Abstract
Clinical outcome post-progression to first-line triplet chemotherapy (CT) plus bevacizumab (FIr-B/FOx) was evaluated in metastatic colorectal cancer (MCRC) patients (pts). Second-line treatment was selected according to fitness, KRAS genotype, previous efficacy and safety. Efficacy was evaluated and compared according to treatment or KRAS genotype, using log-rank analysis. Among 54 pts, median overall survival (OS) post-progression was 12 months, significantly better in 40 (74.1%) treated compared to 14 (25.9%) who died without further treatment. Second-line surgical treatment, 4 pts (7.4%), medical treatment, 36 pts (66.7%): triplet CT plus targeted agent, 10 (18.5%); triplet regimens, 19 (35.2%); doublet/monotherapy, 7 (13%). At follow-up of 14 months, objective response rate (ORR) was 38%, metastasectomies 12.5%, progression-free survival (PFS) 10 months, OS 14 months. According to treatment, ORR, metastasectomies, PFS and OS were significantly favourable in triplet CT plus targeted agent compared to triplet, respectively: 80%, 40%, 13 months, not reached; 28%, 6%, 8 months, 11 months. PFS and OS were significantly worse in c.35 G>A mutant compared to wild-type and/or other mutant patients. Prognosis after progression to first-line FIr-B/FOx may be significantly favourable in MCRC pts re-challenged with intensive regimens, and unfavourable in c.35 G>A KRAS mutant patients.
Keywords: metastatic colorectal cancer, triplet chemotherapy plus bevacizumab, KRAS genotype, re-challenge, c.35 G>A KRAS mutation, post-progression
Introduction
Clinical management of MCRC is faced with different options and lines of treatment strategies according to the fitness of the patients, extension of metastatic disease and KRAS genotype (1–5). First line triplet regimens significantly increased PFS up to 7.2–10.6 months and OS up to 19.9–26.1 months over doublet regimens, also integrated with secondary resection of liver metastases in liver-limited (L-L) disease (2,4,6). After progression to first line treatment strategy, 50–80% MCRC pts receive a second line treatment (4,7–11). Randomized clinical trials and pooled analysis demonstrated that exposure of patients to all three most active chemotherapeutic drugs is associated with the longest OS and similar efficacy (7), regardless of the sequence of administration. OS after progression does not correlate with any second line treatment (8).
Second line irinotecan (CPT-11), in 5-fluorouracil (5-FU) refractory MCRC pts, achieved median PFS of 3–4 months and OS 9.9 months (12,13). Doublet FOLFOX6 or FOLFIRI showed similar efficacy (7), with <2% metastasectomies. FOLFOX4 compared to CPT-11 significantly achieved ORR 28% and PFS 6.2 months, with no difference in OS (14). The addition of oxaliplatin (OXP) to CPT-11 showed significantly increased ORR 22%, PFS 5.3 months and median OS of 13.4 months (15). The addition of bevacizumab (BEV) to FOLFOX4 significantly increased ORR to 22.7%, median PFS 7.3 months, and median OS 12.9 months (16). Among pts treated with first line triplet FOLFOXIRI chemotherapy, ORR was 23%, PFS 5.9 months, OS 13.2 months (10). In EGFR-overexpressing MCRC pts previously treated with 5-FU, CPT-11 and OXP, cetuximab significantly improved ORR, PFS and OS, compared to best supportive care (BSC) (17,18). In CPT-11 or 5-FU/OXP refractory pts, cetuximab addition to CPT-11 showed significantly higher ORR of 22.9% and 16.4%, PFS 4.1 and 4.0 months, respectively (19,20). A significant interaction was demonstrated between KRAS wild-type genotype and effectiveness of cetuximab compared to BSC alone, increasing PFS up to 3.7 months and OS up to 9.5 months (21). Panitumumab confirmed the significantly positive predictive effect of KRAS wild-type status, with ORR of 17%, median PFS 12.3 weeks, median OS 8.1 weeks, compared to mutant genotype (22,23). In KRAS wild-type pts, the addition of panitumumab to FOLFIRI significantly increased ORR of 35% and PFS 5.9 months, with a trend toward increased OS (24).
More intensive first line medical treatment consisting of triplet chemotherapy plus targeted agent can increase activity, thus increasing resection rate of liver metastases and clinical outcome of MCRC pts (1,2,6,25,26). We recently proposed a phase II study of BEV addition to triplet chemotherapy, according to FIr-B/FOx schedule (1) reaching ORR of 82%, 54% liver metastasectomies in L-L disease, median PFS 12 months, median OS 28 months (1,3). KRAS wild-type pts with L-L disease may achieve significantly greater benefit from integration with liver metastasectomies compared to other/multiple metastatic (O/MM) pts, with respect to KRAS mutant pts (3,5).
The present study evaluated clinical outcome of the fit MCRC pts after progression to FIr-B/FOx and, retrospectively, the prognostic relevance of second line treatments and KRAS genotype.
Materials and methods
Patient eligibility
Sixty-seven fit MCRC pts were enrolled in previously reported phase II study (1) and in the expanded clinical program proposing FIr-B/FOx association as first line treatment. Pts had histologically confirmed diagnosis of measurable MCRC, age 18–75 years, World Health Organization (WHO) performance status ≤2, adequate hematological, renal and hepatic functions, life expectancy >3 months. The study was approved by the Local Ethics Committee (Comitato Etico, Azienda Sanitaria Locale n.4 L’Aquila, Regione Abruzzo, Italy) and conducted in accordance with the Declaration of Helsinki. All patients provided written, informed consent. After progression, second-line treatment was selected among medical and/or surgical options available in clinical practice, according to age (< or ≥75 years), patient fitness (performance status, Comorbidity Index Rating Scale), safety of FIr-B/FOx treatment, activity and efficacy of first line treatment [objective response (OR), PFS], KRAS genotype. Pts with performance status 3 were not treated, nor pts with clinical complete response (cCR) until progression.
