Abstract
There is a growing demand for off-the-shelf tissue engineered vascular grafts (TEVGs) for replacement or bypass of damaged arteries in various cardiovascular diseases. Scaffolds from the decellularized tissue skeletons to biopolymers and biodegradable synthetic polymers have been used for fabricating TEVGs. However, several issues have not yet been resolved, which include the inability to mimic the mechanical properties of native tissues, and the ability for long term patency and growth required for in vivo function. Electrospinning is a popular technique for the production of scaffolds that has the potential to address these issues. However, its application to human TEVGs has not yet been achieved. This review provides an overview of tubular scaffolds that have been prepared by electrospinning with potential for TEVG applications.
Keywords: electrospinning, tubular scaffolds, vascular grafts, tissue engineering, burst strength, compliance, mechanical properties
1. Introduction
Each year 1.4 million patients in US need arterial prostheses [1]. The available options for replacement of vascular grafts have limited clinical success with a cost of more than US$25 billion [1]. Particularly, the pathologies affecting small and medium sized blood vessels are the primary cause of death [2, 3]. Atherosclerosis is one of fatal diseases that causes build-up of plaques under the intimal layer, reducing the cross-sectional area available for blood flow and thereby resulting in decreased flow of blood to the tissues downstream to the plaque [4]. Eventually, cardiac and peripheral bypass surgeries become necessary, requiring replacement of a segment of blood vessels. In blue baby syndrome, only one of the two ventricles of an infant functions properly, and a “Fontan Operation” becomes necessary [5]. In “Fontan Operations”, an engineered blood vessel is required to connect the right pulmonary artery with the inferior vena cava so that the deoxygenated blood can bypass the heart and travel straight to the lungs. Similarly coronary artery diseases and peripheral vascular diseases very often require the replacement of diseased and damaged native blood vessels [6]. The currently available options for these transplants are autologous grafts (e.g. coronary artery bypass graft with autologous mammary arteries and saphenous veins), allografts (donor/cadaveric), xenografts (e.g. bovine or porcine pulmonary valve conduit), artificial prostheses or synthetic vascular grafts made of expanded polytetrafluroethylene (ePTFE) and polyethylene terephthalate (PET) [7].
The use of autografts and allografts are limited due to the lack of tissue donors, previous harvesting or anatomical variability [8, 9]. Besides, there are concerns about their long-term functionality due to the use of strong detergents and decellularizing agents. Xenografts suffer from their relatively shorter life span. As an example, a bovine or porcine graft may last for up to fifteen years, which is a major issue for pediatric patients, which will require a new implant replacement at every ten to fifteen years interval. Other issues include poor control over physical and mechanical properties, inflammation and calcification [10, 11].
Synthetic prosthetic grafts are rejected within a few months by the immune system of the body if the diameter of the vessel is smaller than 6 mm. This rejection arises from the associated re-occlusion problem caused by thrombosis, aneurysm, and intimal hyperplasia due to mismatch of compliance (compliance is opposite of the stiffness, measured as the strain/expansion or contraction of the graft with force) [8, 10, 12, 13].
Tissue engineering is an alternative approach for creating new vascular grafts. In this approach cells are seeded or encapsulated in scaffolds fabricated from a biodegradable polymer. In tissue engineering, it is anticipated that cells produce extracellular matrix (ECM) while the polymer is degraded gradually creating the intended tissue. Extensive research has been conducted on TEVG over the past few decades, and as a result significant progress has been made in terms of achieving the remodeling of the tissue in the TEHV constructs similar to the native blood vessels, [14–17] as depicted in Figure 1. Several reviews have already been published that discuss about different methods for tissue engineering of vascular grafts [3, 18], design of natural and artificial arteries as well as vascular networks [19], and application of stem cell and other human cells for tissue engineering of blood vessels [20]. Main approaches for fabrication of scaffolds include methods that are based on molecular self-assembly, hydrogels, solvent casting-particulate leaching technique, thermally induced phase separation and electrospinning process. The latter is a versatile technique for fabrication of nano-micro scale fibers, which has a great potential for mimicking the microenvironment of natural ECM. The success of an implantable tissue engineered vascular graft is governed, among other factors, by the development of a scaffold that mimics the ECM [21]. It is well known that in natural tissues the ECM is a three dimensional (3D) network of 50–500 nm diameter structural protein and polysaccharide fibers. Electrospinning has evolved to allow the fabrication of nanofiber scaffolds in this size range and beyond [13].
Fig. 1.
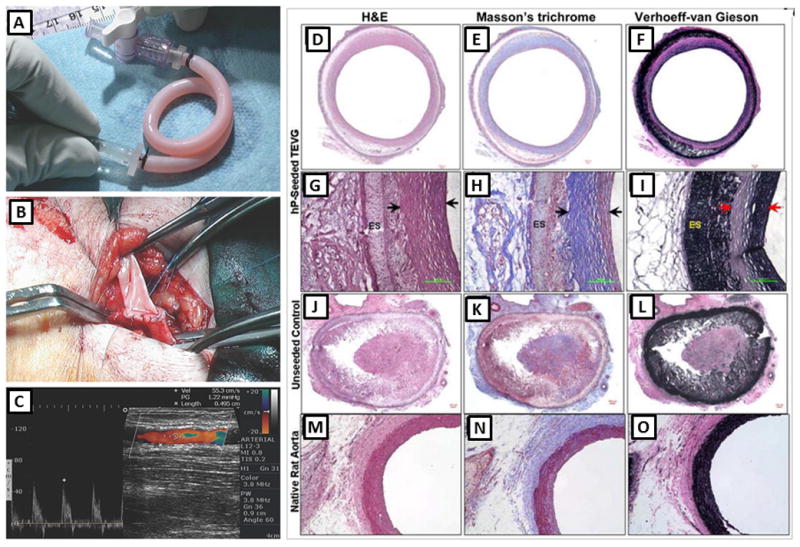
Tissue engineered blood vessels; A–C: the first clinically used sheet-based tissue engineered blood vessel tested on three human patients for application as high pressure arteries, (A) tissue engineered graft was implanted between the axillary vein and the humeral artery as an arteriovenous shunt, (B) the vessel exhibited normal suturing and surgical handling properties, (C) the shunt maintained high flow without signs of aneurysm or restenosis even after 6 months. Reprinted from [14] with permission from Nature Publishing Group. D–O: A comparative histological analysis of human pericyte cell-seeded TEVGs (D–I), unseeded scaffolds (J–L), and native rat aorta (M–O). The Hematoxylin and Eosin (D, G, J and M), (H&E), Masson’s trichrome (E, H, K and N), and Verhoeff-Van Gieson (F, I, L and O) stainings demonstrated remodeling of the tissue in the TEHV construct enriched with collagen and elastin similar to the native aorta. G, H and I are magnified images for the wall cross-section of D, E and F. Arrows indicate the remodeled tissue while ES stands for electrospun scaffold layer. The remaining PEUU material was unspecifically stained in black by Verhoeff-van Gieson stain in I and L. Figures reprinted from [17] with permission from Elsevier Science.
Numerous review articles are available on fundamentals and applications of electrospinning, its historical development, and various modifications [22–30]. These reviews illuminate the capability of electrospinning technique for fabrication of fibers from broad range of polymers including purely natural to synthetic to composites mixtures of ECM analogs and interstitial constituents from organ specific extracts [31–33]. These fibers with nano-micron sized pores and large surface area have been used as catalysts, filtration systems, protective clothing, drug delivery depots, optical wave guides, electronics, and tissue engineering scaffolds [31, 32, 34–39]. The versatility of this technique makes it also possible to process various categories of non-polymeric materials including ceramics and their composite with polymer [34]. Electrospinning is a flexible technique in which the mechanical and biological properties of fibers can easily be tuned by varying the composition of a mixture which is not easily possible in other scaffold fabrication methods.
Due to these advantages, in recent years, the interest in electrospinning technique for the fabrication of tissue engineering scaffolds has increased exponentially. In the current review we present a summary of the most recent application of electrospinning for fabrication of tissue engineered vascular grafts. The scope is limited to small and medium sized blood vessels (diameter ≤ 5 mm), while large blood vessels (diameter > 5 mm), microvasculature and capillary network are beyond the scope of this review. The aspects that are critical for the design of TEVG such as structure of vascular graft and function are also discussed.
2. Structure of Vascular Grafts
The native blood vessels in the human body have complex structures with distinct features. The arterial wall is composed of three different layers: (i) intima, (ii) media and (iii) adventitia, (Figure 2). Intima, the innermost of the three concentric layers, consists of a continuous monolayer of endothelial cells (ECs) directly attached to the basement membrane which is mainly comprised of connective tissues. Media is the middle layer comprised of dense populations of concentrically organized smooth muscle cells (SMCs) with fibers or bands of elastic tissues. Adventitia is consisted of a collagenous ECM that mainly contains fibroblasts and perivascular nerve cells. In smaller arterioles and capillaries some of these layers might be less obvious or absent [3]. An internal and an external elastic lamina separate the intima, media and adventitia from each other. Each layer serves a specific function: the collagenous adventitia functions to add rigidity while the elastic lamina provides elasticity to the vessel walls.
Fig. 2.
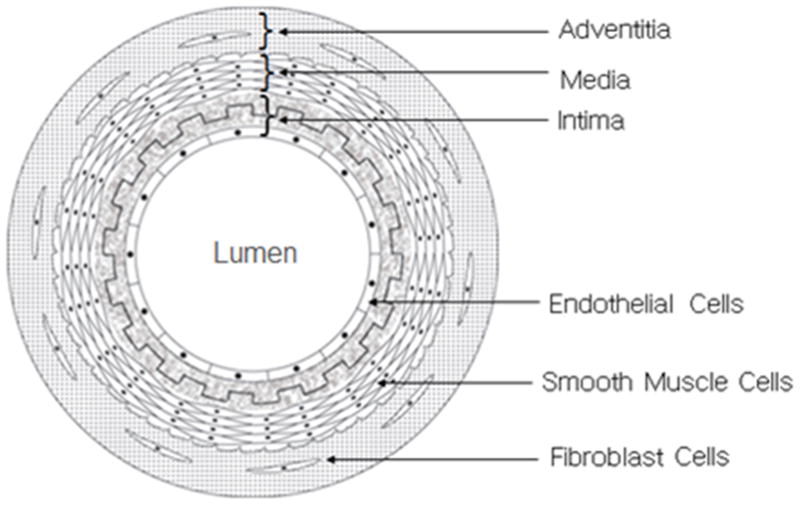
Schematic cross-sectional view of an artery. The arterial wall comprises of three main layers (i) adventitia, (ii) media, and (iii) intima. A layer of endothelial cell covers the internal surface of the lumen while smooth muscle cells and fibroblast cells exist in outer layers.
