Abstract
Background
Lejeunea is a largely epiphytic, subcosmopolitan liverwort genus with a complex taxonomic history. Species circumscriptions and their relationships are subject to controversy; biogeographic history and diversification through time are largely unknown.
Methodology and Results
We employed sequences of two chloroplast regions (trnL-trnF, rbcL) and the nuclear ribosomal ITS region of 332 accessions to explore the phylogeny of the Harpalejeunea-Lejeunea-Microlejeunea complex. Lejeunea forms a well-supported clade that splits into two main lineages corresponding to L. subg. Lejeunea and L. subg. Crossotolejeunea. Neotropical accessions dominate early diverging lineages of both main clades of Lejeunea. This pattern suggests an origin in the Neotropics followed by several colonizations from the Neotropics into the Paleotropics and vice versa. Most Afro-Madagascan clades are related to Asian clades. Several temperate Lejeunea radiations were detected. Eighty two of the 91 investigated Lejeunea species could be identified to species level. Of these 82 species, 54 were represented by multiple accessions (25 para- or polyphyletic, 29 monophyletic). Twenty nine of the 36 investigated species of L. subg. Lejeunea were monoicous and 7 dioicous. Within L. subg. Crossotolejeunea, 15 of the 46 investigated species were monoicous and 31 dioicous. Some dioicous as well as some monoicous species have disjunct ranges.
Conclusions/Significance
We present the first global phylogeny of Lejeunea and the first example of a Neotropical origin of a Pantropical liverwort genus. Furthermore, we provide evidence for the Neotropics as a cradle of Lejeunea lineages and detect post-colonization radiations in Asia, Australasia, Afro-Madagascar and Europe. Dioicy/monoicy shifts are likely non-randomly distributed. The presented phylogeny points to the need of integrative taxonomical studies to clarify many Lejeunea binomials. Most importantly, it provides a framework for future studies on the diversification of this lineage in space and time, especially in the context of sexual systems in Lejeuneaceae.
Introduction
The taxonomic history of the leafy liverwort Lejeunea Lib. is best characterized as a story of controversial opinions on species delimitation and assumed relationships. Libert [1] described the genus based on only two species, Lejeunea calcarea Lib. [nowadays treated as Cololejeunea calcarea (Lib.) Schiffn.] and Lejeunea serpyllifolia Lib., the latter being a synonym of Lejeunea cavifolia (Ehrh.) Lindb. [2], [3]. Soon after Libert's publication, the genus became widely recognized and numerous new species were described [4]. Until the end of the 19th century, the number of Lejeunea species exceeded one thousand [5] but early authors applied a wider genus concept than is accepted today. A good example in this regard is the treatment of Spruce [6] who classified Lejeunea in 39 subgenera. The majority of these subgenera was elevated to genus rank by Schiffner [7]. Subsequently, further new genera were introduced consisting of former Lejeunea species (e.g., [8]–[10]). As a consequence, Lejeunea species sensu Spruce [6] were placed in some 60 different genera [11].
Recent taxonomic and/or molecular phylogenetic studies of Lejeuneaceae led to a considerable reduction of genera [12]–[18]. This trend becomes particularly apparent in Lejeunea since more than a dozen generic names were recently lowered to synonyms of this genus [15], [18], [19]. Lejeunea is currently circumscribed by long-inserted leaves, divided or undivided underleaves, leaf lobules with an unreduced first tooth and a marginal hyaline papilla, small, segmented or homogeneous oil bodies, lack of ocelli, lejeuneoid innovations, unwinged female bracts and inflated perianths with 0–5 smooth or toothed wings [17], [20]. Lejeunea is recognized for its morphological disparity. Diversification time estimates indicated an origin of Lejeunea in the early Cenozoic [21]–[23]. The genus has its centre of diversity in the humid tropics where the species usually grow as epiphytes on stems, branches, twigs and leaves of a large number of cormophytes but also on rock [24]. Although the vast number of species occur exclusively in tropical climates, the genus is also well represented in temperate regions with a humid climate [25], [26].
According to recent estimates the species diversity of Lejeunea may exceed three hundred [27], however, the precise number of species is still unclear due to the limited availability of modern revisionary studies [13], [28]–[30]. According to current knowledge, Lejeunea includes narrow endemics [29] as well as intercontinentally distributed species such as the subcosmopolitan Lejeunea flava (Sw.) Nees [31]. Intercontinental ranges have been accepted for many liverwort species due to an extensive morphological overlap of remote populations and the production of spores and propagules suitable for long-distance dispersal [32]–[34], although molecular phylogenetic studies incorporating multiple accessions of morphologically-typologically circumscribed liverwort species usually demonstrate a considerable genetic variation and a structure that is related to spatial ranges rather than to morphological disparities [35]–[40]. These studies also demonstrated the para- or polyphyly of many morphologically circumscribed liverwort species [36], [41], [42].
The objective of this study is to reconstruct the first comprehensive phylogeny of Lejeunea using chloroplast and nuclear DNA markers. This phylogenetic framework is used to reconstruct the origin of the genus and infer evidence, which supports dispersal between the Neotropics and the Paleotropics [40], respectively the hypothesis of a tropical origin of the extant temperate species diversity [22], [43]. In addition, we infer the evolution of reproductive systems with the focus on monoicy and dioicy in the evolution of Lejeunea. Finally, we test current morphological-typological species concepts by including multiple accessions and examine whether the recovered phylogenetic relationships correspond to/or conflict with morphologically circumscribed taxa.
Results
Phylogeny - Reduced dataset
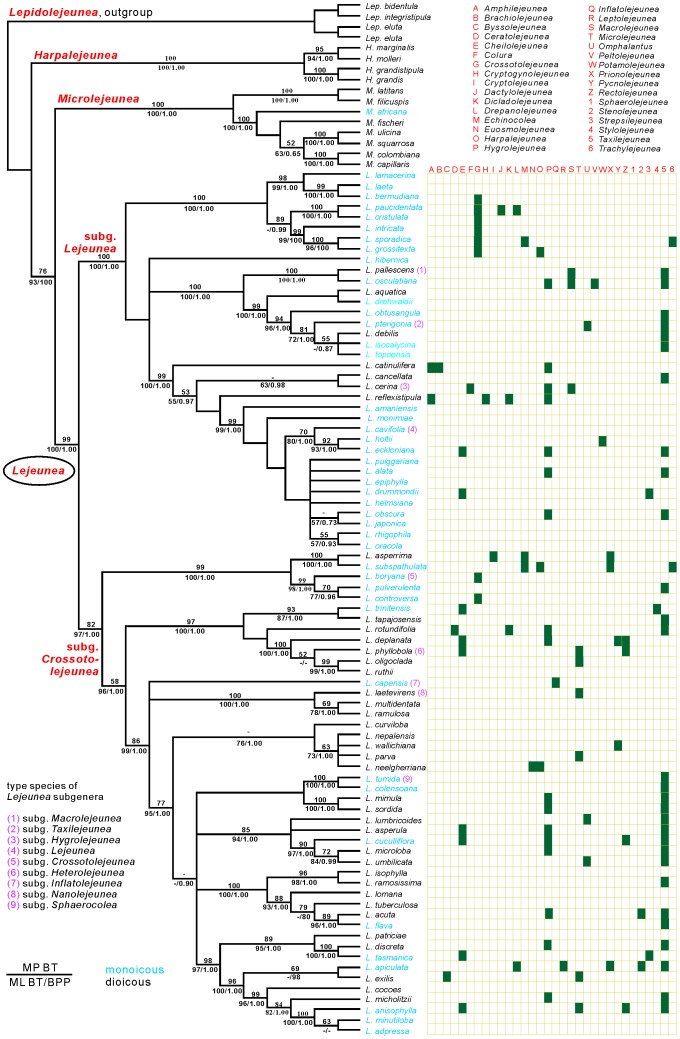
The reduced dataset comprised one accession per ingroup species (specimens identified only to genus level excluded). Of a total of 2,351 character sites, 725 were parsimony informative, 248 unique to a single accession and 1,378 constant. The maximum parsimony (MP) analysis resulted in 4,578 most parsimonious trees with the features: length of 4,016 steps, consistency index of 0.38, and retention index of 0.69 (Figure 1). Bayesian inference of phylogeny and maximum likelihood (ML) analyses recovered consensus trees respectively optimal trees that were highly similar in their topologies to each other as well as to the MP tree. The four representatives of Harpalejeunea (Spruce) Schiffn. formed a clade that was placed sister to a clade comprising two clades of which one included eight Microlejeunea Steph. species whereas the other one was composed by 82 Lejeunea species. The monophyly of Lejeunea achieves bootstrap percentage values (BPVs) of 99 or 100% and a Bayesian Posterior Probability (BPP) of 1.00 (Figure 1). The Lejeunea clade consisted of two main lineages corresponding to Lejeunea subg. Lejeunea (BPV MP 100%, ML 100%, BPP p = 1.00) and L. subg. Crossotolejeunea Spruce (BPV MP 82% ML 97%, BPP p = 1.00). In past classification, the investigated Lejeunea species were alternatively placed in 32 different genera, with up to 6 different treatments per species (Figure 1). Lejeunea species previously treated as Taxilejeunea (Spruce) Schiffn. were diffusely distributed and nested in most Lejeunea clades. Elements of Crossotolejeunea (Spruce) Schiffn. were found in both main lineages of Lejeunea. Twenty nine of the 36 investigated representatives of Lejeunea subg. Lejeunea were monoicous (81%) and 7 (19%) dioicous (Figure 1). Within Lejeunea subg. Crossotolejeunea, 15 of the 46 investigated species were monoicous (33%) and 31 dioicous (67%). Ancestral character reconstruction recovered dioicy as the likely ancestral state of Lepidolejeunea R.M.Schust., Harpalejeunea, and Microlejeunea, whereas the ancestral state of Lejeunea was found to be equivocal in maximum parsimony reconstructions. In maximum likelihood reconstructions, dioicy was found to be ancestral with a probability of 0.75 versus a probability of 0.25 for monoicy. The ancestral state of L. subg. Lejeunea was either resolved as equivocal (50% of most parsimonious trees) or monoicous (50% of most parsimonious trees). ML reconstructions recovered a probability of monoicy of 0.71. Similarly, the ancestral state of subg. Crossotolejeunea was found to be equivocal in all most parsimonious trees but showed a probability of 0.77 to be dioicous (Table 1).