Medical treatment regimens
Medical treatments included: rechallenge of FIr-B/FOx or triplet chemotherapy plus cetuximab; triplet, doublet or mono-chemotherapy regimens. FIr-B/FOx schedule consisted of weekly timed-flat-infusion 5-FU (TFI 5-FU), associated to weekly alternating CPT-11/BEV or L-OXP (1): TFI/5-FU (Fluorouracil Teva; Teva Italia, Milan, Italy), 900 mg/m2/die, over 12 h (from 10:00 p.m. to 10:00 a.m.), on days 1–2, 8–9, 15–16 and 22–23; CPT-11 (Campto; Pfizer, Latina, Italy), 160 mg/m2, days 1 and 15; BEV (Avastin; Roche, Welwyn Garden City, UK), 5 mg/kg, days 1 and 15; l-OXP (Eloxatin; Sanofi-Aventis, Milan, Italy), 80 mg/m2, days 8 and 22; cycles every 4 weeks. Triplet chemotherapy plus cetuximab consisted of: TFI/5-FU, 800 mg/m2/die, days 1–2, 8–9, 15–16 and 22–23; CPT-11, 140 mg/m2, days 1 and 15; l-OXP, 80 mg/m2, days 8 and 22; cetuximab (Erbitux; Merck, Darmstadt, Germany), 400 mg/m2 initial dose, then 250 mg/m2/week; cycles every 4 weeks. Triplet FIr/FOx regimen, doublets and mono regimens were administered according to previously reported schedules (27,28).
Study design
Pts were assessed at the time of progression to first line treatment and every 2–3 cycles of second line treatment. A multidisciplinary team, consisting of a medical oncologist, liver surgeon, radiologist, evaluated resectability, according to previously reported resectability categories (3). Clinical criteria of activity and efficacy were: ORR, resection rate of metastases, PFS, OS. ORR was evaluated according to RECIST criteria (29); pathologic complete response was defined as absence of residual cancer cells in surgically resected specimens. Clinical evaluation of response was made by CT-scan; PET was added based on investigator assessment. Liver metastasectomies were defined as: R0, if radical surgery; R1, if radiofrequency was added. Surgery was recommended >4 weeks after BEV discontinuation. PFS and OS were evaluated using the Kaplan-Meier method (30). PFS was defined, as the length of time from the beginning of treatment and disease progression or death (resulting from any cause) or to the last contact; OS as length of time between beginning of treatment and death or to last contact. Prognostic relevance of second line treatments and of KRAS genotype was retrospectively assessed, using log-rank test to compare PFS and OS (31).
Mutational analysis
KRAS and BRAF genetic analyses were performed on paraffin-embedded tissue blocks from primary tumor and/or metastases, through selection of tumor cells, and DNA extraction, as previously described (5). Genotype status was assessed for KRAS codon 12–13 and BRAF c.1799 T>A (V600E) mutations by SNaPshot® multiplex screening for KRAS mutations and KRAS/BRAF mutations in 36 and 32 samples, respectively (32,33); direct sequencing was performed to detect KRAS mutations in 26 samples. SNaPshot multiplex assay was performed as reported (32,33). Briefly, KRAS exon 2 and BRAF exon 15 were simultaneously PCR-amplified using specific primers and analyzed using the ABI PRISM SNaPshot Multiplex kit (Applied Biosystems, Foster City, CA, USA) with five primers including at their 5′-end an additional tail allowing their simultaneous detection. Sense primers allowing the extension at nucleotides KRAS c.34G, c.35G, c.37G, c.38G and BRAF c.1799T were used and multiplex SNaPshot reaction was performed as reported (32). KRAS exon 2 sequence was performed from PCR-amplified tumor DNA using the Big Dye V3.1 Terminator kit (Applied Biosystems), electrophoresis in ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems), and analysis using the GeneMapper Analysis Software version 4.0 (Applied Biosystems).
Results
Patient demographics
Fifty-four MCRC pts developed disease progression (80.6%), among 67 consecutively treated with first-line FIr-B/FOx regimen. Fourteen pts (25.9%) died without further treatment. Forty pts (74.1%) underwent second line treatment, 4 pts surgical (7.4%), 36 pts medical (66.7%) (Table I). Second line medical treatment were: triplet chemotherapy plus targeted agent, 10 pts (18.5%); triplet regimens, 19 pts (35.2%); doublet regimen, 3 pts (5.6%); mono-therapy, 4 pts (7.4%). Among 51 KRAS evaluated pts (94.4%), 26 wild-type and 25 (49%) mutant, second line treated were 21 (80.8%) and 17 (68%), respectively; death events without further treatment were 5 (19.2%) and 8 (32%), respectively. Cetuximab-containing regimen was also administered in 4 EGFR-overexpressing/KRAS mutant pts, before recommendation of anti-EGFR treatment in KRAS wild-type patients.
Table I.