A confluent monolayer of EC in the lumen of the blood vessel is critical for preventing the clotting of the blood, infection and inflammation of adjacent tissues. The EC monolayer is also important for regulating the gaseous and molecular (oxygen and nutrients) exchange. It also controls the signaling to the media, particularly to its muscular component [4]. The SMCs in the middle layer (media) of the blood vessel walls have a unique contractile function. When the signals from EC or cytokines stimulate the SMCs, the latter cells dilate and contract in a coordinated manner. This leads to the dilation and contraction of the vessels as the pressure in the vessels change. The concentric layer-wise structure of ECM, the spatial organization and alignment of the EC and SMCs, and the interplay between the cells and ECM structures are, therefore, important factors that should be taken into account when designing tissue engineered blood vessels.
3. Functional Requirements for Vascular Grafts
It is crucial that TEVG functions, i.e. gets integrated to the adjacent blood vessels, sustains the load from blood pressure, and allows blood flow without leakage, immediately after implantation. Biocompatibility and bioactivity are other primary requirements for engineering vascular grafts. In addition, the mechanical properties, adhesive ligands, growth factor presentation, transport, and degradation kinetics of the materials used for the scaffold should mimic the relevant ECM environment to a reasonable extent. It is favorable to use cost-effective and cytocompatible materials with tunable properties for a particular tissue [37]. An exact replication of the native tissue structure is not always necessary, however, as a functional construct, it is necessary that an engineered vascular graft fulfills certain basic criteria [3, 14]. For example the burst strength above 260 kPa is required for a TEVG [6, 14], to prevent rupture due to variation in blood pressure. In addition, adequate elasticity is crucial to withstand cyclic loading in which no dilation occurs in constructs within a month of in vitro cyclic loading within physiological ranges [14]. The engineered vascular graft should be compatible with the adjacent host vessel and provide an anti-thrombotic lumen (autologous endothelium) [4]. The ability of the scaffold to provide initial mechanical function for the vascular graft is another important factor, even though the structural and mechanical characteristics of the native vessels are expected to be gradually acquired through remodeling, repair, and growth upon implantation. Finally, it is also important that the implanted vascular grafts minimize intimal hyperplasia and allow for regeneration of arterial tissues.
The uniaxial tensile strength of several native human blood vessels is presented in Table 1. The properties of native grafts, as listed in Table 1, can be used as guidelines to target as properties for tissue engineered grafts. Some safety factors may also be incorporated in the design. There are some apparent discrepancies in literature among the reported values of various mechanical properties for the same tissue by different researchers. For example, the uniaxial tensile values of 43 MPa [40], 4.2 MPa [41], and 2.25 MPa [54] are reported for human saphenous vein in the circumferential direction. This discrepancy is due to the inconsistency in methods for extracting the results from the experimental load-displacement data. The values for the elastic modulus in Donovan et al. [40], for example, was taken as the maximum linear slope of the curve before failure while that in Stekelenburg et al. [41] was taken from the initial linear elastic region of the curve. A reconciliation of literature data is therefore important when comparing experimental results from different groups.
Table 1.
Uniaxial tensile mechanical properties (mean values): Elastic modulus, ultimate tensile stress, tensile strain at failure, and burst strength of some native human blood vessels
| Type of Blood Vessel | Elastic Modulus (MPa) | Ultimate stress (MPa) | Strain at failure (%) | Burst Strength (mmHg) | Ref |
|---|---|---|---|---|---|
| Saphenous Vein (circ) | 43 | 3 | 11 | NA | [40] |
| Saphenous Vein (long) | 130 | 13 | 17 | [40] | |
| Saphenous Vein (circ) | 4.2 | 1.8 | 242 | 1680–3900 | [41] |
| Saphenous Vein (long) | 23.7 | 6.3 | 83 | [41] | |
| Saphenous Vein (circ) | 2.25 | 4 | 180 | 1250 | [54] |
| Left Internal mammery Artery (circ) | 8 | 4.1 | 134 | [41] | |
| Left Internal mammery Artery (long) | 16.8 | 4.3 | 59 | 2000 | [41] |
| Femoral artery (circ) | 9–12 | 1–2 | 63–76 | NA | [108, 109] |
circ: circumferential, long: longitudinal, NA: Not Available
In developing any tissue engineering product including TEVG it is also crucial that researchers take into account the requirements behind the FDA’s approval process to ensure that FDA’s approval can be obtained for releasing the end product in the market and that it can be used in the clinic. Researchers need to contemplate the safety, efficacy, purity and identity of biomaterials in the engineering and design of products.
4. Application of Electrospun Scaffolds in Tissue Engineering of Vascular Grafts
Electrospinning offers the ability to fine-tune mechanical properties during the fabrication process, while also controlling the necessary biocompatibility and structure of the tissue engineered grafts. The ability of electrospinning technique to combine the advantages of synthetic and natural materials makes it particularly attractive for TEVG where a high mechanical durability, in terms of high burst strength and compliance (strain per unit load), is required. In addition, incorporation of natural polymers, with abundance of cell binding sites, can promote the formation of a continuous monolayer of EC in the lumen and proliferation of other cell types in the matrix of the graft’s wall. The electrospinning technique also offers precise control over the composition, dimension, and the alignment of fibers that have impact on the porosity, pore size distribution and the architecture of scaffolds. This method allows for engineering of a wide range of tunable structural and mechanical properties as required for specific applications. Moreover, aligned nanofibers can be used for orienting cells in a specific direction necessary to provide the anisotropy encountered in certain organs including blood vessels. Companies have recently commenced fabrication of electrospun grafts for transplantation of trachea, and other tissue engineered conduits. The world’s first tissue engineered tracheal transplant was successfully used in a clinical trial in Sweden in June 2011 [42].
4.1. Electrospinning Process and Parameters
The electrospinning process is based on stretching of a viscoelastic solution into nano-micro fibers using a high electrostatic force. In-depth reviews about the electrospinning process can be found elsewhere [15–23]. In brief, the material to be electrospun is loaded into a syringe and is pumped at a slow flow rate by a syringe pump (Figure 3a). A high DC voltage is applied to the solution causing a repulsive force within like charges in the liquid. Under the large applied electric field, the tip of the liquid droplet makes a cone shape, also called the Taylor cone. When the applied voltage is high enough to overcome the surface forces acting on the Taylor cone, a narrow jet of liquid generates from the Taylor cone and travels toward the collector. An electrode of either opposite polarity or neutral (grounded) charge is located nearby to attract and collect the fibers. As the liquid jet travels through the ambient toward the collector, the solvent from the fiber jet evaporates and a solid fiber is deposited on the collector. Schematics of three different modified experimental setups of electrospinning method that can be used for fabricating multilayer, composite, and hybrid scaffolds are presented in Figure 3. In one of these setup, a multilayered composite scaffold is prepared by forming an electrospun tubular scaffold using a rotating mandrel as shown in Figure 3a, followed by molding of a concentric layer of hydrogel around the electrospun scaffold. It is also viable to prepare hybrid scaffolds from two types of fibers collected simultaneously by electrospinning using setup in Figure 3b that contains a single mandrel A modified approach has also been developed in which a hydrogel prepolymer is electro sprayed concurrently with the electrospinning of fibers [1, 43] as shown Figure 3c. These modifications allow fabricating scaffolds with multilayer structures, enhancing mechanical and biological properties through the use of hybrid and composite structures. The simultaneous electrospinning - electrospraying approach can be used to combine the advantages of hydrogels and electrospinning, including uniform cell distribution throughout the scaffold, enhanced cell attachment, spreading and proliferation, and improved mechanical properties resulting from electrospun fibers.
Fig. 3.
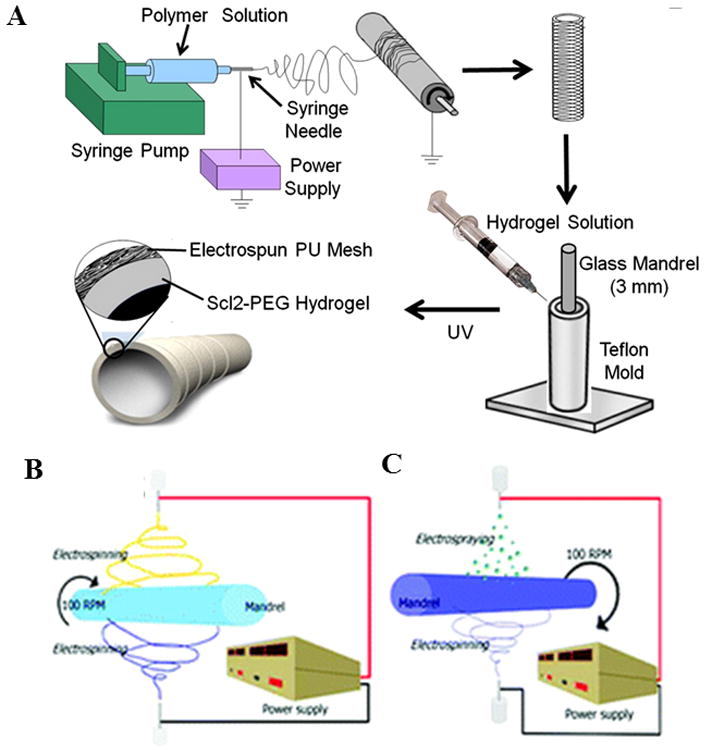
Schematics of various experimental set ups for electrospinning process for fabrication of tubular scaffolds, (A) a multilayer scaffold combining electrospinning and hydrogel fabrication. Adapted from [1] with permission from Elsevier. (B) Co-electrospinning of two polymer solutions concurrently, and (C) electrospinning with simultaneous electrospraying. Adapted from [43] with permission from American Chemical Society.
The morphology of electrospun fibers is affected by various parameters including the density, viscosity, electrical conductivity, molecular weight, surface tension, applied voltage, flow rate, distance of the collector from the tip, and environmental parameters such as humidity and temperature [32, 44–46]. In brief, an increase in the concentration of solute increases the fiber diameter in a power law relationship, which in turn enhances the porosity. As an example, micron size fibers generate a more porous scaffold compared to nano fibers. Similar trends are observed for the effect of polymer molecular weight and viscosity, raising these parameters also increases the fiber diameter, and hence the pore size. However, prior to fiber formation it is critical to determine the range for each of these variables for the formation of uniform, continuous and stable fibers.
An increase in the electrical conductivity of solution generally decreases the fiber diameter. Contradicting results were reported for the effect of applied voltage [32]. While some researchers reported no significant difference in fiber diameter with variation of applied voltage [47], others reported an increase or decrease of size with increase in applied voltage [48]. An increase in flow rate increases the fiber diameter. However, a lower flow rate is commonly used to ensure that the solvents evaporate from fibers during the process. These data demonstrate that by controlling process parameters it is possible to tune the fiber characteristics, hence the mechanical properties. Various studies have been performed to create electrospun scaffolds with sufficient modulus and strength for the engineering of vascular graft that are described in the following section.