Figure 1. Strict consensus of 4578 equally parsimonious trees derived from the reduced dataset.
MP and ML bootstrap percentage values and Bayesian Posterior Probabilities are indicated at branches. Monoicous species are given in blue, dioicous species in black. Type species of subgenera of Lejeunea are marked and alternative genus assignments of Lejeunea species shown.
Table 1. Ancestral character reconstruction of dioicous/monoicous reproductive systems.
| MP Dioicy | MP Monoicy | ML Dioicy | ML Monoicy | |
| Lepidolejeunea | yes | no | 0.93 | 0.07 |
| Harpalejeunea | yes | no | 0.98 | 0.02 |
| Microlejeunea | yes | no | 0.93 | 0.07 |
| Lejeunea | equivocal | equivocal | 0.75 | 0.75 |
| L. subg. Lejeunea | equivocal (50%), no (50%) | equivocal (50%), yes (50%) | 0.29 | 0.71 |
| L. subg. Crossotolejeunea | equivocal | equivocal | 0.77 | 0.23 |
| clade L. lamacerina-L. grossitexta | no | yes | 0.01 | 0.99 |
| clade L. hibernica-L. oracola | no | yes | 0.29 | 0.71 |
| clade L. pallescens- L. topoensis | no | yes | 0.24 | 0.76 |
| clade L. catinulifera-L. oracola | yes | no | 0.75 | 0.25 |
| clade L. amaniensis-L. oracola | no | yes | 0.10 | 0.90 |
| clade L. asperrima-L. controversa | equivocal | equivocal | 0.40 | 0.60 |
| clade L. trinitensis-L. adpressa | yes | no | 0.92 | 0.08 |
| clade L. trinitensis-L. ruthii | yes | no | 0.96 | 0.04 |
| clade L. capensis-L. adpressa | yes | no | 0.97 | 0.03 |
The reconstruction is based on the reduced dataset using Maximum Parsimony (MP) and Maximum Likelihood (ML).
Phylogeny - Large dataset
The large dataset consisted of 2,351 character sites (909 parsimony informative, 1,212 constant). The MP analysis resulted in more than 350,000 equally parsimonious trees with a length of 6,427 steps, a consistency index of 0.30 and a retention index of 0.83 (not depicted).
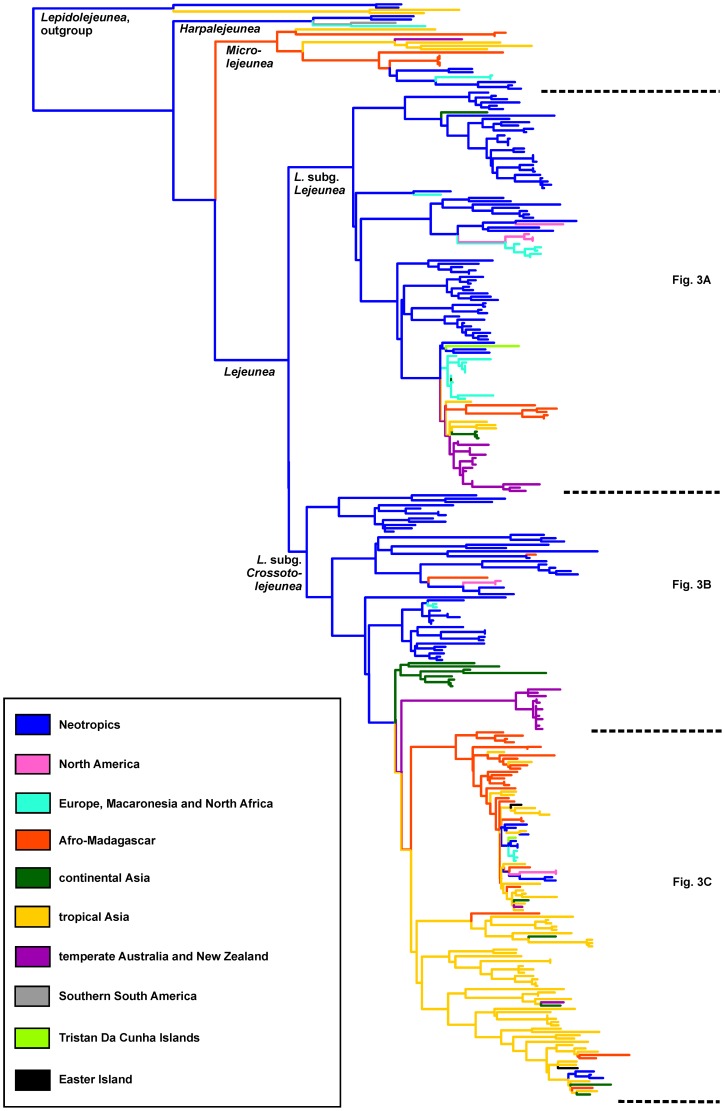
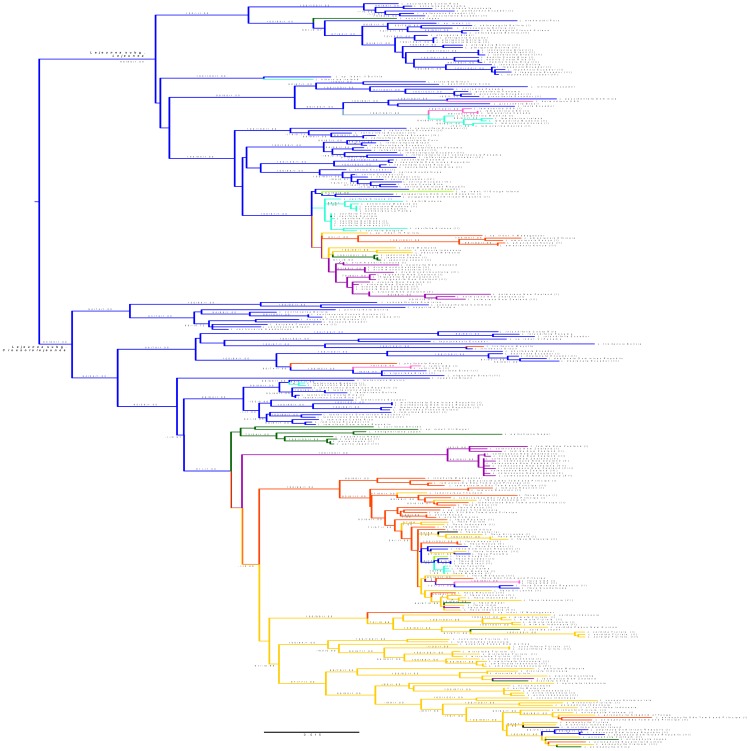
The ML phylogeny based on the large dataset is shown in Figure S1. A condensed version without species labeling is depicted in Figure 2. The Lejeunea clade was pruned and split in three parts, which are depicted in Figure 3, with BPPs and ML/MP BPVs indicated at branches. The phylogeny was consistent to the topology derived from the reduced dataset albeit without good ML BPV for Lejeunea subg. Crossotolejeunea (ML BPV = 65%). Out of the 82 Lejeunea species with reliable species identification, 54 were represented by multiple accessions. Twenty five of these 54 Lejeunea species were resolved as para- or polyphyletic, whereas 29 were monophyletic. Intercontinental ranges of several Lejeunea species were confirmed.
Figure 2. Condensed Maximum Likelihood phylogeny of the Harpalejeunea-Lejeunea-Microlejeunea clade.
Branch colors correspond to the most parsimonious reconstruction of ancestral areas of distribution and provide evidence for a Neotropical origin of Lejeunea.
Figure 3. Pruned Lejeunea clade from Figures 2/S1.
ML and MP bootstrap percentage values as well as Bayesian Posterior Probabilities are indicated at branches. Fifty four Lejeunea species are represented by multiple accessions, 29 of these are monophyletic, 25 para- or polyphyletic.
Biogeography
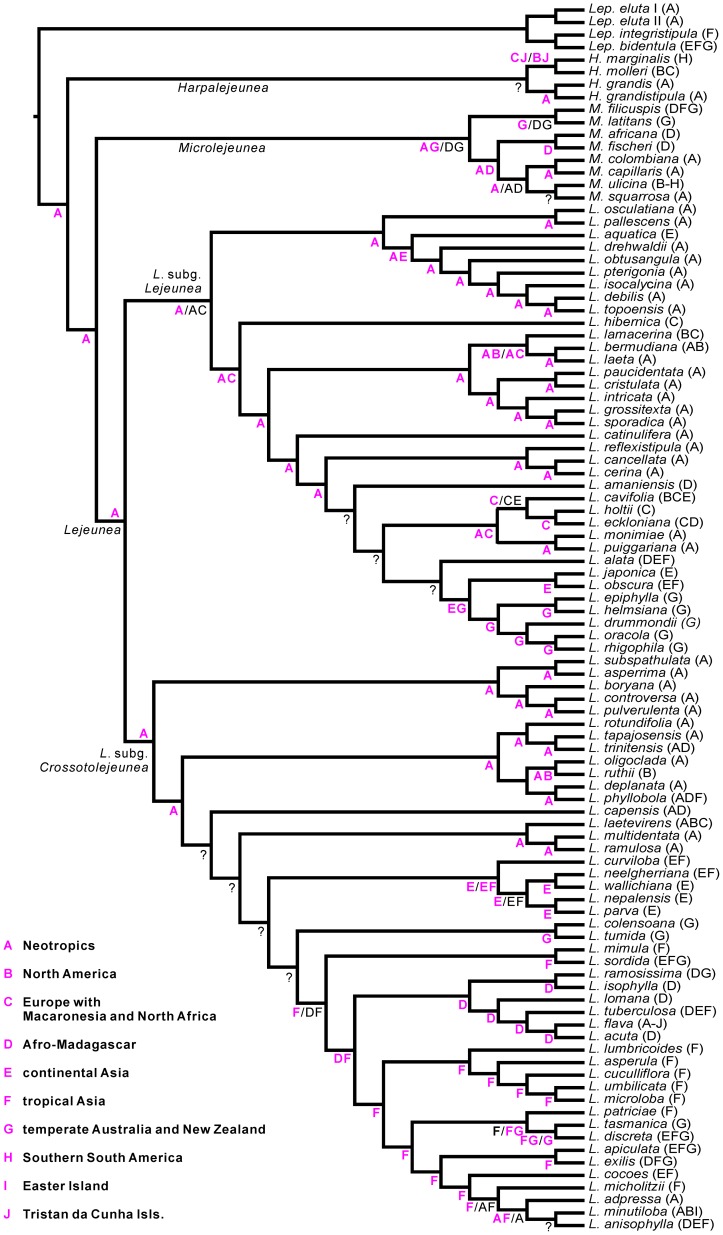
The most parsimonious reconstruction of ancestral areas of distribution based on the large dataset (Figures 2, 3 A–C) indicated a Neotropical origin of Lejeunea as well as of its subgenera Crossotolejeunea and Lejeunea. The S-Diva reconstruction generated from the reduced dataset suggested two scenarios. In scenario one both subgenera originated in the Neotropics, whereas in the other scenario two alternative solutions were found for L. subg. Crossotolejeunea (Figure 4). In the second scenario, L. subg. Crossotolejeunea originated in an area comprising the Neotropics but also Europe plus Macaronesia and North Africa. African and Asian accessions were found to be nested in derived lineages. Lejeunea subg. Crossotolejeunea comprised a species rich radiation in Afro-Madagascar, Africa, and Asia that likely originated from a single colonization of the Paleotropics from the Neotropics. Each four clades of Lejeunea were recovered with occurrences in Australasia or North America respectively, five clades with occurrences in Macaronesia and Atlantic Europe, and seven clades with occurrences in temperate/subtropical Asia (Figure 3). The subcosmopolitan L. flava complex nested in an African lineage. Accessions from Gough Island were resolved in Neotropical lineages; accessions from Easter Island in tropical Asian clades. The African-Neotropical L. trinitensis Lindenb. & Gottsche nested in a Neotropical clade; the Neotropical L. adpressa Nees in a clade dominated by Asian accessions. North American accessions of L. lamacerina (Steph.) Schiffn. are placed sister to European/Macaronesian accessions.