Clinical management of MCRC patients after progression to first-line FIr-B/FOx regimen.
| Overall
|
KRAS genotype
|
||
|---|---|---|---|
| No. of patients (%) | Wild-type (%) | Mutant (%) | |
| Total no. | 54 | 26 | 25 |
| Second line treatment | 40 (74.1) | 21 (80.8) | 17 (68) |
| Medical treatment | 36 (66.7) | 18 (69.2) | 16 (64) |
| Triplet chemotherapy plus targeted agent | 10 (18.5) | 5 (19.2) | 5 (20) |
| Triplet chemotherapy plus bevacizumab | 7 | 3 | 4 |
| Triplet chemotherapy plus cetuximab | 3 | 2 | 1 |
| Triplet regimen | 19 (35.2) | 10 (38.5) | 7 (28) |
| Doublet chemotherapy plus bevacizumab | 5 | 1 | 4 |
| Doublet chemotherapy plus cetuximab | 13 | 8 | 3 |
| Triplet chemotherapy | 1 | 1 | - |
| Doublet regimen | 3 (5.6) | 2 (7.6) | 1 (4) |
| Mono-chemotherapy plus bevacizumab | 3 | 2 | 1 |
| Mono therapy | 4 (7.4) | 1 (3.8) | 3 (12) |
| Mono-chemotherapy | 3 | - | 3 |
| Panitumumab | 1 | 1 | - |
| Surgery | 4 (7.4) | 3 (11.5) | 1 (4) |
| Death events without further treatment | 14 (25.9) | 5 (19.2) | 8 (32) |
Table II describes features of the 40 treated pts: male/female ratio, 26/14; median age, 65 years; young-elderly pts (≥65/<75 years), 18 (45%); metastatic disease metachronous 37.5%, synchronous 62.5%. Metastatic sites: liver 22 pts 55%), lung 17 pts (42.5%), lymph nodes 17 pts (42.5%); local recurrence 13 pts (32.5%). Metastatic site was single in 16 pts 40%), multiple in 24 pts (60%). Single metastatic sites were: liver 9 pts (22.5%), other than liver 7 pts (17.5%). Liver metastases were single in 3 pts (7.5%) and multiple in 20 pts (50%). The features of the patients who died without further treatment were not different from the treated patients. Among 38 second line treated MCRC pts evaluated for KRAS genotype, 21 wild-type (55.3%) and 17 mutant (44.7%), demographic and baseline features were, respectively: male/female ratio, 17/4 and 8/9; metachronous/synchronous metastatic disease, 10/11 (48/52%) and 5/12 (29/71%) pts. Distribution according to extension of metastatic disease, L-L and O/MM, was, respectively: KRAS wild-type, 3 (14%) and 18 (86%); KRAS mutant, 6 (35%) and 11 (65%). KRAS mutations detected in 17 pts were: codon 12, 14 pts (82.3%), specifically c.35 G>A 8 pts (47.7%), c.35 G>T 5 pts (29.4%), c.35 G>C, 1 patient; codon 13, 3 pts (17.6%), c.38 G>A 2 pts (11.7%) and c.37_39 dupl, 1 patient. Twenty-three tumoral samples (62.2%) were analysed for BRAF and no BRAF mutation was detected; 13 out of 21 KRAS wild-type MCRC pts were KRAS and BRAF wild-type.
Table II.
Features of second line treated patients according to KRAS genotype.
| Overall treated
|
KRAS wild-type
|
KRAS mutant
|
|
|---|---|---|---|
| Total no. (%) | Total no. (%) | Total no. (%) | |
| No. of patients | 40 | 21 (55.3) | 17 (44.7) |
| Sex | |||
| Male/female | 26/14 | 17/4 | 8/9 |
| Age, years | |||
| Median | 65 | 64 | 66 |
| Range | 46-74 | 46-73 | 51-74 |
| ≥65 years | 18 (45) | 9 (43) | 8 (47) |
| Metastatic disease | |||
| Metachronous | 15 (37.5) | 10 (48) | 5 (29) |
| Synchronous | 25 (62.5) | 11 (52) | 12 (71) |
| Primary tumor | |||
| Colon | 18 (45) | 6 (29) | 11 (65) |
| Rectum | 22 (55) | 15 (71) | 6 (35) |
| Sites of metastases | |||
| Liver | 22 (55) | 10 (48) | 11 (65) |
| Lung | 17 (42.5) | 10 (48) | 5 (29) |
| Lymph nodes | 17 (42.5) | 10 (48) | 5 (29) |
| Local | 13 (32.5) | 9 (43) | 4 (23) |
| Other | 12 (30) | 6 (29) | 5 (29) |
| No. of involved sites | |||
| 1 | 16 (40) | 9 (43) | 8 (47) |
| ≥2 | 24 (60) | 12 (57) | 9 (53) |