4.2. Electrospun Tubular Scaffolds
Electrospinning technique has been widely used in fabrication of scaffolds, however, with a few exceptions, majority of the investigations have been limited to in vitro characterizations. Tubular scaffolds have been electrospun using a broad range of materials as presented in Table 2. These materials include blends of segmented polyurethane (SPU), styrenated (ST)-gelatin, type I collagen, as well as SPU and polyethylene oxide (PEO) [49], and elastin, type I collagen, and poly (D, L-lactide-co-glycolide) PLGA polymers [50]. The latter composite graft possesses superior biocompatibility, favorable tissue composition and attractive mechanical properties particularly for applications in blood vessels [50]. The poly ε-caprolactone (PCL), collagen, PEO, gelatin and Heprasil grafts have also been fabricated [43], however, their mechanical properties have not been reported [43]. The addition of natural polymers significantly improves cellular attachment and infiltration. Zhu et al. [51] demonstrated that aligned PCL fibers coated with fibrin induce the alignment of human smooth muscle cells (hSMCs) along the direction of these fibers. This scaffold promoted SMC modulation into a contractile phenotype and supported their survival and biological function. The electrospun silk fibronin grafts coated with silk sponge [52] have also been utilized for vascular graft applications. A recent report on small-diameter polyurethane (PU) graft [53] is also noteworthy. Composite scaffolds of poly (ester urethane) urea (PEUU) involving electrospinning and TIPS (thermally induced phase separation) [54–56], bilayer scaffolds of PCL-collagen blend [57], and bilayer scaffold of a stiff circumferentially aligned fibrous PLA outer layer with a randomly oriented elastic PCL inner layer scaffold [58] have also been fabricated using electrospinning. These studies demonstrate the feasibility of fabricating strong and pliable electrospun scaffolds with a multilayer structure similar to that of native blood vessels. Another work using chitosan-collagen-Thermoplastic polyurethane (TPU) nanofiber scaffolds crosslinked by glutaraldehyde (GTA) vapor [59] showed flexible mechanical properties with a high degree of tensile strength, and satisfactory biocompatibility for Schwann cells and endothelial cells. Finally, multi-layered (type I collagen, St-gelatin, segmented polyurathane and poly(ethylene oxide) scaffold [33] present interesting, novel and recent approaches for tissue engineered grafts. Besides, a series of studies also investigated the incorporation of various specialized cells such as macrophages [60] and mast cells [61] in electrospun scaffolds, particularly highlighting the bioresorbable properties of certain polymers, and their applicability in fabrication of vascular grafts promoting the remodeling and regeneration of ECM. Apart from some exceptions, most of these constructs, however, have not been tested in vivo to assess their performance in animals and clinical settings.
Table 2.
A summary of conditions for fabrication of electrospun scaffold based TEVG, including the composition, molecular weight and concentration of solutes, the solvent composition, the mandrel specification and process parameters
| Electrospun Material | Mandrel | Process Parameters | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Material Composition | Molecular Weight (daltons) | Solvent | Concentration (%) | Material | Diameter | Speed (rpm) | Voltage (kV) | Flow rate (ml/hr) | Needle- mandrel distance (cm) | Needle Size (gauge) | |
| Blends of rhTE(5–9%), PCL, (1–5 %) | 70,000–90,000 | HFP | 10 | N/A | 2.8 | N/A | N/A | N/A | N/A | 18 | [8] |
| PLLACL (70:30) | 150,000 | DCM/DMF | 10 | Metallic * | 3 | 150 | 10 | 1 | 10 | N/A | [62] |
| PEG-Sc12 hydrogel with electrospun PEU | 3,400–6,000 | DMAC | 15 | Copper | 4 | N/A | 14 | 1.0 | 35 | 20 | [1] |
| rhTE | N/A | HFP | 15 | Copper SS |
10 4 |
4400 | 18.5 | 2 | 12.5 | 18 | [10] |
| Collage-chitosan-TPU | N/A | HFP/TFA | 6 | AL foil | 6 cm | 4000 | 18 | 1 | 12–15 | N/A | [59] |
| PCL: Silk | 100:0 and | HFP | 65 | AL | 4 | 500, 8000 | 30 25 |
6 | 15 | 18 | [67] |
| PDO: Silk | 50:50 (v:v) | 4 | |||||||||
| PCL | N/A | DMF/Chloroform | 10, 25 | SS | N/A | 400–3000 | 10 | 1.0 | 10 | N/A | [51] |
| PCL (PEUU in HFIP) | N/A | HFIP | 8 | N/A | 4.0 1.0 |
or 250 | 13 | 1.0 | 7 | N/A | [54] |
| Type I collagen, PEO, SPU and ST- gelatin (mPCL/Col) with PEO, or Gelatin, or Heprasil. | N/A | HFIP/THF/Chloroform | 12.5, 10, 5.0 | SS | 3 | 100 | 7.0 30.0 25, 15 |
– 3 | 5.0 30.0 |
– N/A | [49] |
| N/A | HFIP | 20 | N/A | N/A | 100 | 9 | 4.0 | 6 | 19 | [43] | |
| PGA | 67, 100, 143 mg/ml | HFP | N/A | SS | 2 | 500 | 22 | 8–14 | 28 | 18 | [64] |
| PU silk fibroin (from B. Mori) | N/A | HFP | 5 | AL | 4 | 35000 | 12 | 0.8 | 12 | 18 | [53] |
| N/A | HFIP | 3,8 | AL/teflon | 3 | 8 m/min | 20 | 9×10^−3 cm/min | 6 | 20 | [52] | |
| prTE | N/A | HFP | 15 | copper SS |
10 4 |
4400 | 18.5 | 2 | 12.5 | 18 | [10] |
| Biosyn | N/A | HFP | 5, 8, 10, 13, 15, 20 | SS | 4 | 300–3125 | 22 | 1.5 | 22 | 27 | [110] |
HFP: 1,1,1,3,3,3-hexafluro-2-propanol, DCM: dichloromethane, DMF: N,N-dimethylformamide, DMAC: dimethylacetamide, TFA: trifluoroacetic acid, HFIP: 1,1,1,3,3,3-hexafluroisopropanol, THF: tetrahydrofuran, PCL: Poly Capro Lactone, PLLACL: Poly-L-Lactic acid-co-poly-ε-caprolacton, PEG: Polyethylene glycol, PEU: poly (esterurethane), TPU: Thermoplastic polyurethane, PDO: polydioxanone, PEUU: poly (esterurethane) urea, PEO: Polyethylene oxide, PU: poly urethane, prTE: porcine recombinant tropoelastin, SS: Stainless Steel, AL: Aluminum
Acellular electrospun scaffolds have also been used in vivo by Wei et al. [62], Wise et al. [8] and Soletti et al. [63]. Wei et al. [62] fabricated tubular poly-L-lactic acid-co-poly-ε-caprolacton (PLL-CL) scaffolds of 3 mm diameter and implanted them in rabbits for a period of 7 weeks. The structural integrity of these scaffolds was maintained and the grafts showed patency. They also incorporated ECs, obtained from human coronary artery, on the collagen coated scaffolds. Results showed that the employed ECs distributed evenly and spread well throughout the lumen of the scaffolds over 1 to 10 days after seeding [62].
Wise et al. [8] engineered a recombinant human tropoelastin/PCL conduit by electrospinning a 10% total concentration of tropoelastin and PCL solution in 1,1,1,3,3,3-hexafluro-2-propanol. They tailored the mechanical properties of the scaffolds to mimic the elastic modulus, compliance, permeability, and burst pressure of human internal mammary artery. Cellular attachment and proliferation behavior were investigated using human umbilical vein endothelial cells (HUVECs). The acellular graft was also implanted in a rabbit model and explanted after one month followed by post implant mechanical characterization. Enhanced vascular compatibility with reduced platelet attachment and increased endothelialization was observed for electrospun synthetic elastin/PCL conduits compared to elastin free synthetic (PCL) graft. The results showed that the addition of tropoelastin significantly enhanced EC attachment and proliferation. The addition of 5–9% tropoelastin to 5–1% PCL, maintaining a 10% (w/v) total solution concentration, allowed development of TEVG that mimicked the innate vascular grafts properties including elastic modulus, compliance, permeability, and burst pressure of those of human internal mammary artery.
Soletti et al. [63] implanted acellular PEUU grafts in adult rat models as aortic interposition grafts for up to 24 weeks. The inner lining of the grafts was coated with a non-thrombogenic 2-methacryloyloxyethyl phosphorylcholine copolymer. The results showed that the phospholipid coated grafts exhibit significantly decreased platelet adherence and improved patency as compared to the uncoated PEUU grafts. The mechanical properties of the grafts were also compatible with native arterial conduits.
Boland et al. [64], and Valence et al. [65] performed animal studies on cell seeded electrospun scaffolds. Boland et al. [64] fabricated PGA (Polyglycolic acid) scaffolds composed of small diameter fibers through electrospinning and attempted to improve their biocompatibility using acid pre-treatment. Rat cardiac fibroblast cells were seeded into the scaffolds and rat intramuscular implantation was used for assessing the in vivo host response of scaffolds. The results showed improved biocompatibility of PGA as a result of acid pretreatment. In addition, Valence et al. [65] evaluated nano-fiber and micro-fiber based PCL grafts in a rat model of abdominal aortic replacement for up to eighteen months. They evaluated the grafts for compliance, thrombosis, patency, material degradation and tissue regeneration. Reported results showed rapid endothelialization, and good mechanical properties, and patency. However, the regeneration of the vessels wall was poor.
Vorp and colleagues conducted multiple in vivo studies using cell seeded composite bilayer PEUU tubular constructs as aortic interposition grafts in rats [17, 55]. One of their studies [55] involved muscle-derived stem cells obtained from Lewis rat while another one [17] involved use of human pericytes. In both studies in vivo morphological and angiographic analysis revealed higher patency for the cell seeded grafts compared to their acellular counterparts. Histological studies showed evidence of tissue regeneration on the luminal side of the grafts, mimicking that of real blood vessel structure. The stem cells based graft was also shown to be mechanically suitable for such systemic circulations. Despite these encouraging results from the preclinical studies, it is too early to comment on the full potential of these grafts in clinical applications. Future studies should involve longer duration of implantation as well as well as more rigorous functional evaluations.