Figure 4. Ancestral areas of distribution reconstructed using S-DIVA.
The distribution of each species is given in brackets according to the ancestral areas of distribution scheme. Putative ancestral areas of distribution are shown at nodes, in case of alternative results the less likely solution is given in black. Question marks indicate ambiguities [more than two alternative proposals]. The reconstruction points to a Neotropical origin of Lejeunea.
Discussion
Supraspecific classification
Recent molecular phylogenetic studies identified a monophylum with representatives of Harpalejeunea, Microlejeunea and Lejeunea [17]. Furthermore, a recent report showed that the putatively allied genus Bromeliophila R.M.Schust. [20] forms a sister relationship with Prionolejeunea (Spruce) Schiffn. rather than nesting in Lejeunea [44]. Morphologically, Lejeunea differs from the former two genera by a lack of ocelli [the sole representative of Lejeunea with ocelli, L. huctumalcensis Lindenb. & Gottsche, belongs to another main lineage of Lejeuneaceae (Czumay et al., unpublished)]. The monophyly of Harpalejeunea, Microlejeunea and Lejeunea is confirmed in our study, with Microlejeunea placed sister to Lejeunea.
Lejeunea has been classified in some 50 subgenera of which 17 are still accepted as part of Lejeunea. These subgenera are usually defined by one or a few morphological character states, and their recognition and circumscription is subject to controversy. A good example is Lejeunea subg. Taxilejeunea which was alternatively treated as separate genus Taxilejeunea, and as such accepted by several recent authors [9], [12], [45], [46], although the morphology of both genera is largely overlapping [12]. This situation is reflected in our phylogeny, with Taxilejeunea elements in nearly all lineages of Lejeunea (Figure 1). The problematic circumscription of Lejeunea taxa is also reflected in the alternative placement of the 82 identified species of our study in 32 different genera of Lejeuneaceae (Figure 1), with one species in up to six genera (Lejeunea apiculata Sande Lac.). Lejeunea splits into two main clades with heterogeneous morphology. One includes the generitype L. serpyllifolia ( = L. cavifolia) and the types of three further subgenera; the other clade comprises types of four different subgenera, the oldest available subgenus name being L. subg. Crossotolejeunea Spruce (type: L. boryana Mont.) (Figure 1). Lejeunea subg. Crossotolejeunea was proposed for monoicous species with decurved and acuminate leaf apices, and 5-keeled perianths with denticulate and fimbriate keels [6]. A few years later, Crossotolejeunea was raised to generic rank [7]. However, Crossotolejeunea was synonymized with Lejeunea because the diagnostic character combinations were found to be inconsistent among species considered to belong to Crossotolejeunea [13]. The polyphyly of Crossotolejeunea as circumscribed by Spruce [6] is confirmed in the presented study by recovering Crossotolejeunea representatives in both main clades of Lejeunea (Figure 1). However, the presence of the type species L. boryana in the second main clade allows the assignment of L. subg. Crossotolejeunea. Incongruence of morphology-based classifications and molecular phylogenies was reported for a rapidly increasing number of genera of liverworts such as Athalamia Falc. [47], Cololejeunea (Spruce) Schiffn. [48], Diplasiolejeunea (Spruce) Schiffn. [40], Frullania Raddi [49], Plagiochila (Dumort.) Dumort. [50], Porella L. [51], Radula Dumort. [52], Scapania (Dumort.) Dumort. [53], Syzygiella Spruce [54], and Telaranea Schiffn. [55]. Together, these studies clarified the phylogeny of these liverworts and provided the foundation to introduce new classifications using holophyly as the main criterion [40], [52], [56]–[58]. Unfortunately, many of these newly circumscribed taxa lack obvious morphological diagnostic characters hampering assignments of species to these clades using solely morphology.
In this study we propose to assign the two main Lejeunea clades to Lejeunea subg. Crossotolejeunea and Lejeunea subg. Lejeunea but hesitate to establish further supraspecific entities. In our opinion, it is premature to introduce a comprehensive classification of the two subgenera into sections since our Lejeunea sampling is still rather incomplete in the context of taxonomic sampling. In addition, further studies are required to explore the morphological features of species recovered in well supported clades. A good example in this regard is the morphological treatment of L. pulverulenta (Gottsche ex Steph.) M.E.Reiner [46]. In this study, L. pulverulenta was assumed to be aligned with L. controversa Gottsche and L. cerina (Lehm. & Lindenb.) Gottsche et al. based on morphological similarities, e.g. the papillose leaf cells with trigones and intermediate cell wall thickenings. A sister relationship of L. pulverulenta and L. controversa (L. subg. Crossotolejeunea) is confirmed (Figure 1) but L. cerina is found to be nested in Lejeunea subg. Lejeunea instead of L. subg. Crossotolejeunea.
The morphology of many Lejeunea species has not yet been exhaustively studied and our knowledge is often restricted to descriptions of the gross morphology of the gametophyte. Schuster [9], [31] repeatedly pointed to the taxonomical value of character states visible only in living plants, namely the oil bodies, and the sporophytes. Only recently it was shown that the rough surface of Lejeunea species is not necessarily caused by papillae but can also result from the production of surface waxes [59]. We need comprehensive morphological datasets of gametophytes and sporophytes besides expansion of molecular datasets to establish a hierarchical classification of Lejeunea into subgenera and sections. These data will also demonstrate whether clades share certain morphologies or can only be defined by DNA sequence evidence.
Circumscription of species
The present study addressed the reliability of current morphological-typological species concepts in Lejeunea by sampling multiple accessions of several currently accepted species. In the absence of studies on speciation processes and the maintenance of species borders in Lejeunea, we consider three criteria - diagnostic morphology, biogeographic consistency, and reciprocal monophyly - as the most reliable procedure to identify putative species [60]. Congruence between the phylogenies derived from either the nuclear or the chloroplast markers is interpreted as evidence for reproductive isolation. Hence we regard incongruence of morphologically circumscribed taxa with molecular phylogenies as evidence for the limitations of our current morphology-based classification. However, integration of molecular and exhaustive morphological data allows often but not always for a reconsideration of morphological features considered to be of diagnostic importance and result in modified species circumscriptions (e.g., [61]–[64]). These short-term solutions are practical and helpful despite the amount of efforts required. In addition, they may allow to recognize the extent of the failure of current taxonomic practice.
Multiple accessions of 29 Lejeunea species formed monophyletic lineages but 25 species proved to be para- or polyphyletic (Figure 3 A–C). The ratio of nearly 50% rejection of currently accepted species is remarkable and requires further using of more comprehensive datasets and analyses. These datasets may expand not only the number of accessions studied per species but also explore the genetic diversity by employing markers that will allow a more comprehensive study of the genotypic distinction such as ISSRs, AFLPs, and SNPs. Exhaustive studies with such marker-systems hold special promises for lineages with a low clade diversity such as the Lejeunea cavifolia – L. eckloniana Lindenb. – L. holtii Spruce-complex. The high number of non-monophyletic Lejeunea-species indicates that our current morphology-based classification does not adequately consider the possible presence of morphologically cryptic or semicryptic entities, and local endemism [38], [62], [65]. Some studies reported evidence for rather limited morphological variation among Lejeunea species and thus morphologically similar plants may be placed in different main clades of Lejeunea. A good example is the Lejeunea tumida Mitt. complex whose representatives are placed in both main clades of Lejeunea although they were earlier treated as a single species [30], [42]. This observation is consistent with the results available for other genera of Lejeuneaceae, namely Marchesinia Gray [36], Ptychanthus Nees [66], Mastigolejeunea (Spruce) Schiffn. and Thysananthus Lindenb. [67]. All these studies suggest that we currently underestimate Lejeuneaceae species diversity. Examples supporting this notion are reported here with Lejeunea flava and L. laetevirens Nees & Mont., which may in fact represent complexes including several independent entities. Lejeunea flava has been studied exhaustively using morphological evidence and several subspecies or segregates have been proposed [10], [31], [68], [69]. However, we were not able to adopt these taxonomical concepts for our phylogeny (Figure 3 C) although we could recognize some morphological tendencies and found the morphologically well separated species L. acuta Mitt. and L. tuberculosa Steph. nested in the L. flava clade. The L. laetevirens complex is similarly problematic since our phylogeny indicates that several still unrecognized entities hide in L. laetevirens s.l.: A robust clade with Neotropical and Macaronesian accessions of L. laetevirens is placed sister to a Neotropical clade with L. laetevirens morphotypes as well as multiple accessions of L. multidentata M.E.Reiner & Mustelier and L. ramulosa (Herzog) R.M.Schust. The latter two species differ from L. laetevirens by dentate or acute leaves. Lejeunea multidentata was aligned with L. boryana Mont. and L. controversa rather than with L. laetevirens based on shared dull appearance caused by strongly papillose cells [70], [71], however, according to our phylogenies these species are not closely related. An extension of the sampling is necessary to revise the taxonomy of the L. laetevirens clade. The same holds true for the polyphyletic L. anisophylla Nees & Mont. and several other problematic binomials.