| Single metastatic sites | |||
| Liver-limited | 9 (22.5) | 3 (14) | 6 (35) |
| Other than liver | 7 (17.5) | 6 (29) | 2 (12) |
| Lung | 4 (10) | 4 (19) | 1 (6) |
| Lymph nodes | 2 (5) | 2 (9) | - |
| Local | - | - | - |
| Other | 1 (2.5) | - | - |
| Multiple metastatic sites | 24 (60) | 12 (57) | 9 (53) |
| Liver metastases | |||
| Single | 3 (7.5) | 1 (5) | 2 (12) |
| Multiple | 20 (50) | 9 (43) | 9 (53) |
| Previous adjuvant chemotherapy | 7 (17.5) | 5 (24) | 1 (6) |
| FA/5-FU bolus | 4 (10) | 3 (14) | - |
| Capecitabine | - | - | - |
| FOLFOX4 | 3 (7.5) | 2 (9) | 1 (6) |
| Previous radiotherapy | 5 (12.5) | 4 (19) | 1 (6) |
| RT alone | 1 (2.5) | 1 (5) | - |
| RT+CT (5-FU continous infusion) | 2 (5) | 2 (9) | - |
| RT+CT (XELOX) | 2 (5) | 1 (5) | 1 (6) |
Activity and efficacy
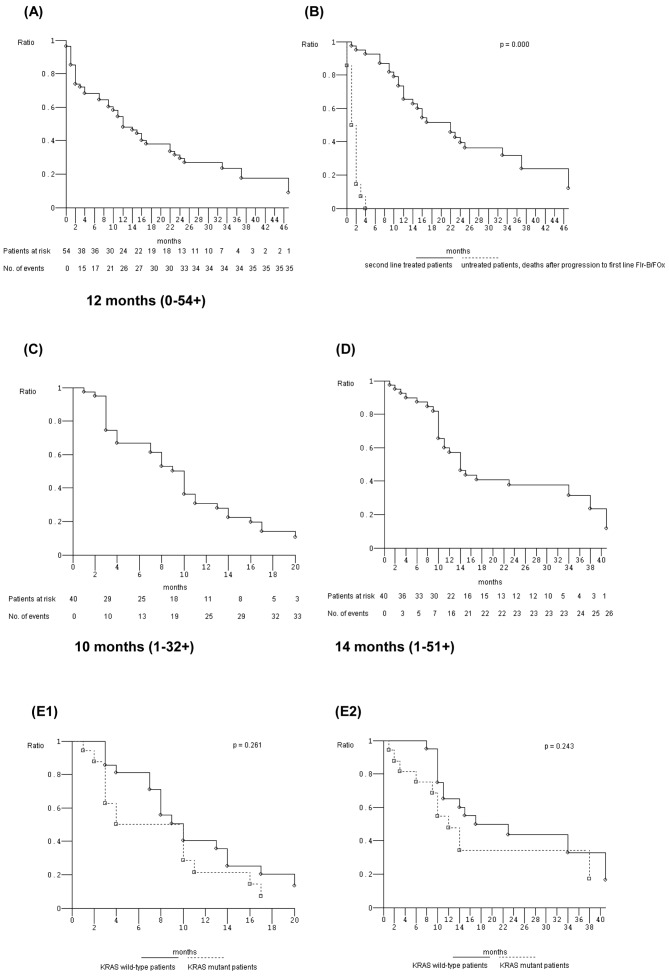
At a median follow-up of 11.5 months, overall OS post-progression to FIr-B/FOx was 12 months (0–54+ months) (Fig. 1A). Among the 40 pts who received second line treatment and the 14 untreated pts, median OS after progression was significantly different: 22 months (1+−54+) and 2 months (0–4 months), respectively (Fig. 1B). Intent-to-treat analysis of 34 evaluable pts (Table IIIA) showed ORR 38% (α 0.05, CI ± 17). We observed 13 objective responses: 10 partial responses (29%) and 3 complete responses (CR 9%); 10 were stable disease (29%); and 11 progressive disease (32%). Disease control rate was 68% (α 0.05, CI ± 16). At median follow-up of 14 months, median PFS was 10 months (1–32+): 33 events occurred (Fig. 1C). Median OS was 14 months (1–51+ months): 26 events occurred (Fig. 1D). Secondary metastasectomies were performed in 5 pts (12.5%): 2 liver resections, 2 peritonectomies, 1 lymph node resection. Two liver metastasectomies (R0) were performed out of 22 pts with liver metastases (9%), and out of 9 pts with L-L disease (22%), without surgery-related complications. A pathologic CR was obtained after 3 cycles of FIr-B/FOx rechallenge inducing a cCR in a c.35 G>T KRAS mutant patient with multiple liver-only metastases. Among 18 evaluable KRAS wild-type pts, ORR was 50% (CI ± 24) (Table IIIA). We observed 9 objective responses: 7 partial responses (39%) and 2 CR (11%); 5 stable diseases (28%); 3 progressive diseases (17%). Disease control rate was 82% (CI ± 19). Metastasectomies were performed in 3 pts (15%). Median PFS was 10 months (3–31+ months), 17 events occurred. Median OS was 17 months (5+−51+ months), 13 events occurred. Among 14 evaluable KRAS mutant pts, ORR was 29% (CI ± 26) (Table IIIA). There were 4 objective responses: 3 partial responses (21%) and 1 CR (7%); 3 stable diseases (21%); 7 progressive diseases (50%). Disease control rate was 50% (CI ± 27). Metastasectomies were performed in 2 pts (12%). Median PFS was 10 months (1–32+ months), 14 events occurred. Median OS was 12 months (1–39+ months), 11 events occurred. KRAS wild-type compared with mutant pts did not show significantly different PFS nor OS (Fig. 1E1 and E2).
Figure 1.
Kaplan-Meier survival estimate. (A) Post-progression from first line FIr-B/FOx regimen overall survival; (B) Post-progression from first line FIr-B/FOx regimen overall survival, second line treated patients versus untreated patients; (C) Second line treatment, overall patients, progression-free survival; (D) Second line treatment, overall patients, overall survival; (E) Second line treatment, KRAS wild-type versus KRAS mutant patients: (E1) Progression-free survival; (E2) Overall survival.