4.3. Mechanical and Biological Properties of Electrospun Scaffolds
A summary of mechanical properties including uniaxial tensile properties and burst strength of electrospun tubular scaffolds is presented in Table 3. In addition to uniaxial tensile properties the burst strength and compliance are also among the important mechanical properties for vascular grafts. However, these latter properties are rarely measured and reported. The mechanical properties of scaffolds are controlled by changing various microstructural parameters such as fiber diameter, porosity, alignment as well as the ratio and spatial distribution of constituent fibers in the case of hybrid scaffolds. In addition, controlled variations of mechanical properties of scaffolds [57] have been used to regulate cell survival, migration and proliferation during tissue formation. For example, Ju et al. [57] used a co-electrospinning technique to fabricate PCL/collagen bilayer scaffolds with an outer layer containing large pores that enhanced SMC infiltration and an inner layer with smaller pore sizes that facilitated EC attachment (Figure 4A–G). Thus the microstructure and mechanical properties of the resultant scaffolds were controlled by the fiber diameter. Increasing the fiber diameter from 0.27 μm to 4.45 μm enhanced the scaffold’s porosity and reduced its Young’s modulus from 2.03 MPa to 0.26 MPa. The fabricated bilayered scaffold supported the infiltration and growth of SMCs within the outer layer and endothelialization of EC in the inner layer on the surface of lumen [57]. In another study, Chen et al. [66] fabricated electrospun collagen/chitosan/TPU nanofibrous scaffolds with functional and structural properties resembling the native ECM where chitosan and collagen were used to provide better cell friendliness, and TPU was used to improve the mechanical properties of the resultant material. The results revealed that the orientation of fibers in these composites influenced the mechanical properties of scaffolds. The tensile strength of scaffolds with random and aligned fibers was 4.6 MPa and 10.3 MPa, respectively. In addition, the mechanical properties of resultant scaffolds were significantly improved by crosslinking the fibers. The tensile strength of glutaraldehyde (GA) crosslinked scaffold with random fibers was 2-fold higher than uncrosslinked samples. The fabricated scaffolds could support the growth and alignment of ECs and were used to form a vascular graft in vitro [66]. Similarly, McClure et al. [67] fabricated tubular scaffolds of aligned and non-aligned fibers composed of different compositions of PCL/polydioxanone (PDO)/silk fibronin. The fiber alignment was controlled by the variation of rotational speed and diameter of mandrels, which resulted in the formation of scaffolds with varying mechanical properties. It was found that increasing the mandrels rotational speed enhanced the stiffness and burst strength of fabricated scaffolds [67]. The tubular scaffolds of aligned PCL/fibrin were also fabricated by Zhu et al. [51]. In their study, aligned PCL fibers were coated with fibrin and the composite constructs were used to align hSMCs along the direction of fibers and to promote SMC modulation into a contractile phenotype. It was found that the aligned PCL/fibrin composite could support the survival, orientation and biological function of hSMCs. In another study, small-diameter vascular grafts were made by coating electrospun silk fibronin with a silk sponge to reinforce the scaffold [52]. Both the circumferential and longitudinal tensile strengths were found to be higher in coated electrospun silk fibronin scaffolds compared to uncoated samples (1.7 MPa and 4.5 MPa compared to 2.9 MPa and 6.6 MPa, respectively). In addition, the results demonstrated that the addition of silk sponges decreased the water permeability of electrospun silk fibrous scaffold within the range suitable for endothelialization [52]. In another variation, Lorenzo et al. [54] fabricated bilayered elastomeric PCL/PEUU scaffolds, containing a highly porous inner layer for cell infiltration and a fibrous reinforced electrospun outer layer, for fabrication of small size vascular grafts. The resultant scaffold had elastic modulus of 1.4 MPa and ultimate tensile stress of 8.3 MPa, which resembled the mechanical properties of native vessels [54]. The fabricated scaffolds also supported the growth and proliferation of stem cells isolated from human skeletal muscle cultured under dynamic conditions for 7 days [54].
Table 3.
Mechanical properties of various electrospun scaffolds for TEVGs including Young’s modulus, ultimate tensile stress, strain to failure and the burst pressure
| Material | Youngs Modulus (MPa) | Ultimate Tensile Stress (MPa) | Strain to failure (%) | Burst Pressure (mmHg) | Ref |
|---|---|---|---|---|---|
| PLLA | 40 | [111] | |||
| PLLA-NaCl | 6 | ||||
| PCL (circ) | 3 | 15 | 300 | [54] | |
| PCL (long) | 4.50 | 17.5 | 250 | ||
| Biosyn® dry 13% (circ) @300rpm | 30 | 7.7 | [110] | ||
| Biosyn® dry 13% (long) @300rpm | 14 | 1750 | |||
| Biosyn® dry 20% (circ) @300rpm | 7.9 | ||||
| Biosyn® dry 20% (long) @300rpm | 10 | 3000 | |||
| Biosyn® dry 13% (circ) @3125rpm | 7 | ||||
| Biosyn® dry 13% (long) @3125rpm | 15 | 2500 | |||
| Biosyn® dry 20% (circ) @3125rpm | 8 | ||||
| Biosyn® dry 20% (long) @3125rpm | 12 | 2600 | |||
| PCL/Collagen, 5% (fiber dia:0.27 μm) | 2.03 | 3.13 | 90 | [57] | |
| PCL/Collagen, 10% (fiber dia:1 μm) | 0.58 | 2.03 | 142 | ||
| PCL/Collagen, 15% (fiber dia:2.4 μm) | 0.45 | 0.71 | 211 | ||
| PCL/Collagen, 15% (fiber dia:4.4 μm) | 0.26 | 0.75 | 734 | ||
| PCL @500rpm | 0.4 | 2 | 175 | 2000 | [67] |
| PCL @8000rpm | 0.5 | 5 | 125 | 3000 | |
| PCL:Silk @500rpm | 0.4 | 1 | 75 | 1000 | |
| PCL:Silk @8000rpm | 0.6 | 2 | 150 | 1900 | |
| PDO @500rpm | 17 | 11 | 150 | 1000 | |
| PDO @8000rpm | 12 | 4 | 75 | 3000 | |
| PDO:Silk @500 | 0.25 | 1 | 100 | 600 | |
| PDO:Silk @8000 | 0.3 | 2 | 125 | 1100 | |
| P(LLA-CL) (long) | 16 | 7 | 289 | [62] | |
| P(LLA-CL) (circ) | 16.6 | 3.90 | 292 | ||
| Silk Fibronin (circ) | 1.7 | 1.74 | [52] | ||
| Silk Fibronin (long) | 4.94 | 2.76 | |||
| Silk Sponge Coated Silk Fibronin (circ) | 2.91 | 3.28 | |||
| Silk Sponge Coated Silk Fibronin (long) | 6.63 | 3.83 | |||
| PLGA random | 134.5 | 2.60 | 14 | [112] | |
| PLGA aligned | 40.4 | 2.10 | 26 | ||
| CCTPU random non-crosslinked | 4.64 | 61 | [59] | ||
| CCTPU random crosslinked | 9.38 | 99 | |||
| CCTPU aligned parallel non-crosslinked | 10.3 | 30 | |||
| CCTPU aligned parallel crosslinked | 14.9 | 59 | |||
| CCTPU aligned perpendicular non-crosslinked | 2.11 | 70 |
circ: circumferential, long: longitudinal, PLLA: Poly-L-Lactide Acid, NaCl: Sodium Chloride, hSV: human saphenous vein, pIMA: porcine Internal mammary artery, PLGA: poly(lactic-co-glycolic acid), CCTPU: collagen–chitosan–thermoplastic polyurethane
Fig. 4.
A PCL/collagen bilayer electrospun scaffold. (A) Macrostructure of the scaffold; (B) fluorescent images of the scaffold; (C–E) SEM images of different layers of the scaffold, (C) outer layer, (D) bilayer structure, and (E) interface between the inner and outer layers; (F–G) fluorescent images of EC and SMC seeded scaffold (F) EC seeded inner layer (green: CD31 expression), indicating the formation of an EC monolayer and (G) SMC seeded outer layer (red: α-SMA expression), demonstrating SMC infiltration into the outer layer (scale bar in F and G: 500 μm). Reproduced from [57] with permission from Elsevier.
McKenna et al. [10] fabricated a tubular construct using electrospun recombinant human tropoelastin (rTE) (Figures 5A–E). The electrospun scaffolds were formed by using 1,1,1,3,3,3-hexafluro-2-propanol (HFP) as the solvent and disuccinimidyl suberate (DSS) as the crosslinking agent to stabilize the fibers in aqueous solution. The fabricated scaffold had an elastic modulus in the range of 0.15–0.91 MPa, and an ultimate tensile strength of 0.36 MPa. The results of in vitro studies demonstrated that the fabricated electrospun rTE scaffolds supported EC growth (endothelial outgrowth cells derived from Porcine bone marrow) with typical EC cobblestone morphology following 48 h in culture (Figure 5C, E), demonstrating the suitability of this biomaterial for application in vascular grafts [10].
Fig. 5.
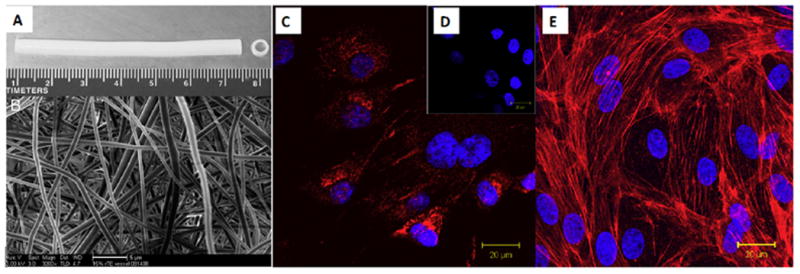
An electrospun tubular scaffold fabricated using recombinant human tropoelastin (rTE), (A) Front view showing the length of the scaffold (7 cm in length, 4 mm inner diameter), (B) The random orientation of the rTE fibers, average diameters: 580 ± 94 nm (scale bar: 5 μm); (C) vWF (red) staining and DAPI (blue) nucleus staining, (D) vWF and DAPI staining of the control sample, (E) The EC monolayer fromed on rTE scaffold (15 wt.%) imaged after 48 hours of culturing. The cytoskeletal actin fiber was stained with rhodamine phalloidin (red) and the nuclei were stained using DAPI. Reprinted from [10] with permission from Elsevier.
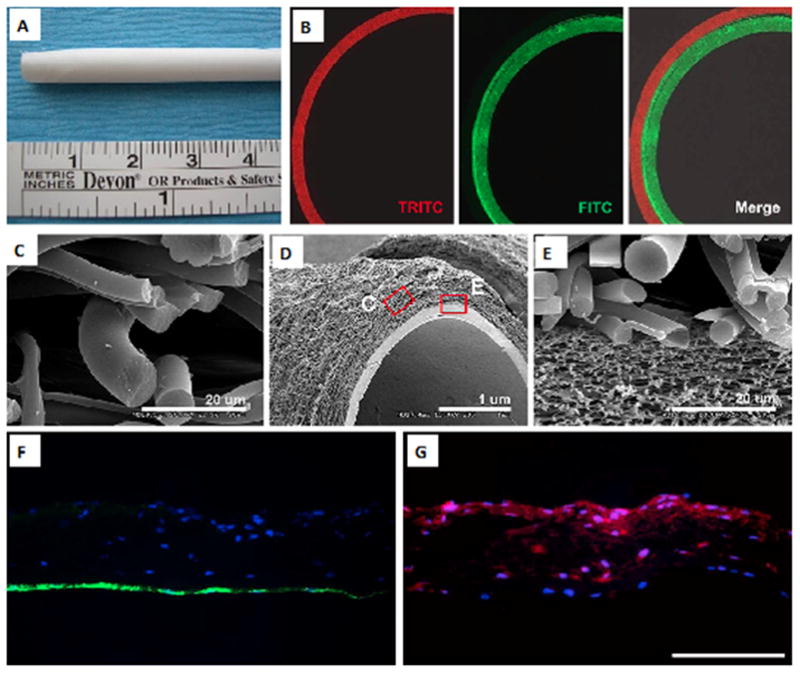
Electrospinning can also be modified to control the alignment of cells using specific surface topographies such as microfibers and microgrooves. For example, Uttayarat and colleagues [53] combined electrospinning with the spin casting method to pattern microfibers and microgrooves on the electrospun polyurethane (PU) tubular scaffold (Figure 6A–F), and observed the orientation of ECs on these micropatterned surfaces. The fabricated micropatterned PU grafts promoted the alignment of EC and formation of cytokine-responsive EC monolayers (Figure 7A–G). Besides, the elastic modulus of the grafts in the axial direction was also in a range similar to those of native vascular grafts.
