native Db. We show further that a deletion construct of the Db gene, which consists of exon 1 linked to exons 4-8, expresses a truncated Db antigen lacking domains 1 and 2 [Db-(1 + 2)] at the cell surface after transfection into the R1E line. Previous biochemical and crystallographic data have indicated that domain 3 is associated with beta 2m; unexpectedly, Db-(1 + 2) does not associate with beta 2m when the mouse beta 2mb gene is transfected into the R1E transfectant expressing the truncated Db. This suggests that interactions with domains 1 and 2 are important for the paired association of domain 3 and beta 2m in the native Db antigen.
Full text
PDF
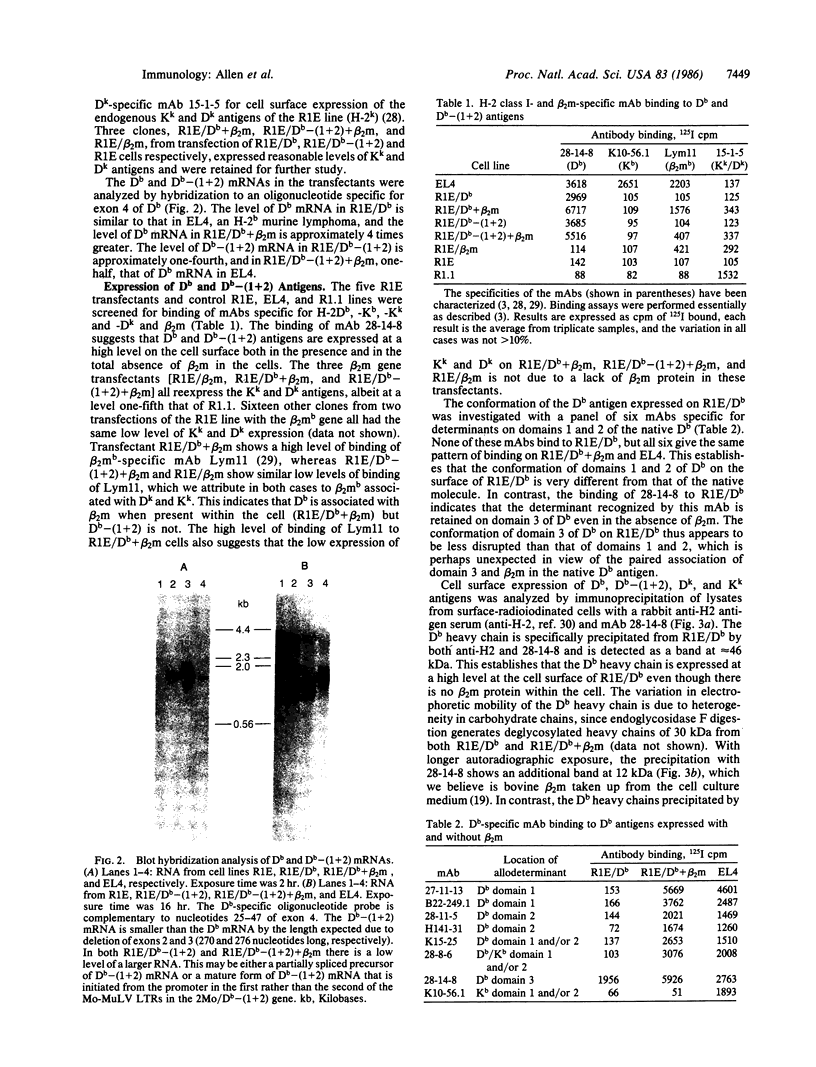
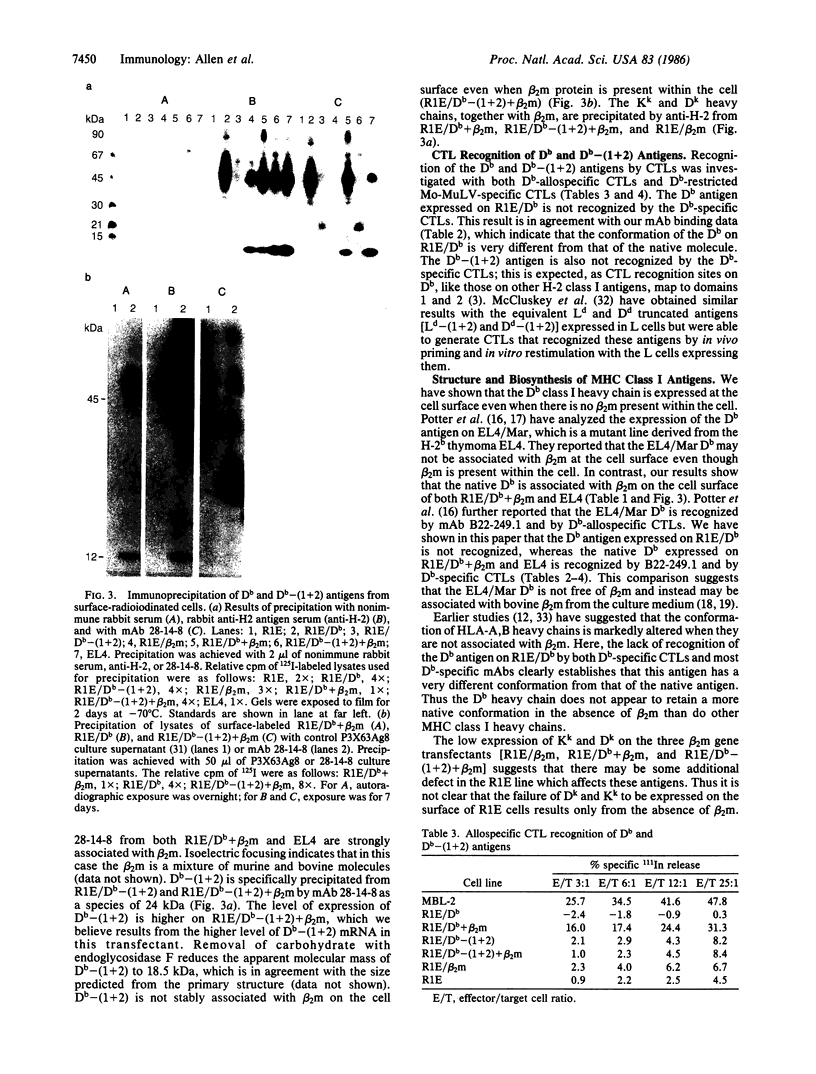

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Strominger J. L., Wiley D. C. Crystallization and X-ray diffraction studies on the histocompatibility antigens HLA-A2 and HLA-A28 from human cell membranes. J Mol Biol. 1985 Nov 5;186(1):205–210. doi: 10.1016/0022-2836(85)90271-2. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Retrovirus-induced changes in major histocompatibility complex antigen expression influence susceptibility to lysis by cytotoxic T lymphocytes. J Immunol. 1985 Oct;135(4):2287–2292. [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Taketani S., Biddison W. E., Strong D. M., Strominger J. L. Comparative structural analysis of HLA-A2 antigens distinguishable by cytotoxic T lymphocytes: variants M7 and DR1. Biochemistry. 1982 Nov 23;21(24):6313–6321. doi: 10.1021/bi00267a042. [DOI] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Peterson P. A. Reactions and crossreactions of a rabbit anti-H2 antigen serum. Scand J Immunol. 