Prognostic relevance of second line treatments and of c.35 G>A KRAS mutation
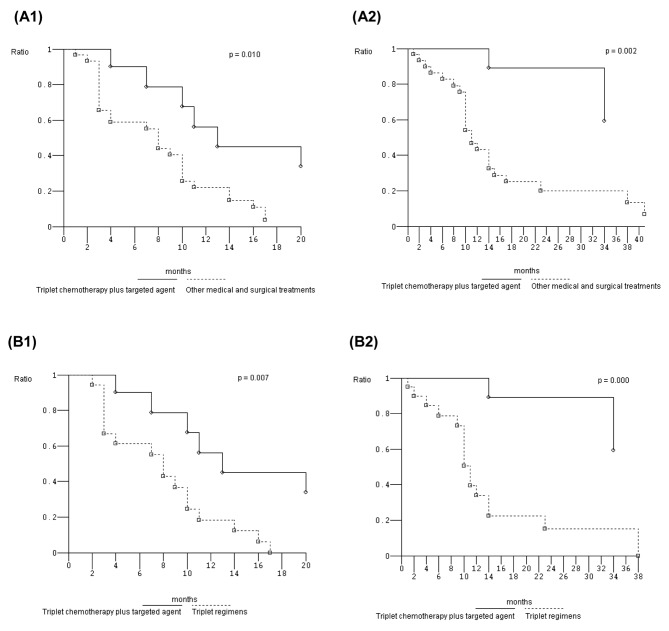
Among 10 pts treated with triplet chemo-therapy plus targeted agent (Table IIIB), ORR was 80% (α 0.05, CI ± 26). We observed 8 objective responses: 5 partial responses (50%) and 3 CR (30%); 1 stable disease (10%); 1 progressive disease (10%). Median PFS was 13 months (4–32+): 6 events occurred. Median OS was not reached (6+−39+ months), at median follow-up of 31.5 months; 2 events occurred for a 2-year OS 80%. Secondary metastasectomies were performed in 4 pts (40%). Among 19 pts treated with triplet regimens (Table IIIB), ORR was 28% (α 0.05, CI ± 21). We observed 5 partial responses (28%); 6 stable diseases (33%); 7 progressive diseases (39%). Median PFS was 8 months (1+−17): 17 events occurred. Median OS was 11 months (1+−38 months): 16 events occurred. Among 7 pts treated with doublet or mono-regimens, we observed 6 progressive diseases (86%), median PFS 4 months (1–17 months), median OS 10 months (1–17 months). Among 4 pts who underwent surgery as second line treatment, median PFS was 14 months (3–14); median OS 41 months (10–42+ months). Eighteen pts (45%) received a third line treatment. PFS and OS were significantly different in pts treated with triplet chemotherapy plus targeted agent compared to other second line treatments (p=0.010 and 0.002, respectively), and to triplet regimens (p=0.007 and 0.000, respectively) (Fig. 2).
Figure 2.
Kaplan-Meier survival estimate. (A) Second line treatment, triplet chemotherapy plus targeted agent versus other medical and surgical treatments. (B) Second line treatment, triplet chemotherapy plus targeted agent versus triplet regimens. (1) Progression-free survival; (2) Overall survival.
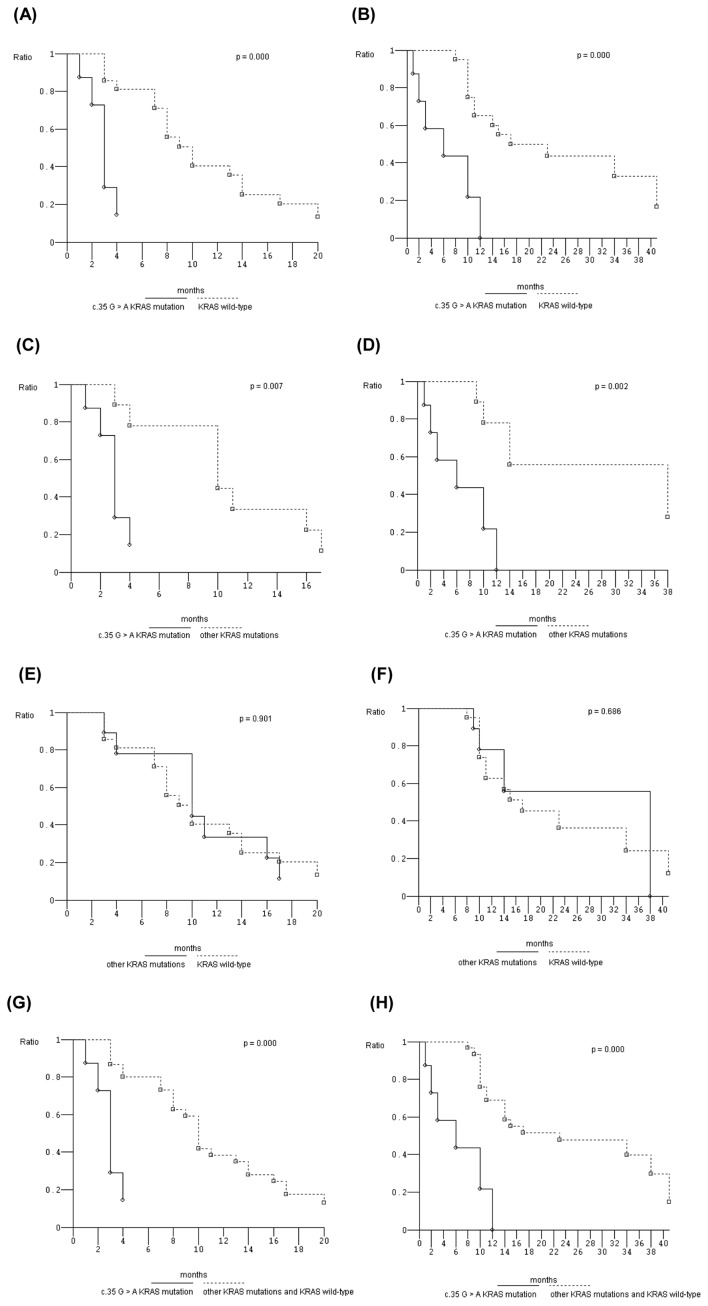
Retrospective analysis of clinical outcome in c.35 G>A KRAS mutant pts showed significantly worse PFS and OS compared to KRAS wild-type pts (p=0.000, and 0.000, respectively) (Fig. 3A and B), and to other than c.35 G>A KRAS mutant pts (p=0.007, and 0.002, respectively) (Fig. 3C and D). No different clinical outcomes were reported in other than c.35 G>A KRAS mutant compared to wild-type pts (Fig. 3E and F). PFS and OS were also significantly worse in c.35 G>A KRAS mutant pts compared to other than c.35 G>A KRAS mutant plus KRAS wild-type pts (Fig. 3G and H).