Fig. 6.
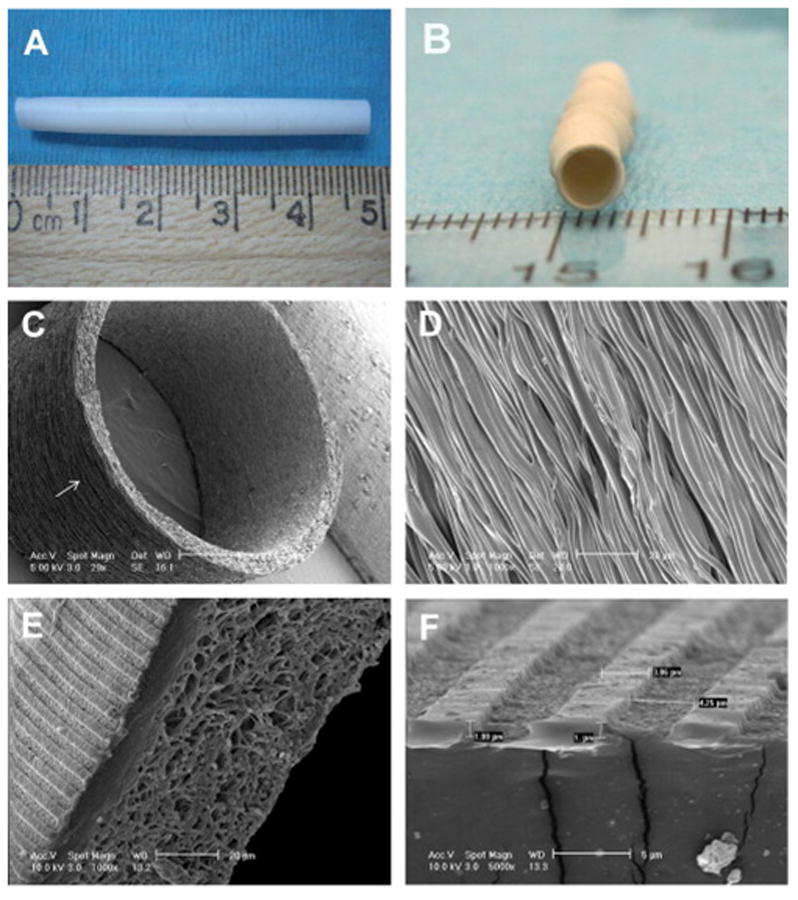
Electrospun PU graft with micropatterns on the luminal surface, (A) length of the graft: 48 mm, (B) diameter of the graft: 4 mm. (C, D) SEM images showing the circumferential alignment of the microfibers (arrow). (D) Enlarged view of the microfibers on the graft exterior. (E, F) SEM images of the hybrid graft showing microgrooves on the luminal surface and mesh of microfibers on the exterior. The dimension in “F” are ridge width = 3.6 ± 0.2, channel width = 3.9 ± 0.1 and channel depth = 0.9 ± 0.03 μm. Adapted from [53] with permission from Elsevier.
Fig. 7.
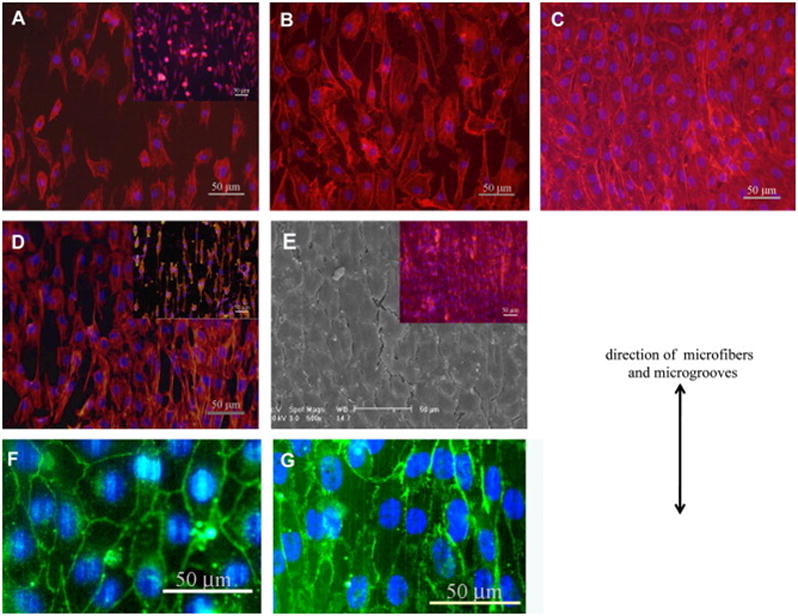
Endothelial cell morphology on the micropatterned scaffold: (A–C) adhesion and spreading of cells inside microfiber patterned electrospun graft. Fluorescently stained cell nuclei (blue) and actin filaments (red) of BAECs or EA.hy926 cells (inset) at (A) 2 hours, (B) 3rd day, and (C) 5th day after seeding. (D, E) adhesion and spreading of cells inside microgrooves patterned hybrid grafts. (D) Fluorescence image of BAECs or EA.hy926 EC (inset) on 3rd day after seeding. (E) The confluent monolayer of BAEC and overlaid fluorescence image of a EA.hy926 EC monolayer (inset). (F, G) VE-cadherin staining (green) of (F) BAEC and (G) EA.hy926 monolayers on microgrooves on 7th day after seeding. The direction of microfibers and microgrooves is shown using the double-headed arrow. Reprinted from [53] with permission from Elsevier.
5. Challenges with Electrospinning and potential solutions for its application in tissue engineering Scaffolds
Even though electrospinning process has numerous advantages over other methods, challenges still remain that need to be overcome prior to the clinical use of electrospinning in TEVGs. The major issues associated for the application of electrospun scaffolds, particularly for TEVG include: lack of adequate cell penetration in thick scaffolds due to small pore sizes; poor surface properties that may have negative impact on the cell viability, proliferation and growth; shortage of favorable cells [4, 68]; poor control over mechanical properties and degradation; and biological response of the tissues/cells in the body to TEVGs. Here we briefly discuss the potential approaches for overcoming some of these challenges.
5.1. Ensuring adequate cell penetration by increasing the pore size and porosity
Cellular infiltration and factors such as diffusion of metabolites, nutrients, and waste are often limited due to the small pore size of electrospun scaffolds. For synthetic polymers such as polyurethane [69], PLGA [70], or for natural proteins such as gelatin [71], pore size and porosity in general are linked to cellular activity in many different types of cells [72–74]. The pore density and pore size of electrospun scaffolds can be controlled by adjusting process parameters during fiber production [71, 75]. Numerous groups have found that factors affect the diameter of fiber also increase the pore size [49, 76]. However, nano sized range promotes cell adhesion and proliferation as opposed to fibers with a larger diameter [77, 78]. Recent reports have shown that the pore size of the fibers do not have a correlation with the pore diameter as it was initially believed. Soliman et al. observed that among three different PCL fiber diameters of 0.3, 2.6 and 5.2 μm, immortalized murine mesenchymal stem cell (mTERT-MSC) adhesion and viability was greater on 2.6 μm diameter unlike the expected larger diameter counterpart [79].
Apart from controlling the physical parameters during fiber production, post processing techniques have also been investigated. Some of these methods have been adopted from hydrogel fabrication techniques [80]. One such method of increasing the porosity is salt leaching or sacrificial leaching method. In this method, salt particles or some other type of sacrificial polymers are initially added and dissolved or leached out after the creation of polymer network [81]. However, the major drawbacks of this method include inability to precisely control the delivery of salt particles, hence producing non-uniform distribution of pores resulting in heterogeneous cell spreading. Instead of dissolving the sacrificial polymers or particles that are introduced or co-electrospun, cryogenic electrospinning involves lyophilizing in which ice crystals are introduced during the electrospinning process producing pore sizes from 10 to 500 μm [82]. In this method, a controlled humidity fiber collection device maintained at low-temperature allows the deposition of ice particles and polymer nano-microfibers concurrently. The embedded ice particles in the scaffold serve as templates for pores. The method was successfully applied to electrospinning of polyesters and polyurethanes suggesting a broad range of applicability for the method, and its non-dependence on material type. It is important to note that new and standardized techniques are required for measurement of pore size of electrospun scaffolds to properly quantify porosity and pore size distribution.
5.2. Uniform Cell Distribution by a Hybrid Method: Electrospinning with co-Electrospraying of Cells
Traditionally, electrospun scaffolds are fabricated by electrospinning followed by cell seeding on the surface of the scaffold. However, cell infiltration into the scaffold is relatively poor. To overcome this challenge a hybrid approach has been developed that involves integration of these two steps where the cells are seeded at the same time as the fibers are formed [68, 83]. Stankus et al. [68] employed electrospraying of vascular SMCs simultaneously with electrospinning of elastomeric PEUU in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) solvent. To evaluate the potential cytotoxic effect on cells, they tested two different methods for spraying the SMCs using: (i) a nozzle under pressure, and (ii) electro-spraying. While a significant decrease in the viability resulted from spraying SMCs through the nozzle, no significant reduction was observed in cell adhesion, viability, spreading and proliferation due to the electro-spraying process. The low cell viability in the former process was attributed to the presence of high shear force and exposure to an organic solvent. Other researchers have also reported a negative impact of aerosol spraying on cell viability as a result of nozzle diameter, spray pressure, and solution viscosity [84]. Furthermore, Stankus et al. [68] developed a new approach to overcome these issues in which cells were first suspended in 3 wt% bovine skin gelatin prior to spraying. The addition of gelatin to the media also increased the viscosity of the solution; therefore, minimized the negative impact of shear force on cells. Their design was based on their hypothesis that the repulsion between electrospraying streams and positively charged electrospun fibers prevents cells from exposure to a solvent prior to deposition. In addition, due to the use of a relatively large tip-to-collector distance (e.g. 23 cm), the PEUU electrospun fibers were solvent- free by the time they deposited [68] on the collector. Their results showed that the cell viability was improved; however, the gelation within the fiber network caused the disruption of mechanical integrity of the PEUU matrices.