Dispersal biogeography
Liverworts produce spores and small propagules that are capable for distribution through air currents over larger distances [72], [73]. However, population studies of liverworts generally show a spatial distribution of genetic diversity that does not correspond to a general panmixis hypothesis [74], [75]. Thus, the current distribution of liverworts is not random and biogeographic studies frequently recover conserved biogeographic patterns that can be interpreted by considering the combination of processes such as occasional long distance dispersal, frequent dispersal over short distances, local extinction, and local diversification [76], [77]. The reported distribution of Lejeunea suggests that this genus is not an exception and that conserved spatial patterns exist. Although the limited availability of lejeuneoid fossils prevents us from a detailed reconstruction of divergence times (the two Miocene fossils Lejeunea sp. [78] and Lejeunea palaeomexicana Grolle [79] cannot be assigned to any of our Lejeunea clades) an early Cenozoic origin of the genus can be assumed based on the existing estimates [21]–[23]. This time frame provides information about the position of the continents which is important in distinguishing between establishment via long-distance dispersal versus vicariance as the preferred explanation for the observed disjunct ranges. Dispersal over larger distances seems to occur only infrequently in Lejeunea, as is indicated by the clear geographical structure of disjunct species as well as multi-species clades. A good example is the L. lamacerina clade that splits into a North American and a European/Macaronesian lineage, without any evidence of recent geneflow. The unsatisfactory taxonomy of many other investigated clades hampers similar statements, however, the long branches in many morphologically circumscribed species and their para- or polyphyly provide evidence for local diversification/speciation. Evidence for lacking or restricted geneflow between distant liverwort populations has been demonstrated several times [74], [80] and can also be concluded for Lejeunea. Local diversification subsequent to successful long-distance dispersal seems to dominate the evolutionary history of Lejeunea. Accordingly, the majority of the investigated Lejeunea species has regional distribution ranges but about 23% of the identified species are more widespread and occur in at least two of our ten putative areas of endemism. Examples include the Neotropical-Macaronesian range of L. laetevirens, the Neotropical-Asian range of L. trinitensis Lindenb. & Gottsche (Figure 3 B) and the African-Asian range of L. anisophylla (Figure 3 C).
Neotropical origin
The early diverging lineages of both main clades of Lejeunea occur predominantly in the Neotropics. Thus, our reconstructions revealed a Neotropical origin of Lejeunea with subsequent dispersal into other tropical as well as temperate regions. A Neotropical origin has been shown for several lineages of angiosperms, namely Burmanniaceae [81], Burseraceae [82], Gentianaceae [83] and Malpighiaceae [84]. It has also been discussed for the grammitid clades of polygrammoid ferns [85], [86] and the Neotropical-African liverwort Bryopteris (Nees) Lindenb. [87] but has not yet been proposed for any subcosmopolitan liverwort genus based on molecular data. This is partly caused by the limited access to comprehensive phylogenies of species-rich liverwort genera [40], [49], [51]–[54], [76], [77], [88]. The lejeuneoid genus Diplasiolejeunea shows a somewhat different pattern with a deep split into a Paleotropical and a Neotropical clade [40], but a few Pantropical species soften this otherwise strict separation by indicating occasional intercontinental dispersal events. In contrast to the pattern in Diplasiolejeunea both main clades of Lejeunea show a more even representation of putative regions of endemism, indicating that long distance dispersal is more frequent in Lejeunea than in Diplasiolejeunea as long as we assume similar ages for both genera.
Our topologies point to several dispersal events from the Neotropics into Africa (L. trinitensis, L. phyllobola Nees & Mont.). This pattern is not uncommon in leafy liverworts and has been recovered for Herbertus juniperoideus (Sw.) Grolle [77], Marchesinia brachiata (Sw.) Schiffn. [36], Plagiochila boryana Steph. [56] and the genus Bryopteris [87]. The subcosmopolitan L. flava complex appears to have originated in Africa and subsequently colonized large parts of the tropics and adjacent regions, with several dispersal events between the Old and the New World. This pattern of older spatial separations followed by young inter-continental dispersals was reported for a few plants such as the fern genus Nephrolepis Schott [89] and the pantropical liverwort Plagiochila sect. Vagae Lindenb. [56]. Our phylogenies support close relationships of African and Asian Lejeunea floras, however, the Neotropical L. adpressa is of Paleotropical, most likely Asian, origin (Figure 3 C). Lejeunea-accessions from the Polynesian Easter Island are likewise related to Asian clades whereas the Lejeunea accessions from Gough Island (Southern Atlantic Ocean) are nested in Neotropical lineages. A South American origin of Gough Island liverworts has already been demonstrated for the genus Herbertus [90]. The Macaronesian accessions of L. laetevirens are nested in a Neotropical clade, indicating dispersal from the Neotropics into Macaronesia. This pattern seems to be common in leafy liverworts and has also been reconstructed for species of Plagiochila [91] and Leptoscyphus Mitt. [92].
The tropics as a cradle and museum
Lejeunea has its centre of diversity in the humid lowlands and lower montane sites of the tropics; its diversity in temperate regions is considerably lower. This pattern is consistent with the widely recognized latitudinal biodiversity gradient [43], [93]–[96]. Various hypotheses have been introduced to explain the origin of this gradient (see [43] for review) of which some involve the rather controversial concept of niche conservatism. So far, very little attention has been given to latitudinal biodiversity gradients in seed-free land plants, but is starting to be explored in ferns (see [97]) and here in the liverwort genus Lejeunea. In accordance with the general hypothesis of a latitudinal diversity gradient, Lejeunea includes only a few temperate lineages, which are in each case nested in tropical clades.
The pattern observed for Lejeunea appears to be consistent with the role of the tropics as a cradle and museum of diversity [98]–[100], and mirrors observations for the whole family Lejeuneaceae [22]. Liverwort families with a centre of diversity in the tropical highlands can show considerably different patterns and may have entered the tropics from temperate regions [76]. Interestingly, temperate species were not always found to possess a tropical sister species but evidence for several radiations in temperate regions were discovered, including two multi-species clades with occurrences in Australasia, one with occurrences in temperate Asia, and two with occurrences in Macaronesia and Atlantic Europe (Figs 3 A–C). The discovery of these clades provides opportunities to test some of the arguments concerning the origin of the latitudinal diversity gradient such as niche conservatism and different speciation rates [97], [101]. The recovery of radiations in the temperate climate zones of Australasia resembles the recent report of a New Zealand radiation of grammitid ferns [102]. Grammitid ferns share with Lejeunea their origin in tropical regions and their preference to climates with high humidity. These examples may indicate the possibility of high speciation rates in temperate climates caused by ecological opportunities. The observed change in the climatic niche preferences is again consistent with reports in tree ferns growing in the wet temperate climates of Australasia [103].
Sexual systems in a largely epiphytic genus
About two third of liverworts are dioicous [104] whereupon the distribution of dioicous and monoicous species differs from genus to genus. The speciose genus Plagiochila is a prime example of a completely dioicous group whereas monoicous species dominate in Cololejeunea (Spruce) Schiffn., Riccia L. and Riccardia Gray [24], [105]. The evolution of sexual systems has so far been studied for only two genera of liverworts using a phylogenetic framework: the largely epiphytic leafy liverworts Radula Dumort. and Diplasiolejeunea [40], [106]. Only 16 of the ca. 200 Radula species are monoicous whereas monoicy and dioicy is more evenly distributed in Diplasiolejeunea. Single monoicous species of Radula were resolved in several otherwise dioicous clades, a similar supposedly random pattern was observed in Diplasiolejeunea. Monoicy in Radula was also not correlated with obligate epiphytism but occurred in facultative epiphytic lineages [106].
In Lejeunea we observed an uneven distribution of sexual systems (Figure 1). Lejeunea subg. Lejeunea is dominated by monoicous species whereas dioicous species dominate in L. subg. Crossotolejeunea. Similarly to the situation in Radula, some monoicous species clustered in clades dominated by dioicous species, in particular in L. subg. Crossotolejeunea. However, monoicous species are the most frequent in L. subg. Lejeunea and our character reconstruction (Table 1) recovered some indications for the transition from dioicy to monoicy in the early diversification of the genus. We also found evidence for a rather frequent change of the reproductive system during the history of the genus with a minimum number of character state changes: five times in L. subg. Lejeunea and nine times in L. subg. Crossotolejeunea.
Monoicous species are potentially capable to produce sporophytes through self-fertilization. On one hand this may allow a more frequent establishment of new populations via long distance dispersal, but on the other hand this may result in invariable genotypes, accumulation of genetic load, and limited adaptation to new environments [105]. However, dioicy is not necessarily a barrier to regular sporophyte development. Many Plagiochila species frequently produce sporophytes as do at least some dioicous species of Frullania and Porella [50], [105]. Thus, future studies need to explore the accumulation of genetic load, effective population size, and the temporal stability of habitats as factors that shape the evolution of reproductive systems in Lejeunea.
According to existing data, both dioicous and monoicous Lejeunea species are able to form disjunct ranges. However, disjunctions over large distances might not necessarily be the result of spore dispersal but could also be caused by vegetative reproduction through propagules. Vegetative reproduction plays an important role in the range formation of liverworts and enhances the chances of establishing in a new environment, especially for dioicous species. A dioicous long-distance disperser is trapped in a very small area unless it is able to colonize its new environment through vegetative distribution. Accordingly the likelihood of the arrival of spores of the other sex clearly increases with range expansion through vegetative distribution. However, Lejeunea includes only few species that frequently produce propagules [107], despite wide species distribution ranges. A further aspect may be variation in the extinction risks caused by the different sexual systems but very little evidence exists to evaluate this factor.