Figure 3.
Kaplan-Meier survival estimate. (A) Progression-free survival of c.35 G>A KRAS mutant patients versus KRAS wild-type patients; (B) Overall survival of c.35 G>A KRAS mutant patients versus KRAS wild-type patients; (C) Progression-free survival c.35 G>A KRAS mutant patients versus other KRAS mutant patients; (D) Overall survival c.35 G>A KRAS mutant patients versus other KRAS mutant patients; (E) Progression-free survival, other KRAS mutant patients versus KRAS wild-type patients; (F) Overall survival, other KRAS mutant patients versus KRAS wild-type patients; (G) Progression-free survival, c.35 G>A KRAS mutant patients versus other KRAS mutant plus KRAS wild-type patients; (H) Overall survival, c.35 G>A KRAS mutant patients versus other KRAS mutant plus KRAS wild-type patients.
Discussion
Among fit MCRC pts treated with first line FIr-B/FOx regimen, adding BEV to triplet chemotherapy, 74.1% underwent a second line treatment, in the range of reported 50–80% (7–11); 25.9% died without receiving further antitumoral treatment. Median OS post-progression to FIr-B/FOx was 12 months, including untreated pts and significantly better in second line treated patients. At median follow-up of 14 months, the 34 evaluable pts treated with re-challenge of triplet chemotherapy plus targeted agent (18.5%), triplet (35.2%) or less intensive regimens (13%), reported an overall ORR of 38%, median PFS 10 months, median OS 14 months. Secondary metastasectomies were performed in 12.5% (22% of L-L disease), all previously challenged with first-line FIr-B/FOX regimen and secondary surgery. Doublet FOLFOX4 schedule, or OXP associated to CPT-11 reported significantly increased ORR of 22 and 28%, and PFS 6.2 and 5.3 months, compared to CPT-11 alone, respectively. Median OS was 13 months, significantly increased only with OXP/CPT-11 regimen (14,15). Randomized studies of cetuximab plus CPT-11 in EGFR-overexpressing pts, previously treated with CPT-11 or with 5-FU/OXP, respectively showed significantly improved ORR of 16.4 and 22.9% and PFS 4 months (12,20). Triplet FOLFOX4-BEV association, after progression to 5-FU/CPT-11, demonstrated significantly increased ORR 22.7%, median PFS 7.3 months, and median OS 12.9 months (16). Recently, FOLFIRI-aflibercept, after progression to OXP-containing chemotherapy, gained significantly increased median OS 13.5 months (34). A randomized trial reported that BEV associated with 5-FU-based chemotherapy, after first line BEV-containing regimen, significantly improved clinical outcome (35). In KRAS wild-type pts, triplet panitumumab/FOLFIRI regimen reported significantly increased ORR of 35% and median PFS 5.9 months (23,24). Thus, OS after progression does not correlate with any second line treatment (8) in clinical trials and few secondary resections of metastases were reported after second line treatment (7).
Retrospective analysis of 32 pts (24%) achieving OR and progressed >3 months, who were re-challenged with FOLFOXIRI, reported significantly longer PFS (8.2 months) and OS (19.3 months), with respect to doublet regimens (10,11). In our present analysis, second line triplet regimens, proposed to 19 pts (47.5%) achieved ORR 28%, secondary metastasectomies 6%, median PFS 8 months, median OS 11 months. Re-challenge of triplet chemotherapy associated to targeted agent, proposed to 10 pts (25%), with previous OR, long PFS (≥10 months), off-treatment interval ≥3 months and no previous limiting toxicities, achieved ORR 80%, that correlated with 40% secondary surgical resections, median PFS 13 months, and 2-year OS 80% (median OS not reached at median follow-up 31.5 months). PFS and OS were significantly favourable in pts treated with triplet chemotherapy plus targeted agent compared to triplet regimens. Present data confirm that re-challenge of intensive medical treatment is feasible in a selected subgroup of MCRC pts, with high activity, efficacy and effectiveness of secondary metastasectomies. Prospective studies will address if medical and surgical re-challenge can be the standard multidisciplinary second line strategy.
Direct comparison of PFS and OS in KRAS wild-type compared to mutant pts failed to significantly differentiate prognosis in second line, as it was previously reported in first line treated MCRC pts (5,36,37). In KRAS mutant pts harbouring the prevalent c.35 G>A transversion, median PFS and OS were significantly worse compared to KRAS wild-type pts and/or other than c.35 G>A KRAS mutant pts, due to increased aggressiveness and resistance to medical treatment (38). Present data confirm our recent findings of significantly worse prognosis of c.35 G>A KRAS mutant pts treated with first line FIr-B/FOx (39), even in a small cohort of MCRC patients. Here we report for the first time the c.35 G>A KRAS mutant genotype as prognostic biomarker of unfavourable clinical outcome, significantly related to worse efficacy (PFS) of second line treatments. Further prospective studies will confirm prognostic and predictive value of c.35 G>A KRAS mutation in MCRC patients.