In another study, López-Rubio et al. [83] successfully incorporated strain B. animalis Bb12 bacteria in electrospun fibers of poly(vinyl alcohol) PVA (molecular weight: 146,000–186,000) during the electrospinning process. Even though this latter study is not directly relevant to fabrication of TEVGs, it does prove the feasibility of the hybrid method for simultaneous seeding of living cells during electrospinning of scaffolds. The authors, in this latter study, used distilled water as the solvent for PVA, and skimmed milk as the suspending medium for B. animalis Bb12 cells. A coaxial stainless steel needle arrangement was used where the B. animalis Bb12 cells were pumped through the inner needle (diameter: 0.8 mm), and the PVA solution was pumped through the outer needle (diameter: 1.5 mm). The process parameters, i.e. the flow rate, the voltage, and the distance of the collector from the needle tip were set at 60 μl/h to 300 μl/h, 11 kV, and 13 cm respectively. For SEM observation, the electrospun fibers were dried overnight at 38 °C while for viability testing aliquots of the PVA/Bb12 fibers were pre-stored at three temperatures (−20 °C, 4 °C and 20 °C) and the viability tests were performed for several months. The results showed that the cells were maintained viable even after 40 days and 130 days storage at room and low temperatures, respectively.
These studies demonstrate the feasibility of using in situ cell seeding in the electrospinning process, without any apparent adverse effect of osmotic change and electrostatic field generated during the process on cell viability. Further research will be needed to determine the optimum conditions for each new type of cells, polymers and solvent systems and to achieve the desirable physical properties for fibers with high cellular viability. In addition, in the design of these electrospinnning systems extra precautions will be necessary for establishing a sterile environment to prevent contamination and its negative impact on cell viability. Despite these issues, in situ cell seeding during the electrospinning has significant potential, although a concerted effort is needed for addressing the current challenges associated with this technique to broaden its applications in the future.
5.3. Creating cell friendly surface properties
Even though the commonly used polymers in electrospinning present favorable mechanical properties, usually their cell adhesion, spreading, migration and proliferation properties are relatively poor. These poor surface properties arise from the fact that many solvents used in the electrospinning process are cytotoxic and they make the surfaces of the scaffolds non adhesive to cells. Fortunately, the toxic effect of the solvent can be removed by completely removing the solvent and the surface properties can be improved by applying some bioactive molecules or other types of treatments. Common approaches to modify the surface of nanofibers include (1) plasma treatment, (2) wet chemical modification, (3) surface graft polymerization, and (4) co-electrospinning of surface active agents with polymers [85].
Plasma treatment of polymer substrates temporarily changes the surface chemical composition of the electrospun fibers. By selecting nitrogen, oxygen or air plasma, it is possible to introduce diverse functional groups that can generate carboxyl or amine reactive groups. Subsequently, various types of ECM components such as elastin, gelatin or collagen can be adhered to the surface, which in turn enhances cell attachment [86]. The disadvantages of plasma treatment include the relatively high cost and poorly defined chemistry, the need for a vacuum environment, and the difficulty in generating plasma in small pores and within long, small-diameter tubing. Besides, the effect of plasma treatment in terms of increasing the wettability/hydrophilicity or other types of surface functionalization remains effective only for a few minutes from the time of plasma treatment. The modified surface must be bonded to some other components within a few minutes from the treatment.
A number of groups have used wet chemistry to induce surface modifications on nanofiber scaffolds [87, 88]. This usually involves acidic or basic treatment that causes partial hydrolysis of the scaffold surfaces. Most commonly these modifications alter surface wetting properties and create new functionalities [89]. However, in this case, the parameters such as concentration of hydrolyzing agents and duration of the hydrolysis need to be closely monitored as not to change the bulk material properties. This approach modifies the scaffolds by chemical scission of ester linkages that is random, and that offers flexibility for modification of the surfaces of thick nanofibrous meshes. Zhu et al. reported that wettability modification followed by immobilization of bio macromolecules on electrospun PCL membrane improved the biocompatibility of scaffolds for human ECs [90].
Another technique for scaffold modification is based on surface graft polymerization [91]. The method is mostly based on creation of free radicals on the surface using UV radiation or plasma treatment to help in polymerization. This approach alters surface hydrophilicity. Recently however, more applications have been geared towards attaining multi-functional groups on the surface, which could then be used for further covalent attachment of biologically relevant molecules [92–94]. In a study, Ma et al. utilized the graft polymerization method to immobilize gelatin on the nanofiber surfaces without causing structural damage to the bulk phase [92]. They observed that the surface density of carboxylic acid correlated with increasing reaction times and the monomer concentration of electrospun polyethylene terephthalate (PET) nanofibers.
An alternative method is based on co-electrospinning of a second cell friendly material with the main bulk polymer as a means of surface modification [95, 96]. Similarly, specific nanoparticles and/or relevant polymer segments can be directly conjugated to the scaffold surface during the electrospinning process. Other non-covalent approaches for modifying surface properties of tissue engineered scaffolds often involve physical surface adsorption, adapted from drug loading applications. Most commonly, electrostatic, hydrophobic and van der Waals interactions, play key roles in driving molecular surface adsorption [97]. These methods have been utilized as a way to benefit from favorable growth factor and cellular interactions [98, 99]. However, in tissue engineering applications, chemical immobilization of biologically active molecules is favored over physical immobilization since they are not easily leached over prolonged periods of time. On the other hand, direct conjugation can pose several limitations most notably due to sterically buried bioactive molecules within the mesh structure. This limited exposure prevents cells from easily recognizing the biological signals and ligands inhibiting favorable cell adhesion and proliferative properties of engineered scaffolds.
Kim et al. used covalent immobilization of Gly-Arg-Gly-Asp-Tyr (GRGDY), a cell adhesive peptide, and observed enhanced attachment of NIH 3T3 cells where conjugation was achieved via surface-amine groups on PLGA nanofibers [100]. This surface modification of RGD peptide not only significantly improved cell attachment but also resulted in enhanced proliferation. The same type of approach has been developed and applied to covalent conjugation of several growth factors that could induce wound healing. In a report by Choi et al, the authors used recombinant human epidermal growth factor (rhEGF) immobilization with a poly(ethylene glycol) (PEG) linker on PCL nanofibers [101] for wound healing applications.
The study by Araujo et al accomplished improved scaffold properties as a result of calcium phosphate surface functionalization [102]. Similarly, others have employed collagen modification for improving attachment and proliferation of stem cells [103]. Alternatively, electrospun nanofibers can be conjugated to cell adhesion proteins for enhanced cultivation of epithelial cells [104].
One recent report of high interest involved cultivation of ECs on poly(L-lactic acid)-co-poly(ε-caprolactone) (P(LLA-CL)) mesh [104]. The polymeric nanofibrous meshes could either be fabricated as aligned or randomly distributed followed by collagen surface modification. In the case of human coronary artery ECs, proliferation was directed by the direction of the aligned surface-modified nanofibers.
5.4. Sourcing of Required Cell Types: Stem cells in Electrospun Tubular Scaffolds
Another challenge that remains with tissue engineering of blood vessel substitutes is the need for endothelial, smooth muscle and other necessary cell sources [4]. However, stem cells and progenitor cells have emerged as potential solution to this cell source issue. Krawiec et al. [20] recently presented a detailed review on this topic where they discussed the application of (1) bone marrow mono nuclear progenitor cells, (2) messenchymal stem cells and (3) endothelial precursor cells (EPCs) in tissue engineered blood vessels. However, majority of the reported studies have utilized either decellularized tissue scaffolds or other non-electrospinning techniques. Exploring the potential of direct use of stem cells in electrospun scaffolds, and their differentiation into various vascular cells upon seeding on the scaffolds for tissue engineered conduits have the potential to resolve the cell source issue.
One study dealing with human adipose tissue-derived stem cells (hASCs) was reported by Zonari et al. [105]. The authors’ goal was to enhance the formation of a vascular network using differentiated endothelial cells for engineering of bone tissue. They prepared an electrospun mesh from polyhydroxyalkanoates (PHAs) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate (PHB-HV), which were biocompatible and biodegradable polymers meant to help in differentiating stem cells into EC. By culturing in media supplemented with VEGF and bFGF they demonstrated improved differentiation and expression of EC markers, e.g. vWF and VE-Cadherin by using these scaffolds.
In another interesting study, Zhang et al. [106] seeded bone marrow derived MSCs onto electrospun poly (propylene carbonate) (PPC) scaffolds (2 mm lumen diameter) and analyzed them using SEM, and hematoxylin and eosin (H&E) staining. They demonstrated that at day 14 cells integrated well into the scaffolds, leading to the formation of a 3D cellular network implying that MSCs had favorable interactions with synthetic PPC scaffold. Based on endothelial nitric-oxide synthase (eNOS) staining and expression, they concluded that the cells seeded on the inner surface were able to migrate into the scaffold.
Similarly, Centola et al. [107] developed a novel technique that combined electrospinning with fused deposition modeling and fabricated tubular electrospun scaffolds. The new approach allowed them to reinforce the scaffolds using a helical coil of PCL and improve mechanical properties. After seeding with human MSCs the group was able to observe the scaffold’s ability to differentiate the MSCs into vascular EC.
Finally, ensuring the safety, efficacy, purity and consistency in the quality of the TEVG products, like those for any other tissue engineered products would pose numerous challenges. This issue is specifically important for “off-the-shelf” product as in case of “off-the-shelf” cell seeded TEVG, maintaining the viability during the marketing and storage conditions will be challenging. The design of an acellular product might be more convenient and will also take shorter period to receive approval by regulatory agencies such as Food Drug Administration (FDA). It is intuitive that a more convenient method would be to manufacture and market the scaffolds and the cells under separate packaging which could be stored along with suitable bioreactors in various distribution centers of the products, whereby cells can be seeded into the scaffolds and the products can be preconditioned in the bioreactors as necessary, prior to in vivo implantation.
6. Conclusions and Future Work
Electrospinning has become a popular technique for the fabrication of tissue engineering scaffolds, and has a great potential for fabrication of TEVG scaffolds with the required structural and functional complexities. This graft should be comprised of a tri-layer structure with sufficient mechanical strength and elasticity as well as biocompatibility, bioactivity, and antithrombotic properties. Extensive research has been conducted on electrospun scaffolds in recent years. However, a human implantable TEVG with electrospun scaffold is yet to be achieved. There are only a limited number of studies that attempted to assess cell seeded electrospun scaffolds or acellular electrospun scaffolds in animals, and none has approached clinical trials. Although the high cost of animal studies is one of the hurdles, we believe another obstacle that impedes the progress, is the lack of efforts in evaluating the dynamic mechanical performance of scaffolds. In vitro experiments using pulsatile fluid flow loops simulating actual blood flow in vessels can be employed for evaluating the performance of the scaffolds and tissue engineered vascular grafts prior to animal studies to minimize the risk of failure and optimize the composition and properties of TEVG designed by electrospinning of various materials. Computational multi-physics simulations can also be employed to expedite the critical physical characterization prior to in vivo tests. Further research in these directions will enable screening the most promising materials from a broad range of biomaterials. Since the primary requirements for TEVG scaffold design also include the antithrombogenicity and the ability to promote the regeneration and remodeling of vascular tissues, future work should include longer-term implantation with thorough investigations of cytocompatibility, cell-matrix interactions, and ECM production.