Schuster [31] emphasizes the importance of monoicy for species colonizing unstable epiphytic habitats but many Lejeunea species are dioicous. This trend is even more evident in the sister genus Microlejeunea which is nearly completely dioicous [24], despite its general preference for epiphytic habitats. The same applies to Radula. Devos et al. [106] speculate that dioicous epiphytes often distribute vegetatively, not only through specialised propagules but also through unspecialized gametophyte fragments, and that they are often not strictly depending on epiphytic environments. Kraichak [108] reinforces this argument by demonstrating a correlation of reproduction through asexual propagules and an epiphyllous mode of life in Lejeuneaceae.
Currently the importance of monoicy for an epiphytic mode of life and long distance dispersal is rather unclear since the available studies point to more complex interrelationships. Future studies should not only focus on an extension of the phylogenetic sampling and improvements of the underlying taxonomy but also on the ecological ranges of disjunct liverworts. Intercontinentally distributed Diplasiolejeunea species have broader ecological amplitudes compared to geographically more restricted species [40], allowing for the colonization of a larger number of environments and enhancing the chance of a permanent establishment. We also need comprehensive studies on the resistance of spores and vegetative propagules of liverworts against drought and frost and the ability of sporophyte production under suboptimal climatic conditions.
Perspectives
Lejeunea is a prime example to illustrate the current state of affairs in liverwort classification. After three centuries of morphology-based research a plethora of taxa have been proposed in this genus, of which only a small part has been included in modern revisions, reflecting the limited number of liverwort specialists dealing with these taxonomically difficult plants. Our molecular data add to growing evidence that not all biologically relevant entities can be detected using solely morphology, and that the acceptance of a considerable intraspecific morphological variation may lead to an underestimation of the actual number of biological species [109], [110]. Thus, concepts considering cryptic and semi-cryptic species may provide more realistic estimates than the current practice. Based on our topology it is possible to identify species complexes that are not yet properly understood and that need to be studied using extended datasets. We urgently need molecular studies incorporating numerous accessions of morphologically circumscribed species from throughout their range. Only combined molecular-morphological studies will allow to understand range formation and to establish more natural species circumscriptions [111]. These studies will also facilitate estimates of the real number of biological species of liverworts. It is not unlikely that a portion of these species will not exhibit morphological disparities or can at best been identified using statistical methods and larger series of reference specimens [112]. In such a situation, reference sequences ( = DNA barcodes) are the most promising approach to obtain reliable identifications of these plants [113]. However, the establishment of the DNA barcodes needs to go hand-in-hand with critical taxonomic revision of species-rich genera like Lejeunea. The reported phylogeny provides the framework enabling the design and management of these studies because the major task of taxonomic revisions can be separated in groups of species belonging to the same clade.
Materials and Methods
Taxon sampling and outgroup selection
Taxa studied, including GenBank accession numbers and voucher details, are listed in Table S1. Ingroup taxa were selected according to availability and to represent the morphological variation and geographical distribution of Lejeunea. Representatives of the sister genera Harpalejeunea and Microlejeunea [17] were included to test the Lejeunea genus concept. Multiple accessions of several species were used to explore intraspecific genetic variation. Representatives of Lepidolejeunea were selected as outgroup species based on the analyses of [14] and [17]. Altogether 332 accessions from the herbaria AK, DUKE, EGR, GOET, JE, L, or NSW were used for this study.
DNA extraction, PCR amplification and sequencing
Upper parts of a few gametophytes were isolated from herbarium specimens. Total genomic DNA was extracted using Invisorb Spin Plant Mini Kit (Invitek, Berlin, Germany) prior to amplification. Protocols for PCR were carried out as described in previous publications: rbcL gene and trnL-F region from [114], and nrITS1-5.8S-ITS-2 region from [87]. Bidirectional sequences were generated using a MegaBACE 1000 automated sequencing machine using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequencing primers were those used for PCR. Newly generated sequences were assembled and edited using SeqAssem [115]. Seven hundred and nineteen sequences were newly generated for this study; 175 sequences were downloaded from Genbank.
Phylogenetic analyses
All sequences were aligned manually in Bioedit version 7.0.5.2 [116] resulting in a rbcL alignment with 895 positions, trnL-F 441 and an nrITS alignment with 1,015 putatively homologous sites. Ambiguous positions were excluded from all alignments and lacking data were coded as missing. Two datasets were compiled and analysed separately: dataset 1 ( = large dataset) included all studied accessions, whereas dataset 2 ( = reduced dataset) included only one accession per identified ingroup species. Accessions identified only to genus level were excluded from dataset 2. Phylogenetic trees based on the reduced dataset were used to visualize the current supraspecific classification of Lejeunea and to explore the evolution of monoicy/dioicy.
Maximum parsimony (MP) analyses were carried out with PAUP* version 4.0b10 [117]. MP heuristic searches of the comprehensive and the reduced datasets were conducted with the following options implemented: heuristic search mode, 1,000 random-addition-sequence replicates, tree bisection-reconnection (TBR) branch swapping, MULTrees option on, and collapse zero-length branches off. All characters were treated as equally weighted and unordered. Non-parametric bootstrapping values [118] were generated as heuristic searches with 1,000 replicates, each with ten random-addition replicates. The number of rearrangements was restricted to ten millions per replicate. Bootstrap percentage values (BPV)≥70 were regarded as good support [119]. Where more than one most parsimonious tree was found, trees were summarized as strict consensus tree(s). The three genomic regions and the combined chloroplast DNA dataset vs nrITS dataset were first analysed separately to check for topological incongruence. The consensus trees of the non-parametric bootstrap analyses were compared by eye to identify conflicting nodes supported by at least 70% [120]. The trees gave no evidence of incongruence. Accordingly, the datasets were combined.
The program jModeltest 0.1.1 [121] was used to select a best-fit model of sequence evolution for the maximum likelihood (ML) analyses of the each genomic region, using the Akaike information criterion. The following models were chosen for the respective data divisions: (rbcL) TPM1uf+I+G; (trnL-F) TVM+G and (nrITS) TIM3+G. A partitioned ML bootstrap analysis was conducted using the program Garli 2.0 [122]. The analysis was run until five million generations were completed without significant improvement (ln L increase of 0.01) to the topology. Node support was evaluated through 200 bootstrap replicates in which each repetition terminated after 100,000 generations were completed without topological improvements.
Bayesian inference was implemented in the program MrBayes 3.2 [123] allowing different models for each partition. Bayesian searches were carried out with four simultaneous Markov chains, ten million generations, and sampling every 1000th generation. The first 25% of trees were discarded as burn-in. Bayesian posterior probability (BPP) confidence values were generated from trees saved after this initial burn-in. Values were regarded as significant when BPP≥0.95 [124].
Ancestral areas of distribution
Data on distribution ranges of the investigated taxa were obtained from the literature. Given the wide distribution ranges of some species, the putative distribution range of endemism was coded as covering ten possible areas: Neotropics, North America, Southern South America, Europe with North Atlantic Islands (e.g. Macaronesia) and North Africa (Africa north of the Sahara), Afro-Madagascar (sub-Saharan Africa, Madagascar, Mascarenes, Seychelles, and São Tomé), continental Asia (comprising temperate and subtropical regions), tropical Asia (including Melanesia and tropical Australia), temperate Australia and New Zealand, Tristan da Cunha Islands and Easter Island. Ancestral areas of distribution were reconstructed using two different approaches. The first approach was based on the large dataset and considers the presence of several unidentified species with unclear distribution ranges. To overcome this problem, the putative region of endemism ( = the ten regions mentioned above, see also Fig. 2) of every accession was coded rather than the species range. Subsequently we reconstructed ancestral areas of distribution using MP criteria as implemented in Mesquite ver. 2.75 [125] based on the ML topology.
In the second approach we used dataset 2 including each one accession per identified species and a coding of the complete species range. Ancestral areas of distribution were reconstructed using S-DIVA [126] as implemented in RASP 2.0 based on 7,500 Bayesian trees from the reduced dataset.
Evolution of reproductive systems
The occurrence of dioicous/monoicous reproductive systems was scored by evaluating the information provided in the literature for each species included in dataset 2 [13], [28], [31], [45], [68], [127]–[137]. In case both character states were indicated (L. hibernica Grolle, [131]), the species was scored as monoicous. These efforts resulted into a matrix of two character states without any polymorphic or unknown character states. To explore the evolution of this character, we used the results of the MP analyses of the reduced dataset. Maximum parsimony character reconstructions were carried out using Mesquite 2.75. The character states were plotted over all most parsimonious trees recovered in the MP analysis of the reduced dataset. Nodes absent from some of these trees were ignored. In addition, we carried out maximum likelihood analyses using the MK model [138] and the strict consensus tree obtained from the most parsimonious tree set.
Supporting Information
Maximum Likelihood phylogeny of the Harpalejeunea-Lejeunea-Microlejeunea clade. ML and MP bootstrap percentage values as well as Bayesian Posterior Probabilities are indicated at branches. Branch colors correspond to the most parsimonious reconstruction of ancestral areas of distribution (see Figure 2).
(TIF)
Taxa used in the present study. Information about the origin of the studied material, vouchers, as well as GenBank accession numbers is included. New sequences in bold face.
(DOC)
Acknowledgments
We thank the curators and directors of the herbaria cited in the text for the loan of specimens and permission for destructive sampling.