In conclusion, clinical outcome of MCRC progressing to first line FIr-B/FOx regimen may be significantly favourable in pts re-challenging triplet chemotherapy associated with targeted agent compared to other second line treatments and significantly worse in c.35 G>A mutant compared to wild-type and other mutant KRAS patients.
Table III.
Prognostic relevance.
| A, Activity, efficacy and effectiveness of second line after FIr-B/FOx regimen according to KRAS genotype
| ||||||
|---|---|---|---|---|---|---|
| All
|
KRAS wild-type
|
KRAS mutant
|
||||
| Intent-to-treat analysis
|
Intent-to-treat analysis
|
Intent-to-treat analysis
|
||||
| No | % | No | % | No | % | |
| Enrolled patients | 40 | 100 | 21 | 100 | 17 | 100 |
| Evaluable patients | 34 | 89 | 18 | 86 | 14 | 93 |
| Objective response | 13 | 38 (CI ± 17) | 9 | 50 (CI ± 24) | 4 | 29 (CI ± 25) |
| Partial response | 10 | 29 | 7 | 39 | 3 | 21 |
| Complete response | 3 | 9 | 2 | 11 | 1 | 7 |
| Stable disease | 10 | 29 | 5 | 28 | 3 | 21 |
| Progressive disease | 11 | 32 | 3 | 17 | 7 | 50 |
| Median PFS, months | 10 | 10 | 10 | |||
| Range | 1–32+ | 3–31+ | 1–32+ | |||
| Progression events | 33 | 82.5 | 17 | 81 | 14 | 82 |
| Median OS, months | 14 | 17 | 12 | |||
| Range | 1–51+ | 5+−51+ | 1–39+ | |||
| Deaths | 26 | 65 | 13 | 62 | 11 | 65 |
| Metastasectomies | 5 | 12.5 | 3 | 15 | 2 | 12 |
| Peritoneal carcinomatosis | 2 | 1 | 1 | |||
| Liver | 2 | 1 | 1 | |||
| Lymph nodes | 1 | 1 | - | |||
| Pathologic complete responses | 1 | 20 | - | - | 1 | 50 |
| PFS, progression-free survival; OS, overall survival. | ||||||
| B, Activity, efficacy and effectiveness of second line intensive treatments
| ||||
|---|---|---|---|---|
| Intent-to-treat analysis
|
||||
| Triplet chemotherapy plus targeted agent
|
Triplet regimen
|
|||
| No. | % | No. | % | |
| Enrolled patients | 10 | 100 | 19 | 100 |
| Evaluable patients | 10 | 100 | 18 | 95 |
| Objective response | 8 | 80 (CI ± 26) | 5 | 28 (CI ± 21) |
| Partial response | 5 | 50 | 5 | 28 |
| Complete response | 3 | 30 | - | - |
| Stable disease | 1 | 10 | 6 | 33 |
| Progressive disease | 1 | 10 | 7 | 39 |
| Median PFS, months | 13 | 8 | ||
| Range | 4–32+ | 1+−17 | ||
| Progression events | 6 | 60 | 17 | 89 |
| Median OS, months | NR | 11 | ||
| Range | 6+−39+ | 1+−38 | ||
| Deaths | 2 | 20 | 16 | 84 |
| Metastasectomies | 4 | 40 | 1 | 6 |
| Peritoneal | 1 | 1 | ||
| Liver | 2 | - | ||
| Lymph nodes | 1 | - | ||
| Pathologic complete responses | 1 | 25 | - | - |
| PFS, progression-free survival; OS, overall survival. NR, not reached. | ||||
Acknowledgments
We thank Gino Coletti and Antonella Dal Mas, Pathology Department, S. Salvatore Hospital, L’Aquila, Italy, for collection and assembly of biological materials and Daniela Di Giacomo, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy, for contribution to molecular genetic analysis. G.B. is a PhD student in Biotechnology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, funded by the University of L’Aquila, Italy.
References
- 1.Bruera G, Santomaggio A, Cannita K, Lanfiuti Baldi P, Tudini M, De Galitiis F, Mancini M, Marchetti P, Antonucci A, Ficorella C, Ricevuto E. ‘Poker’ association of weekly alternating 5-Fluorouracil, Irinotecan, Bevacizumab and Oxaliplatin (FIr-B/FOx) in first line treatment of metastatic colorectal cancer: a phase II study. BMC Cancer. 2010;10:67. doi: 10.1186/1471-2407-10-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruera G, Ricevuto E. Intensive chemotherapy of metastatic colorectal cancer: weighing between safety and clinical efficacy. Evaluation of Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–52. doi: 10.1517/14712598.2011.582462. Expert Opin Biol Ther 11: 821–824, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bruera G, Cannita K, Giuliante F, Lanfiuti Baldi P, Vicentini R, Marchetti P, Nuzzo G, Antonucci A, Ficorella C, Ricevuto E. Effectiveness of liver metastasectomies in patients with metastatic colorectal cancer treated with FIr-B/FOx triplet chemotherapy plus bevacizumab. Clin Colorectal Cancer. 2012;11:119–126. doi: 10.1016/j.clcc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Ficorella C, Bruera G, Cannita K, Porzio G, Lanfiuti Baldi P, Tinari N, Natoli C, Ricevuto E. Triplet chemotherapy in patients with metastatic colorectal cancer: toward the best way to safely administer a highly active regimen in clinical practice. Clin Colorectal Cancer. 2012;11:229–237. doi: 10.1016/j.clcc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Bruera G, Cannita K, Di Giacomo D, Lamy A, Troncone G, Dal Mas A, Coletti G, Frébourg T, Sabourin JC, Tosi M, Ficorella C, Ricevuto E. Prognostic value of KRAS genotype in metastatic colorectal cancer (MCRC) patients treated with intensive triplet chemotherapy plus bevacizumab (FIr-B/FOx) according to extension of metastatic disease. BMC Med. 2012;10:135. doi: 10.1186/1741-7015-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, Salvatore L, Cremolini C, Stasi I, Brunetti I, Fabbri MA, Pugliesi M, Trenta P, Granetto C, Chiara S, Fioretto L, Allegrini G, Crinò L, Andreuccetti M, Falcone A. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21–30. doi: 10.1093/jnci/djq456. [DOI] [PubMed] [Google Scholar]