1978 Apr;7(4):265–276. doi: 10.1111/j.1365-3083.1978.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey J., Bluestone J. A., Coligan J. E., Maloy W. L., Margulies D. H. Serologic and T cell recognition of truncated transplantation antigens encoded by in vitro deleted class I major histocompatibility genes. J Immunol. 1986 Feb 15;136(4):1472–1481. [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Boyer C., Verhulst A. M., Golstein P., Rajan T. V. Expression of H-2Db on the cell surface in the absence of detectable beta 2 microglobulin. J Exp Med. 1984 Jul 1;160(1):317–322. doi: 10.1084/jem.160.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Zeff R. A., Schmitt-Verhulst A. M., Rajan T. V. Molecular analysis of an EL4 cell line that expresses H-2Db but not H-2Kb or beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 May;82(9):2950–2954. doi: 10.1073/pnas.82.9.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F., Berissi H., Weissenbach J., Maroteaux L., Fellous M., Revel M. The beta2-microglobulin mRNA in human Daudi cells has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2(2):239–243. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada N., Kimura S., Hatzfeld A., Hämmerling U. Ly-m11: the H-3 region of mouse chromosome 2 controls a new surface alloantigen. Immunogenetics. 1980;11(5):441–449. doi: 10.1007/BF01567813. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Nathenson S. G. Intramolecular organization of Class I H-2 MHC antigens; localization of the alloantigenic determinants and the beta 2 m binding site to different regions of the H-2 Kb glycoprotein. J Immunol. 1983 Mar;130(3):1419–1425. [PubMed] [Google Scholar]