Funding Statement
This study was supported by the Deutsche Forschungsgemeinschaft [HE 3584/2 and 4]. SD got support from the China Scholarship Council (scholarship number 2010697001). This is publication number 120 from the Courant Research Centre Geobiology that is funded by the German Excellence Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Libert M (1820) Sur un genre nouveau d'Hépatiques, Lejeunia . Ann Gen Sci Phys 6: 372–374. [Google Scholar]
- 2. Bonner CEB, Miller HA (1960) Studies in Lejeuneaceae, I. The typification of Lejeunea . Bryologist 63: 217–225. [Google Scholar]
- 3. Grolle R (1971) Miscellanea hepaticologica 111–120. Trans Brit Bryol Soc 6: 258–265. [Google Scholar]
- 4.Gottsche CM, Lindenberg JBG, Nees ab Esenbeck CG (1844–1847) Synopsis Hepaticarum. Hamburg: Meissner. 834 p. [Google Scholar]
- 5. Reiner-Drehwald ME (1999) Catalogue of the genus Lejeunea Lib. (Hepaticae) of Latin America. Bryophyt Biblioth 54: 1–101. [Google Scholar]
- 6. Spruce RM (1884) Hepaticae Amazonicae et Andinae. Tribus I: Jubuleae. Trans Proc Bot Soc Edinburgh 15: 1–308. [Google Scholar]
- 7.Schiffner V (1893) Hepaticae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien 1. Engelmann: Leipzig. pp. 97–141.
- 8. Evans AW (1904) Hepaticae of Puerto Rico IV. Odontolejeunea, Cyclolejeunea and Prionolejeunea . Bull Torr Bot Club 31: 183–226. [Google Scholar]
- 9. Schuster RM (1994) Studies on Lejeuneaceae, I. Preliminary studies on new genera and subgenera of Lejeuneaceae. J Hattori Bot Lab 75: 211–235. [Google Scholar]
- 10. Reiner-Drehwald ME (2000) On Potamolejeunea and Neopotamolejeunea gen. nov. (Lejeuneaceae, Hepaticae). Nova Hedwigia 71: 447–464. [Google Scholar]
- 11. Gradstein SR, Reiner-Drehwald ME, Jost L (2004) The systematic position and distribution of Myriocolea irrorata (Lejeuneaceae), an endangered liverwort of the Ecuadorian Andes. J Hattori Bot Lab 95: 235–248. [Google Scholar]
- 12. Reiner-Drehwald ME (2005) On Lejeunea rotundifolia and Dicladolejeunea (Lejeuneaceae, Jungermanniopsida). Syst Bot 30: 687–692. [Google Scholar]
- 13. Reiner-Drehwald ME, Goda A (2000) Revision of the genus Crossotolejeunea (Lejeuneaceae, Hepaticae). J Hattori Bot Lab 89: 1–54. [Google Scholar]
- 14. Wilson R, Gradstein SR, Schneider H, Heinrichs J (2007) Unravelling the phylogeny of Lejeuneaceae (Jungermanniopsida): evidence for four main lineages. Molec Phylogen Evol 43: 270–282. [DOI] [PubMed] [Google Scholar]
- 15. Heinrichs J, Dong S, Feldberg K, Schäfer-Verwimp A, Schmidt AR (2012) Sphaerolejeunea (Lejeuneaceae, Porellales) is a synonym of Lejeunea . Phytotaxa 69: 7–15. [Google Scholar]
- 16. Heinrichs J, Dong S, Yu Y, Schäfer-Verwimp A, Pócs T, et al. (2012) A 150 year old mystery solved: Transfer of the rheophytic liverwort Myriocolea irrorata to Colura . Phytotaxa 66: 55–64. [Google Scholar]
- 17. Dong S, Schäfer-Verwimp A, Pócs T, Feldberg K, Czumaj A, et al. (2013) Size doesn't matter – recircumscription of Microlejeunea (Lejeuneaceae, Porellales) based on molecular and morphological evidence. Phytotaxa 85: 41–55. [Google Scholar]
- 18. Ye W, Wei YM, Schäfer-Verwimp A, Zhu RL (2013) Phylogenetic position of Oryzolejeunea (Lejeuneaceae, Marchantiophyta): Evidence from molecular markers and morphology. J Syst Evol 51: 468–475. [Google Scholar]
- 19. Pócs T (2011) What is Cladolejeunea Zwickel? New or little known epiphyllous liverworts, XV. Acta Biol Plant Agr 1: 53–62. [Google Scholar]
- 20. Gradstein SR (2013) A classification of Lejeuneaceae based on molecular and morphological evidence. Phytotaxa 100: 6–20. [Google Scholar]
- 21. Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H (2007) Evolution of leafy liverworts (Jungermanniidae, Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon 56: 31–44. [Google Scholar]
- 22. Wilson R, Heinrichs J, Hentschel J, Gradstein SR, Schneider H (2007) Steady diversification of derived liverworts under Tertiary climatic fluctuations. Biol Lett 3: 566–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper ED, Henwood MJ, Brown EA (2012) Are the liverworts really that old? Cretaceous origins and Cenozoic diversifications in Lepidoziaceae reflect a recurrent theme in liverwort evolution. Biol J Linn Soc 107: 425–441. [Google Scholar]
- 24. Gradstein SR, Churchill SP, Salazar-Allen N (2001) Guide to the bryophytes of tropical America. Mem New York Bot Gard 86: 1–577. [Google Scholar]
- 25. Glenny D (1998) A revised checklist of New Zealand liverworts and hornworts. Tuhinga 10: 119–149. [Google Scholar]
- 26. Grolle R, Long DG (2000) An annotated check-list of the Hepaticae and Anthocerotae of Europe and Macaronesia. J Bryol 22: 103–140. [Google Scholar]
- 27. Lee GE, Gradstein SR (2013) Distribution and habitat of the Malaysian species of Lejeunea (Marchantiophyta: Lejeuneaceae), with description of Lejeunea tamaspocsii sp. nov. Pol Bot J 58: 59–69. [Google Scholar]
- 28. Ilkiu-Borges AL (2005) A taxonomic revision of Echinocolea (Lejeuneaceae, Hepaticae). Nova Hedwigia 80: 45–71. [Google Scholar]
- 29. Renner MAM, Brown EA, Wardle GM (2009) Lejeunea pocsii R.M. Schust. is a heterotypic synonym of L. helmsiana (Steph.) Steph. (Lejeuneaceae, Marchantiophyta). Nova Hedwigia 89: 335–348. [Google Scholar]
- 30. Renner MAM, Brown EA, Wardle GM (2010) The Lejeunea tumida species group (Lejeuneaceae, Jungermanniopsida) in New Zealand. Austral Syst Bot 23: 443–462. [Google Scholar]
- 31.Schuster RM (1980) The Hepaticae and Anthocerotae of North America. Volume 4. New York: Columbia University Press. 1344 p. [Google Scholar]
- 32.Herzog T (1926) Geographie der Moose. Jena: Fischer. 439 p. [Google Scholar]
- 33.Grolle R (1969) Großdisjunktionen in Artarealen lateinamerikanischer Lebermoose. In: Fittkau EJ, editor. Biogeography and Ecology in South America. The Hague: Junk. pp. 562–582. [Google Scholar]
- 34. Gradstein SR, Pócs T, Váňa J (1983) Disjunct Hepaticae in tropical America and Africa. Acta Bot Hung 29: 127–171. [Google Scholar]
- 35. Fuselier L, Davison PG, Clements M, Shaw B, Devos N, et al. (2009) Phylogeographic analyses reveal distinct lineages of Metzgeria furcata and M. conjugata (Metzgeriaceae) in Europe and North America. Biol J Linn Soc 98: 745–756. [Google Scholar]
- 36. Heinrichs J, Klugmann F, Hentschel J, Schneider H (2009) DNA taxonomy, cryptic speciation and diversification of the Neotropical-African liverwort, Marchesinia brachiata (Lejeuneaceae, Porellales). Molec Phylogen Evol 53: 113–121. [DOI] [PubMed] [Google Scholar]
- 37. Heinrichs J, Kreier H-P, Feldberg K, Schmidt AR, Zhu RL, et al. (2011) Formalizing morphologically cryptic biological entities: New insights from DNA taxonomy, hybridization, and biogeography in the leafy liverwort Porella platyphylla (Jungermanniopsida, Porellales). Amer J Bot 98: 1252–1262. [DOI] [PubMed] [Google Scholar]
- 38. Ramaiya M, Johnston MG, Shaw B, Heinrichs J, Hentschel J, et al. (2010) Morphologically cryptic biological species within the liverwort, Frullania asagrayana . Amer J Bot 97: 1707–1718. [DOI] [PubMed] [Google Scholar]
- 39. Laenen B, Désamoré A, Devos N, Shaw AJ, Gonzáles-Mancebo JM, et al. (2011) Macaronesia: a source of hidden genetic diversity for post-glacial recolonization of western Europe in the leafy liverwort Radula lindenbergiana . J Biogeogr 38: 631–639. [Google Scholar]
- 40. Dong SS, Schäfer-Verwimp A, Meinecke P, Feldberg K, Bombosch A, et al. (2012) Tramps, narrow endemics and morphologically cryptic species in the epiphyllous liverwort Diplasiolejeunea . Molec Phylogen Evol 65: 582–594. [DOI] [PubMed] [Google Scholar]
- 41. Feldberg K, Groth H, Wilson R, Schäfer-Verwimp A, Heinrichs J (2004) Cryptic speciation in Herbertus (Herbertaceae, Jungermanniopsida). Range and morphology of Herbertus sendtneri inferred from nrITS sequences. Pl Syst Evol 249: 247–261. [Google Scholar]
- 42. Renner MAM, Brown EA, Wardle GM (2011) The Lejeunea tumida species group is positively polyphyletic (Lejeuneaceae: Jungermanniopsida). Austral Syst Bot 24: 10–18. [Google Scholar]
- 43. Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, et al. (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Letters 10: 315–331. [DOI] [PubMed] [Google Scholar]
- 44. Heinrichs J, Czumaj A, Dong S, Scheben A, Schäfer-Verwimp A, et al. (2013) The Bromeliaceae tank dweller Bromeliophila (Lejeuneaceae, Porellales) is a member of the Cyclolejeunea-Prionolejeunea clade. Pl Syst Evol doi 10.1007s0060601308604/s00606-013-0860-4 [Google Scholar]
- 45. Reiner-Drehwald ME (2000) Las Lejeuneaceae (Hepaticae) de Misiones, Argentina VI. Lejeunea y Taxilejeunea . Trop Bryol 19: 81–131. [Google Scholar]
- 46. Reiner-Drehwald ME (2005) Taxilejeunea pulverulenta (Lejeuneaceae, Jungermanniopsida), a poorly known species from the Neotropics, is transferred to Lejeunea . Cryptog Bryol 26: 59–65. [Google Scholar]