The review of available literature reveals that the mechanical properties of scaffolds are often reported in terms of uniaxial tensile or compressive mechanical properties such as Young’s modulus, ultimate tensile strength and ultimate strain. However, for application in vascular grafts, the dynamic mechanical properties of scaffolds are equally or even more important. Therefore, the dynamic mechanical properties including the burst strength, and dynamic compliance as well as dynamic flexure, fatigue behavior, fracture behavior, relaxation and creep of electrospun scaffolds and tissue engineered grafts deserve more attention.
While there are some intrinsic limitations of electrospun scaffolds such as inadequate pore size and poor cell attachment and spreading; these limitations can be overcome by using various post-fabrication surface treatment approaches. Alternatively, it is also possible to overcome some of these limitations using composite and hybrid scaffolds of multiple fibers electrospun concurrently or in multilayer structure. However, it is important to conduct further research to overcome many of these limitations, particularly the issue of increasing porosity with minimal impact on fiber diameter of scaffolds.
Simultaneous co-electrospraying of cells with electrospinning of fibers has been successfully demonstrated and has strong prospects for use in tissue engineering of vascular grafts. We hope that focusing future efforts along this line will result in further advancements in the field. The use of stem cells and progenitor cells in electrospun scaffolds and differentiating them in situ into various vascular cells such as ECs or SMCs can potentially solve the cell sourcing issue, and require further research.
In the development of an “off-the-shelf” TEVG product, researchers need to take into account the requirements behind the FDA’s approval as well as the challenges associated with maintaining the viability and quality of the TEVG product during marketing and shelf life. We believe, it will be convenient to design the product manufacturing in such a way that the scaffolds and the cells can be marketed and stored under separate packaging whereby prior to implantation, the cells can be seeded into the scaffolds and the TEVG products can be preconditioned in vitro as necessary.
In summary, the electrospinning technique has a great potential to meet the needs of a suitable scaffold for TEVG, however further advancements in the field is necessary before a gold standard can be established by using electrospun scaffolds for human implants for medium and small size tissue engineered blood vessels. Tissue engineering is a multidisciplinary field and it is important to involve disparate researchers with knowledge in advance biomaterial, cell biology, and mechanotransduction to develop a mechanically long-lasting and functionally viable TEVG that can be transplanted in human.
Acknowledgments
A.H. acknowledges Natural Sciences and Engineering Research Council of Canada (NSERC) postdoctoral fellowship. N.A. acknowledges the support from the National Health and Medical Research Council. A.P. acknowledges postdoctoral award from Fonds Québécois de la Recherche sur la Nature et les Technologies (FRQS, Canada). A.K. acknowledges funding from the National Science Foundation CAREER Award (DMR 0847287), the office of Naval Research Young National Investigator Award, the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392), and the Presidential Early Career Award for Scientists and Engineers (PECASE). Authors also acknowledge the help of Arash Nasajpour, a summer student in Ali Khademhosseini lab in organizing some of the data in Tables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browning MB, Dempsey D, Guiza V, Becerra S, Rivera J, Russell B, et al. Multilayer vascular grafts based on collagen-mimetic proteins. Acta Biomaterialia. 2011;8:1010–21. doi: 10.1016/j.actbio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Tu JV, Pashos CL, Naylor CD, Chen EL, Normand SL, Newhouse JP, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada. New England Journal of Medicine. 1997;336:1500–5. doi: 10.1056/NEJM199705223362106. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliffe A. Tissue engineering of vascular grafts. Matrix Biology. 2000;19:353–7. doi: 10.1016/s0945-053x(00)00080-9. [DOI] [PubMed] [Google Scholar]
- 4.Stegemann JP, Kaszuba SN, Rowe SL. Review: Advances in vascular tissue engineering using protein-based Biomaterials. Tissue Engineering. 2007;13:2601–13. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolgin E. Taking tissue engineering to heart. Nature Medicine. 2011;17:1032–5. doi: 10.1038/nm0911-1032. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z, Niklason LE. Blood Vessels Engineered from Human Cells. Trends in Cardiovascular Medicine. 2006;16:153–6. doi: 10.1016/j.tcm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Bouten CVC, Dankers PYW, Driessen-Mol A, Pedron S, Brizard AMA, Baaijens FPT. Substrates for cardiovascular tissue engineering. Advanced Drug Delivery Reviews. 2011;63:221–41. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Ng MKC, Weiss AS. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomaterialia. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Veith FJ, Moss CM, Sprayregen S, Montefusco C. Preoperative saphenous venography in arterial reconstructive surgery of the lower extremity. Surgery. 1979;85:253–6. [PubMed] [Google Scholar]
- 10.McKenna KA, Hinds MT, Sarao RC, Wu P-C, Maslen CL, Glanville RW, et al. Mechanical property characterization of electrospun recombinant human tropoelastin for vascular graft biomaterials. Acta Biomaterialia. 2011;8:225–33. doi: 10.1016/j.actbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhoff G, Stock U, Karim N, Mertsching H, Timke A, Meliss RR, et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits - In vivo restoration of valve tissue. Circulation. 2000;102:50–5. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- 12.Klinkert P, Post PN, Breslau PJ, van Bocke JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. European Journal of Vascular and Endovascular Surgery. 2004;27:357–62. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. Journal of Pathology. 2000;190:292–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.L’Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology Insight: the evolution of tissue-engineered vascular grafts - from research to clinical practice. Nature Clinical Practice Cardiovascular Medicine. 2007;4:389–95. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 15.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nature Medicine. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L’Heureux N. Tissue engineering of a completely biological & autologous human blood vessel for adult arterial revascularization. Faseb Journal. 2007;21:A141-A. [Google Scholar]
- 17.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235–44. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClure MJ, Wolfe PS, Rodriguez IA, Bowlin GL. Bioengineered vascular grafts: Improving vascular tissue engineering through scaffold design. Journal of Drug Delivery Science and Technology. 2011;21:211–27. [Google Scholar]
- 19.Shadwick RE. Mechanical design in arteries. 1999:3305–13. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 20.Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials. 2012;33:3388–400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Advanced Drug Delivery Reviews. 2007;59:1413–33. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603–21. [Google Scholar]
- 23.Boudriot U, Dersch R, Greiner A, Wendorff JH. Electrospinning approaches toward scaffold engineering - A brief overview. Artificial Organs. 2006;30:785–92. doi: 10.1111/j.1525-1594.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 24.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. International journal of nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–97. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Burger C, Hsiao BS, Chu B. Nanofibrous materials and their applications. 2006:333–68. [Google Scholar]
- 27.Tan S, Huang X, Wu B. Some fascinating phenomena in electrospinning processes and applications of electrospun nanofibers. Polymer International. 2007;56:1330–9. [Google Scholar]
- 28.Greiner A, Wendorff JH, Yarin AL, Zussman E. Biohybrid nanosystems with polymer nanofibers and nanotubes. Applied Microbiology and Biotechnology. 2006;71:387–93. doi: 10.1007/s00253-006-0356-z. [DOI] [PubMed] [Google Scholar]
- 29.Dersch R, Graeser M, Greiner A, Wendorff JH. Electrospinning of nanofibres: Towards new techniques, functions, and applications. Australian Journal of Chemistry. 2007;60:719–28. [Google Scholar]
- 30.Greiner A, Wendorff JH. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angewandte Chemie - International Edition. 2007;46:5670–703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 31.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907–14. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhardwaj N, Kundu SC. Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances. 2010;28:325–47. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Simpson DG, Bowlin GL. Tissue-engineering scaffolds: can we re-engineer mother nature? Expert Review of Medical Devices. 2006;3:9–15. doi: 10.1586/17434440.3.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Mouthuy PA, Ye H. 5.04 - Biomaterials: Electrospinning. In: Murray M-Y, editor. Comprehensive Biotechnology. 2. Burlington: Academic Press; 2011. pp. 23–36. [Google Scholar]
- 35.Welle A, Kröger M, Döring M, Niederer K, Pindel E, Chronakis IS. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials. 2007;28:2211–9. doi: 10.1016/j.biomaterials.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. Journal of Applied Polymer Science. 2005;96:557–69. [Google Scholar]
- 37.Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma Z, Ramaseshan R. Electrospun nanofibers: Solving global issues. Materials Today. 2006;9:40–50. [Google Scholar]
- 38.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. Journal of Controlled Release. 2003;89:341–53. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 39.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. Journal of Materials Science: Materials in Medicine. 2005;16:1099–104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 40.Donovan DL, Schmidt SP, Townshend SP, Njus GO, Sharp WV. Material and structural characterization of human saphenous vein. Journal of Vascular Surgery. 1990;12:531–7. [PubMed] [Google Scholar]
- 41.Stekelenburg M, Rutten MCM, Snoeckx LHEH, Baaijens FPT. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts. Tissue Engineering - Part A. 2009;15:1081–9. doi: 10.1089/ten.tea.2008.0183. [DOI] [PubMed] [Google Scholar]
- 42.Jungebluth P, Alici E, Baiguera S, Blanc KL, Blomberg P, Bozoky B, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: A proof-of-concept study. The Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 43.Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097–103. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 44.Sill TJ, von Recum HA. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Soliman S, Sant S, Nichol JW, Khabiry M, Traversa E, Khademhosseini A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. Journal of Biomedical Materials Research Part A. 2011;96A:566–74. doi: 10.1002/jbm.a.33010. [DOI] [PubMed] [Google Scholar]
- 46.Gaumer J, Prasad A, Lee D, Lannutti J. Structure-function relationships and source-to-ground distance in electrospun polycaprolactone. Acta Biomaterialia. 2009;5:1552–61. doi: 10.1016/j.actbio.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–23. [Google Scholar]
- 48.Meechaisue C, Dubin R, Supaphol P, Hoven VP, Kohn J. Electrospun mat of tyrosine-derived polycarbonate fibers for potential use as tissue scaffolding material. Journal of Biomaterials Science, Polymer Edition. 2006;17:1039–56. doi: 10.1163/156856206778365988. [DOI] [PubMed] [Google Scholar]
- 49.Kidoaki S, Kwon IK, Matsuda T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials. 2005;26:37–46. doi: 10.1016/j.biomaterials.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 50.Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–94. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Zhu YB, Cao Y, Pan J, Liu YX. Macro-Alignment of Electrospun Fibers For Vascular Tissue Engineering. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2010;92B:508–16. doi: 10.1002/jbm.b.31544. [DOI] [PubMed] [Google Scholar]