- 7.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 10.Masi G, Marcucci L, Loupakis F, Cerri E, Barbara C, Bursi S, Allegroni G, Brunetti IM, Murr R, Ricci S, Cupini S, Andreuccetti M, Falcone A. First-line 5-fluorouracil/folinic acid, oxaliplatin and irinotecan (FOLFOXIRI) does not impair the feasibility and the activity of second line treatments in meta-static colorectal cancer. Ann Oncol. 2006;17:1249–1254. doi: 10.1093/annonc/mdl119. [DOI] [PubMed] [Google Scholar]
- 11.Fornaro L, Vasile E, Masi G, Loupakis F, Baldi GG, Allegrini G, Salvatore L, Cremolini C, Cupini S, Cortesi E, Tuzi A, Granetto C, Brunetti IM, Ricci S, Falcone A. Outcome of second-line treatment after first-line chemotherapy with the GONO FOLFOXIRI regimen. Clin Colorectal Cancer. 2012;11:71–76. doi: 10.1016/j.clcc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Pyrhönen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21:807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 14.Kim GP, Sargent DJ, Mahoney MR, Rowland KM, Jr, Philip PA, Mitchell E, Mathews AP, Fitch TR, Goldberg RM, Alberts SR, Pitot HC. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. J Clin Oncol. 2009;27:2848–2854. doi: 10.1200/JCO.2008.20.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller DG, Rothenberg ML, Wong AO, Koralewski PM, Miller WH, Jr, Bodoky G, Habboubi N, Garay C, Olivato LO. Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol. 2008;26:4544–4550. doi: 10.1200/JCO.2008.17.1249. [DOI] [PubMed] [Google Scholar]
- 16.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., III Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 17.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 18.Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 19.Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Cetuximab monotherapy and Cetuximab plus Irinotecan in Irinotecan refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 20.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA., III EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 21.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from Cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 23.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 24.Peeters M, Price YJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJA, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 25.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, Ciarlo A, Del Monte F, Cortesi E, Amoroso D, Granetto C, Fontanini G, Sensi E, Lupi C, Andreuccetti M, Falcone A. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 26.Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I, Cognetti F. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadiuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–1547. doi: 10.1038/sj.bjc.6605940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ficorella C, Ricevuto E, Morelli MF, Morese R, Cannita K, Cianci G, Di Rocco ZC, De Galitiis F, De Tursi M, Tinari N, Iacobelli S, Marchetti P. Increased tolerability of bimonthly 12-hour timed flat infusion 5-fluorouracil/irinotecan regimen in advanced colorectal cancer: a dose-finding study. Oncol Rep. 2006;5:1345–1350. doi: 10.3892/or.15.5.1345. [DOI] [PubMed] [Google Scholar]
- 28.Morelli MF, Santomaggio A, Ricevuto E, Cannita K, De Galitiis F, Tudini M, Bruera G, Mancini M, Pelliccione M, Calista F, Guglielmi F, Martella F, Lanfiuti Baldi P, Porzio G, Russo A, Gebbia N, Iacobelli S, Marchetti P, Ficorella C. Triplet schedule of weekly 5-fluorouracil and alternating irinotecan or oxaliplatin in advanced colorectal cancer: a dose-finding and phase II study. Oncol Rep. 2010;23:1635–1640. doi: 10.3892/or_00000805. [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 31.Peto R, Peto J. Asymptomatically efficient rank invariant test procedures. J Roy Statist Soc A. 1972;135:185–206. [Google Scholar]
- 32.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboué R, Tuech JJ, Queniet AM, Paillot B, Sabouirin JC, Michot F, Michel P, Frebourg T. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamy A, Blanchard F, Le Pessot F, Sesboué R, Di Fiore F, Bossut J, Fiant E, Frébourg T, Sabourin JC. Metastatic colorectal cancer KRAS genotyping in routine practice: results and pitfalls. Mod Pathol. 2011;24:1090–1100. doi: 10.1038/modpathol.2011.60. [DOI] [PubMed] [Google Scholar]
- 34.Van Cutsem E, Tabernero J, Lakomy R, Prener H, Prausova J, Macarulla T, Ruff P, Van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 35.Arnold D, Andre T, Bennouna J, Sastre J, Osterlund PJ, Greil R, Van Cutsem E, Von Moos R, Reyes-Rivera I, Bendahmane B, Kubicka S. Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized phase III intergroup study (TML study) J Clin Oncol. 2012;30(Suppl):CRA3503. [Google Scholar]
- 36.Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ, Koeppen H. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 37.Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 38.Guerrero S, Casanova I, Farrè L, Mazo A, Capellà G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6756. [PubMed] [Google Scholar]
- 39.Bruera G, Cannita K, Di Giacomo D, Lamy A, Frébourg T, Sabourin JC, Tosi M, Ficorella C, Ricevuto E. Worse prognosis of KRAS c.35 G>A mutant metastatic colorectal cancer (MCRC) patients treated with intensive triplet chemotherapy plus bevacizumab (FIr-B/FOx) BMC Med. 2013;11:59. doi: 10.1186/1741-7015-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]