- 47. Rubasinghe SCK, Milne R, Forrest LL, Long DG (2011) Realignment of the genera of Cleveaceae (Marchantiopsida, Marchantiaceae). Bryologist 114: 116–127. [Google Scholar]
- 48. Yu Y, Pócs T, Schäfer-Verwimp A, Heinrichs J, Zhu RL, et al. (2013) Evidence for rampant homoplasy in the phylogeny of the epiphyllous liverwort genus Cololejeunea (Lejeuneaceae). Syst Bot 38: 553–563. [Google Scholar]
- 49. Hentschel J, von Konrat MJ, Pócs T, Schäfer-Verwimp A, Shaw AJ, et al. (2009) Molecular insights into the phylogeny and subgeneric classification of Frullania Raddi (Frullaniaceae, Porellales). Molec Phylogen Evol 52: 142–156. [DOI] [PubMed] [Google Scholar]
- 50. Heinrichs J (2002) A taxonomic revision of Plagiochila sect. Hylacoetes, sect. Adiantoideae and sect. Fuscoluteae in the Neotropics with a preliminary subdivision of Neotropical Plagiochilaceae into nine lineages. Bryophyt Biblioth 58: 1–184, Append. 1–5. [Google Scholar]
- 51. Hentschel J, Zhu R-L, Long DG, Davison PG, Schneider H, et al. (2007) A phylogeny of Porella (Porellaceae, Jungermanniopsida) based on nuclear and chloroplast DNA sequences. Molec Phylogen Evol 45: 693–705. [DOI] [PubMed] [Google Scholar]
- 52. Devos N, Renner MAM, Gradstein SR, Shaw J, Vanderpoorten A (2011) Molecular data challenge traditional subgeneric divisions in the leafy liverwort Radula . Taxon 60: 1623–1632. [Google Scholar]
- 53. Heinrichs J, Bombosch A, Feldberg K, Kreier HP, Hentschel J, et al. (2012) A phylogeny of the northern temperate leafy liverwort genus Scapania (Scapaniaceae, Jungermanniales). Molec Phylogen Evol 62: 973–985. [DOI] [PubMed] [Google Scholar]
- 54. Feldberg K, Váňa J, Long DG, Shaw AJ, Hentschel J, et al. (2010) A phylogeny of Adelanthaceae (Jungermanniales, Marchantiophyta) based on nuclear and chloroplast DNA markers, with comments on classification, cryptic speciation and biogeography. Molec Phylogen Evol 55: 293–304. [DOI] [PubMed] [Google Scholar]
- 55. Cooper ED, Henwood MJ, Brown EA (2012) A molecular phylogeny of the Lepidozia generic complex supports re-circumscription of the Lepidozioideae. Molec Phylogen Evol 65: 10–22. [DOI] [PubMed] [Google Scholar]
- 56. Heinrichs J, Lindner M, Gradstein SR, Groth H, Buchbender V, et al. (2005) Origin and subdivision of Plagiochila (Jungermanniidae: Plagiochilaceae) in tropical Africa based on evidence from nuclear and chloroplast DNA sequences and morphology. Taxon 54: 317–333. [Google Scholar]
- 57. Feldberg K, Váňa J, Hentschel J, Heinrichs J (2010) Currently accepted species and new combinations in Jamesonielloideae (Adelanthaceae, Jungermanniales). Cryptog Bryol 31: 141–146. [Google Scholar]
- 58. Váňa J, Hentschel J, Müller J, Heinrichs J (2012) Taxonomic novelties in Scapania . Phytokeys 10: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heinrichs J, Reiner-Drehwald ME (2012) Surface wax in Dinckleria, Lejeunea and Mytilopsis (Jungermanniidae). Cryptog Bryol 33: 81–86. [Google Scholar]
- 60. De Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56: 879–886. [DOI] [PubMed] [Google Scholar]
- 61. Szweykowski J, Buczkowska K, Odrzykoski IJ (2005) Conocephalum salebrosum, (Marchantiopsida, Conocephalaceae) – a new holarctic liverwort species. Pl Syst Evol 253: 133–158. [Google Scholar]
- 62. Feldberg K, Váňa J, Schulze C, Bombosch A, Heinrichs J (2011) Morphologically similar but genetically distinct: on the differentiation of Syzygiella concreta and S. perfoliata (Adelanthaceae subfam. Jamesonielloideae). Bryologist 114: 686–695. [Google Scholar]
- 63. Forrest LL, Salazar Allen N, Gudiño JA, Korpelainen H, Long DG (2011) Molecular and morphological evidence for distinct species in Dumortiera (Dumortieraceae). Bryologist 114: 102–115. [Google Scholar]
- 64. Patiño J, Devos N, Vanderpoorten A, Schäfer-Verwimp A, Renner M (2013) The identity of Radula carringtonii Jack. J Bryol in press. [Google Scholar]
- 65. Kreier HP, Feldberg K, Mahr F, Bombosch A, Schmidt AR, et al. (2010) Phylogeny of the leafy liverwort Ptilidium: cryptic speciation and shared haplotypes between the Northern and Southern Hemispheres. Molec Phylogen Evol 57: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 66. Ahonen I, Sass-Gyarmati A, Pócs T (2005) Molecular, morphological and taxonomic evaluation of the Ptychanthus striatus (Lejeuneaceae, Marchantiophyta) complex. Acta Bot Hung 47: 225–246. [Google Scholar]
- 67. Sukkharak P, Gradstein SR, Stech M (2011) Phylogeny, taxon circumscriptions, and character evolution in the core Ptychanthoideae (Lejeuneaceae, Marchantiophyta). Taxon 60: 1607–1622. [Google Scholar]
- 68. Jones EW (1968) African Hepatics. XIX. The Lejeunea flava complex. Trans Brit Bryol Soc 5: 548–562. [Google Scholar]
- 69. Ah-Peng C, Bardat J, Ellis L (2005) Additions to the bryoflora of Réunion Island (France). Lindbergia 30: 43–45. [Google Scholar]
- 70. Reiner-Drehwald ME, Martinez KM (2004) On Lejeunea multidentata, a new species from Cuba (Jungermanniopsida: Lejeuneaceae). J Bryol 26: 103–106. [Google Scholar]
- 71. Reiner-Drehwald ME (2010a) On Lejeunea subgenus Nanolejeunea (Lejeuneaceae, Jungermanniopsida). Nova Hedwigia Beih 138: 117–128. [Google Scholar]
- 72. Zanten BO van, Gradstein SR (1988) Experimental dispersal geography of Neotropical liverworts. Nova Hedwigia Beih 90: 41–94. [Google Scholar]
- 73.Zanten BO van, Pócs T (1981) Distribution and dispersal of bryophytes. In: Fittkau EJ, editor. Advances in Bryology. Vaduz: Cramer. pp. 479–562. [Google Scholar]
- 74. Pohjamo M, Korpelainen H, Kalinauskaite N (2008) Restricted gene flow in the clonal hepatic Trichocolea tomentella in fragmented landscapes. Biol Conserv 141: 1204–1217. [Google Scholar]
- 75. Vanderpoorten A, Gradstein SR, Carine MA, Devos N (2010) The ghosts of Gondwana and Laurasia in modern liverwort distributions. Biol Rev 85: 471–487. [DOI] [PubMed] [Google Scholar]
- 76. Heinrichs J, Lindner M, Groth H, Hentschel J, Feldberg K, et al. (2006) Goodbye or welcome Gondwana? Insights into the phylogenetic biogeography of the leafy liverwort Plagiochila with a description of Proskauera, gen. nov. (Plagiochilaceae, Jungermanniales). Pl Syst Evol 258: 211–226. [Google Scholar]
- 77. Feldberg K, Hentschel J, Wilson R, Rycroft DS, Glenny D, et al. (2007) Phylogenetic biogeography of the leafy liverwort Herbertus (Jungermanniales, Herbertaceae) based on nuclear and chloroplast DNA sequence data: correlation between genetic variation and geographical distribution. J Biogeogr 34: 688–689. [Google Scholar]
- 78. Reiner-Drehwald ME, Schmidt AR, Heinrichs J (2012) The genus Lejeunea in Miocene amber from the Dominican Republic. Cryptog Bryol 33: 33–38. [Google Scholar]
- 79. Grolle R (1984) Lejeunea palaeomexicana n. sp., das erste Moos aus mexikanischem Bernstein. Stuttg Beitr Naturk, Ser B 108: 1–7. [Google Scholar]
- 80. Korpelainen H, von Crautlein M, Laaka-Lindberg S, Huttunen S (2011) Fine-scale spatial genetic structure of a liverwort (Barbilophozia attenuata) within a network of ant trails. Evol Ecol 25: 45–57. [Google Scholar]
- 81. Merckx V, Chatrou LW, Lemaire B, Sainge MN, Huysmans S, et al. (2008) Diversification of myco-heterotrophic angiosperms: Evidence from Burmanniaceae. BMC Evol Biol 8: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weeks A, Daly DC, Simpson BB (2005) The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Molec Phylogen Evol 35: 85–101. [DOI] [PubMed] [Google Scholar]
- 83. Merckx VSFT, Kissling J, Hentrich H, Janssens SB, Mennes CB, et al. (2013) Phylogenetic relationships of the mycoheterotrophic genus Voyria and the implications for the biogeographic history of Gentianaceae. Amer J Bot 100: 712–721. [DOI] [PubMed] [Google Scholar]
- 84. Davis CC, Bell CD, Mathews S, Donoghue MJ (2002) Laurasian migration explains Gondwanan disjunctions: Evidence from Malpighiaceae. Proc Nat Acad Sci U S A 99: 6833–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider H, Kreier HP, Janssen T, Otto E, Muth H, et al.. (2010) Key innovations versus key opportunities: identifying causes of rapid radiations in derived ferns. In: Glaubrecht M, editor, Evolution in Action. Berlin: Springer. pp. 61–75. [Google Scholar]
- 86. Rouhan G, Labiak PH, Randrianjohany E, Rakotondrainibe F (2012) Not so Neotropical after all: the grammitid fern genus Leucotrichum (Polypodiacae) is also paleotropical, as revealed by a new species from Madagascar. Syst Bot 37: 331–338. [Google Scholar]
- 87. Hartmann FA, Wilson R, Gradstein SR, Schneider H, Heinrichs J (2006) Testing hypotheses on species delimitations and disjunctions in the liverwort Bryopteris (Jungermanniopsida: Lejeuneaceae). Int J Pl Sci 167: 1205–1214. [Google Scholar]
- 88. Schaumann F, Frey W, Pfeiffer T, Stech M (2005) Molecular circumscription, intrafamilial relationships and biogeography of the Gondwanan liverwort family Pallaviciniaceae. Pl Syst Evol 252: 27–48. [Google Scholar]