- 52.Sato M, Nakazawa Y, Takahashi R, Tanaka K, Sata M, Aytemiz D, et al. Small-diameter vascular grafts of Bombyx mori silk fibroin prepared by a combination of electrospinning and sponge coating. Materials Letters. 2010;64:1786–8. [Google Scholar]
- 53.Uttayarat P, Perets A, Li MY, Pimton P, Stachelek SJ, Alferiev I, et al. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomaterialia. 2010;6:4229–37. doi: 10.1016/j.actbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR, et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomaterialia. 2009;6:110–22. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. In Vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Engineering - Part A. 16:1215–23. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 31:8235–44. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31:4313–21. doi: 10.1016/j.biomaterials.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Vaz CM, van Tuijl S, Bouten CVC, Baaijens FPT. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomaterialia. 2005;1:575–82. doi: 10.1016/j.actbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Huang C, Chen R, Ke Q, Morsi Y, Zhang K, Mo X. Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids and Surfaces B: Biointerfaces. 2010;82:307–15. doi: 10.1016/j.colsurfb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Garg K, Sell SA, Madurantakam P, Bowlin GL. Angiogenic potential of human macrophages on electrospun bioresorbable vascular grafts. Biomedical Materials. 2009:4. doi: 10.1088/1748-6041/4/3/031001. [DOI] [PubMed] [Google Scholar]
- 61.Garg K, Ryan JJ, Bowlin GL. Modulation of mast cell adhesion, proliferation, and cytokine secretion on electrospun bioresorbable vascular grafts. Journal of Biomedical Materials Research - Part A. 2011;97 A:405–13. doi: 10.1002/jbm.a.33073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He W, Ma ZW, Teo WE, Dong YX, Robless PA, Lim TC, et al. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. Journal of Biomedical Materials Research Part A. 2009;90A:205–16. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 63.Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR, et al. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. Journal of Biomedical Materials Research - Part A. 96 A:436–48. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2004;71B:144–52. doi: 10.1002/jbm.b.30105. [DOI] [PubMed] [Google Scholar]
- 65.de Valence S, Tille J-C, Mugnai D, Mrowczynski W, Gurny R, Möller M, et al. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Chen F, Su Y, Mo XM, He CL, Wang HS, Ikada Y. Biocompatibility, Alignment Degree and Mechanical Properties of an Electrospun Chitosan-P(LLA-CL) Fibrous Scaffold. Journal of Biomaterials Science-Polymer Edition. 2009;20:2117–28. doi: 10.1163/156856208X400492. [DOI] [PubMed] [Google Scholar]
- 67.McClure MJ, Wolfe PS, Simpson DG, Sell SA, Bowlin GL. The use of air-flow impedance to control fiber deposition patterns during electrospinning. Biomaterials. 2009;33:771–9. doi: 10.1016/j.biomaterials.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Stankus JJ, Guan JJ, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Tienen TG, Heijkants RGJC, Buma P, De Groot JH, Pennings AJ, Veth RPH. Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002;23:1731–8. doi: 10.1016/s0142-9612(01)00280-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X, Cui W, Li X, Jin Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules. 2008;9:1795–801. doi: 10.1021/bm800476u. [DOI] [PubMed] [Google Scholar]
- 71.Powell HM, Boyce ST. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal-epidermal skin substitutes. Journal of Biomedical Materials Research - Part A. 2008;84:1078–86. doi: 10.1002/jbm.a.31498. [DOI] [PubMed] [Google Scholar]
- 72.Sisson K, Zhang C, Farach-Carson MC, Chase DB, Rabolt JF. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. Journal of Biomedical Materials Research - Part A. 94:1312–20. doi: 10.1002/jbm.a.32756. [DOI] [PubMed] [Google Scholar]
- 73.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–76. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 74.Zeltinger J, Sherwood JK, Graham DA, Müeller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Engineering. 2001;7:557–72. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 75.Liao S, Li B, Ma Z, Wei H, Chan C, Ramakrishna S. Biomimetic electrospun nanofibers for tissue regeneration. Biomedical Materials. 2006;1:R45–R53. doi: 10.1088/1748-6041/1/3/R01. [DOI] [PubMed] [Google Scholar]
- 76.Eichhorn SJ, Sampson WW. Statistical geometry of pores and statistics of porous nanofibrous assemblies. Journal of the Royal Society Interface. 2005;2:309–18. doi: 10.1098/rsif.2005.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen M, Patra PK, Warner SB, Bhowmick S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Engineering. 2007;13:579–87. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 78.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 79.Soliman S, Pagliari S, Rinaldi A, Forte G, Fiaccavento R, Pagliari F, et al. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomaterialia. 2010;6:1227–37. doi: 10.1016/j.actbio.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 80.Rnjak-Kovacina J, Weiss AS. Increasing the Pore Size of Electrospun Scaffolds. 2011:110804115837005. doi: 10.1089/ten.teb.2011.0235. [DOI] [PubMed] [Google Scholar]
- 81.Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Engineering - Part B: Reviews. 16:371–83. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simonet M, Schneider OD, Neuenschwander P, Stark WJ. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polymer Engineering and Science. 2007;47:2020–6. [Google Scholar]
- 83.López-Rubio A, Sanchez E, Sanz Y, Lagaron JM. Encapsulation of living bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromolecules. 2009;10:2823–9. doi: 10.1021/bm900660b. [DOI] [PubMed] [Google Scholar]
- 84.Veazey WS, Anusavice KJ, Moore K. Mammalian cell delivery via aerosol deposition. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;72B:334–8. doi: 10.1002/jbm.b.30159. [DOI] [PubMed] [Google Scholar]
- 85.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Advanced Drug Delivery Reviews. 2009;61:1033–42. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Baker SC, Atkin N, Gunning PA, Granville N, Wilson K, Wilson D, et al. Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials. 2006;27:3136–46. doi: 10.1016/j.biomaterials.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 87.Yuan X, Mak AFT, Yao K. Surface degradation of poly(L-lactic acid) fibres in a concentrated alkaline solution. Polymer Degradation and Stability. 2003;79:45–52. [Google Scholar]
- 88.Nam YS, Yoon JJ, Lee JG, Park TG. Adhesion behaviours of hepatocytes cultured onto biodegradable polymer surface modified by alkali hydrolysis process. Journal of Biomaterials Science, Polymer Edition. 1999;10:1145–58. doi: 10.1163/156856299x00801. [DOI] [PubMed] [Google Scholar]
- 89.Croll TI, O’Connor AJ, Stevens GW, Cooper-White JJ. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules. 2004;5:463–73. doi: 10.1021/bm0343040. [DOI] [PubMed] [Google Scholar]
- 90.Zhu Y, Gao C, Liu X, Shen J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules. 2002;3:1312–9. doi: 10.1021/bm020074y. [DOI] [PubMed] [Google Scholar]
- 91.Kou RQ, Xu ZK, Deng HT, Liu ZM, Seta P, Xu Y. Surface modification of microporous polypropylene membranes by plasma-induced graft polymerization of α-allyl glucoside. Langmuir. 2003;19:6869–75. [Google Scholar]
- 92.Ma Z, Kotaki M, Yong T, He W, Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26:2527–36. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 93.Mori M, Uyama Y, Ikada Y. Surface modification of polyethylene fiber by graft polymerization. Journal of Polymer Science, Part A: Polymer Chemistry. 1994;32:1683–90. [Google Scholar]
- 94.Liu ZM, Xu ZK, Wang JQ, Wu J, Fu JJ. Surface modification of polypropylene microfiltration membranes by graft polymerization of N-vinyl-2-pyrrolidone. European Polymer Journal. 2004;40:2077–87. [Google Scholar]
- 95.He W, Yong T, Teo WE, Ma Z, Ramakrishna S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: Potential vascular graft for blood vessel tissue engineering. Tissue Engineering. 2005;11:1574–88. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- 96.Schaub TF, Kellogg GJ, Mayes AM, Kulasekere R, Ankner JF, Kaiser H. Surface modification via chain end segregation in polymer blends. Macromolecules. 1996;29:3982–90. [Google Scholar]
- 97.Yoshida M, Langer R, Lendlein A, Lahann J. From advanced biomedical coatings to multi-functionalized biomaterials. Polymer Reviews. 2006;46:347–75. [Google Scholar]
- 98.McGonigle JS, Tae G, Stayton PS, Hoffman AS, Scatena M. Heparin-regulated delivery of osteoprotegerin promotes vascularization of implanted hydrogels. Journal of Biomaterials Science, Polymer Edition. 2008;19:1021–34. doi: 10.1163/156856208784909381. [DOI] [PubMed] [Google Scholar]
- 99.Lode A, Reinstorf A, Bernhardt A, Wolf-Brandstetter C, König U, Gelinsky M. Heparin modification of calcium phosphate bone cements for VEGF functionalization. Journal of Biomedical Materials Research - Part A. 2008;86:749–59. doi: 10.1002/jbm.a.31581. [DOI] [PubMed] [Google Scholar]
- 100.Kim TG, Park TG. Biomimicking extracellular matrix: Cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Engineering. 2006;12:221–33. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- 101.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29:587–96. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Araujo JV, Martins A, Leonor IB, Pinho ED, Reis RL, Neves NM. Surface controlled biomimetic coating of polycaprolactone nanofiber meshes to be used as bone extracellular matrix analogues. Journal of Biomaterials Science, Polymer Edition. 2008;19:1261–78. doi: 10.1163/156856208786052335. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Guo Y, Wang H, Shi D, Liang C, Ye Z, et al. Electrospun nanofibers immobilized with collagen for neural stem cells culture. Journal of Materials Science: Materials in Medicine. 2008;19:847–54. doi: 10.1007/s10856-007-3087-5. [DOI] [PubMed] [Google Scholar]
- 104.Zhu Y, Leong MF, Ong WF, Chan-Park MB, Chian KS. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials. 2007;28:861–8. doi: 10.1016/j.biomaterials.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 105.Zonari A, Novikoff S, Electo NRP, Breyner NlM, Gomes DA, Martins A, et al. Endothelial Differentiation of Human Stem Cells Seeded onto Electrospun Polyhydroxybutyrate/Polyhydroxybutyrate-Co-Hydroxyvalerate Fiber Mesh. PLoS ONE. 2012;7:e35422. doi: 10.1371/journal.pone.0035422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J, Qi H, Wang H, Hu P, Ou L, Guo S, et al. Engineering of vascular grafts with genetically modified bone marrow mesenchymal stem cells on poly (propylene carbonate) graft. Artificial Organs. 2006;30:898–905. doi: 10.1111/j.1525-1594.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 107.Centola M, Rainer A, Spadaccio C, De Porcellinis S, Genovese JA, Trombetta M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication. 2010;2:014102, 11. doi: 10.1088/1758-5082/2/1/014102. [DOI] [PubMed] [Google Scholar]
- 108.Yamada H. Mechanical properties of circulatory organs and tissues. In: Evans FG, editor. Strength of Biological Materials. New York: Robert E. Krieger Publishing Company, Huntington; 1970. pp. 106–37. [Google Scholar]
- 109.Fung YC. Blood flow in arteries. New York, NY: Springer-Verlag; 1984. [Google Scholar]
- 110.Thomas V, Donahoe T, Nyairo E, Dean DR, Vohra YK. Electrospinning of Biosyn (R)-based tubular conduits: Structural, morphological, and mechanical characterizations. Acta Biomaterialia. 2011;7:2070–9. doi: 10.1016/j.actbio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Wright LD, Andric T, Freeman JW. Utilizing NaCl to increase the porosity of electrospun materials. Materials Science & Engineering C-Materials for Biological Applications. 2011;31:30–6. [Google Scholar]
- 112.Subramanian A, Krishnan UM, Sethuraman S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomedical Materials. 2011;6:art. no.025004. doi: 10.1088/1748-6041/6/2/025004. [DOI] [PubMed] [Google Scholar]