- 89. Hennequin S, Hovenkamp P, Christenhusz MJM, Schneider H (2010) Phylogenetics and biogeography of Nephrolepis - a tale of old settlers and young tramps. Bot J Linn Soc 164: 113–127. [Google Scholar]
- 90. Heinrichs J, Feldberg K, Kreier H-P, Váňa J (2010) DNA-based identification of Herbertus species on Gough Island, South Atlantic Ocean. Cryptog Bryol 31: 67–74. [Google Scholar]
- 91. Heinrichs J, Lindner M, Groth R, Renker C (2005) Distribution and synonymy of Plagiochila punctata (Taylor) Taylor, with hypotheses on the evolutionary history of Plagiochila sect. Arrectae (Plagiochilaceae, Hepaticae). Pl Syst Evol 250: 105–117. [Google Scholar]
- 92. Vanderpoorten A, Long DG (2006) Budding speciation and neotropical origin of the Azorean endemic liverwort, Leptoscyphus azoricus . Molec Phylogen Evol 40: 73–83. [DOI] [PubMed] [Google Scholar]
- 93. Wiens JJ, Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19: 639–644. [DOI] [PubMed] [Google Scholar]
- 94. Ricklefs RE (2010) Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proc Nat Acad Sci U S A 107: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K (2010) Is there a latitudinal gradient in the importance of biotic interactions. Ann Rev Ecol Evol Syst 40: 245–269. [Google Scholar]
- 96. Romdal TS, Araujo MB, Rahbeck C (2013) Life on a tropical planet: niche conservatism and the global diversity gradient. Global Ecol Biogeogr 22: 344–350. [Google Scholar]
- 97. Schneider H, He LJ, Marquardt J, Wang L, Heinrichs J, et al. (2013) Exploring the origin of the latitudinal diversity gradient: contrasting the sister fern genera Phegopteris and Pseudophegopteris . J Syst Evol 51: 61–70. [Google Scholar]
- 98. Jablonski D, Roy K, Valentine JW (2006) Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314: 102–106. [DOI] [PubMed] [Google Scholar]
- 99. Marshall CR (2006) Fossil record reveals tropics as cradle and museum. Science 313: 66–67. [DOI] [PubMed] [Google Scholar]
- 100. McKenna DD, Farell BD (2006) Tropical forests are both evolutionary cradles and museums of leaf beetle diversity. Proc Natl Acad Sci U S A 103: 10 947–10 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moreau CS, Bell CD (2013) Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution doi: 10.111/evo.12105 [DOI] [PubMed] [Google Scholar]
- 102. Perrie LR, Parris BS (2012) Chloroplast DNA sequences indicate the grammitid ferns (Polypodiacae) in New Zealand belong to a single clade, Notogrammitis gen. nov. New Zealand J Bot 50: 475–472. [Google Scholar]
- 103. Bystriakova N, Schneider H, Coomes D (2011) Evolution of the climatic niche in scaly tree ferns (Cyatheaceae, Polypodiopsida). Bot J Linn Soc 165: 1–19. [Google Scholar]
- 104.Vanderpoorten A, Goffinet B (2009) Introduction to Bryophytes. Cambridge: Cambridge University Press. 303 p. [Google Scholar]
- 105.Longton RE, Schuster RM (1983) Reproductive biology. In: Schuster RM, editor, New manual of bryology. Nichinan: Hattori Botanical Laboratory. pp. 386–462. [Google Scholar]
- 106. Devos N, Renner MAM, Gradstein SR, Shaw J, Laenen B, et al. (2011) Evolution of sexual systems, dispersal strategies and habitat selection in the liverwort genus Radula . New Phytologist 192: 225–236. [DOI] [PubMed] [Google Scholar]
- 107. Reiner-Drehwald ME (2010) A taxonomic revision of Lejeunea deplanata (Lejeuneaceae, Marchantiophyta) from tropical America. Nova Hedwigia 91: 519–532. [Google Scholar]
- 108. Kraichak E (2012) Asexual propagules as an adaptive trait for epiphylly in tropical leafy liverworts (Lejeuneaceae). Amer J Bot 99: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 109. Heinrichs J, Hentschel J, Bombosch A, Fiebig A, Reise J, et al. (2010) One species or at least eight? Delimitation and distribution of Frullania tamarisci (L.) Dumort. (Jungermanniopsida, Porellales) inferred from nuclear and chloroplast DNA markers. Molec Phylogen Evol 56: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 110. Hutsemekers V, Vieira CC, Ros RM, Huttunen S, Vanderpoorten A (2012) Morphology informed by phylogeny reveals unexpected patterns of species differentiation in the aquatic moss Rhynchostegium riparioides s.l. Molec Phylogen Evol 62: 748–755. [DOI] [PubMed] [Google Scholar]
- 111. Heinrichs J, Hentschel J, Feldberg K, Bombosch A, Schneider H (2009) Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. J Syst Evol 47: 497–508. [Google Scholar]
- 112. Renner MAM, Brown EA, Wardle GM (2013) Averaging v. outlier removal. Decrypting variance among cryptic Lejeunea species (Lejeuneaceae: Jungermanniopsida) using geometric morphometrics. Austral Syst Bot 26: 13–30. [Google Scholar]
- 113. Hollingworth PM, Forrest LL, Spouge JL, Hajibabaei M, Ratnasingham S, et al. (2009) A DNA barcode for land plants. Proc Nat Acad Sci U S A 31: 12794–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gradstein SR, Wilson R, Ilkiu-Borges AL, Heinrichs J (2006) Phylogenetic relationships and neotenic evolution of Metzgeriopsis (Lejeuneaceae) based on chloroplast DNA sequences and morphology. Bot J Linn Soc 151: 293–308. [Google Scholar]
- 115.Hepperle D (2004) SeqAssem©. A sequence analysis tool, contig assembler and trace data visualization tool for molecular sequences. Win32-Version. Distributed by the author via: http://www.sequentix.de.
- 116. Hall TA (1999) BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95–98. [Google Scholar]
- 117.Swofford DL (2000) PAUP*, phylogenetic analyses using parsimony (* and other methods), version 4.01b10. Sunderland, Sinauer Associates. [Google Scholar]
- 118. Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 119. Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing the confidence in phylogenetic analysis. Syst Biol 42: 182–192. [Google Scholar]
- 120. Mason-Gamer RJ, Kellogg EA (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Syst Biol 45: 524–545. [Google Scholar]
- 121. Posada D (2008) jModelTest: Phylogenetic model averaging. Molec Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 122.Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. GARLI version 2.0 available online at https://code.google.com/p/garli/.
- 123. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 124. Larget B, Simon DL (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molec Biol Evol 16: 750–759. [Google Scholar]
- 125.Maddison DR, Maddison WP (2004) Mesquite, version 2.75 Tucson, Arizona. Available at http://mesquiteproject.org.
- 126. Yu Y, Harris AJ, He XJ (2010) S-DIVA (statistical dispersal-vicariance analysis): a tool for inferring biogeographic histories. Molec Phylogen Evol 56: 848–850. [DOI] [PubMed] [Google Scholar]
- 127.Stephani F (1898–1925) Species Hepaticarum 1–6. Geneva: Georg & Cie. 4354 p. [Google Scholar]
- 128. Grolle R (1966) Lebermoose aus Neuguinea. 3. Stenolejeunea . J Hattori Bot Lab 29: 75–78. [Google Scholar]
- 129. Mizutani M (1971) Lejeunea from the Himalayan region. J Hattori Bot Lab 34: 445–457. [Google Scholar]
- 130.Reiner-Drehwald ME (1995) La familia Lejeuneaceae (Hepaticae) en Misiones, Argentina: estudio taxonómico-floristico. PhD thesis. 237 p.
- 131.Paton JA (1999) The liverwort flora of the British Isles. Colchester: Harley Books. 626 p. [Google Scholar]
- 132. Zhu R-L, Grolle R (2003) Taxonomy and distribution of Lejeunea exilis (Lejeuneaceae, Hepaticae). Ann Bot Fennici 40: 101–106. [Google Scholar]
- 133. Wigginton MJ, editor. E. W. Jones's liverwort and hornwort flora of West Africa. Scripta Bot Belg 30: 1–443. [Google Scholar]
- 134. Reiner-Drehwald ME, Schäfer-Verwimp A (2008) Lejeunea oligoclada and L. rionegrensis (Lejeuneaceae) in tropical America: new data on morphology and geographical distribution. Nova Hedwigia 87: 175–184. [Google Scholar]
- 135. Reiner-Drehwald ME, Schäfer-Verwimp A (2008) On Inflatolejeunea, Lejeunea species with eplicate perianths and Lejeunea talamancensis sp. nov. from Costa Rica (Lejeuneaceae). Nova Hedwigia 87: 387–420. [Google Scholar]
- 136. Gradstein SR, Ilkiu-Borges AL (2009) Guide to the plants of central French Guiana. Part 4. Liverworts and hornworts. Mem New York Bot Gard 76: 1–140. [Google Scholar]
- 137. Renner MAM (2013) Lejeunea subelobata and Lejeunea drummondii (Jungermanniopsida) in Australasia. Polish Bot J 58: 193–203. [Google Scholar]
- 138. Lewis PO (2001) A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50: 913–925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum Likelihood phylogeny of the Harpalejeunea-Lejeunea-Microlejeunea clade. ML and MP bootstrap percentage values as well as Bayesian Posterior Probabilities are indicated at branches. Branch colors correspond to the most parsimonious reconstruction of ancestral areas of distribution (see Figure 2).
(TIF)
Taxa used in the present study. Information about the origin of the studied material, vouchers, as well as GenBank accession numbers is included. New sequences in bold face.
(DOC)