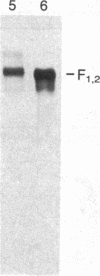
Abstract
A cDNA clone representing the mRNA coding sequence of the fusion glycoprotein (F) gene of human respiratory syncytial virus (RSV) was constructed and inserted into the thymidine kinase gene of vaccinia virus (WR strain) under the control of a vaccinia virus promoter. The resulting recombinant vaccinia virus, vaccinia F, expressed the F1 and F2 cleavage products (48 and 20 kDa, respectively) of the F glycoprotein in cell culture. F1 and F2 were indistinguishable from their authentic RSV counterparts with respect to glycosylation, disulfide linkage, electrophoretic mobility, cell-surface expression, and antigenic specificity. Cotton rats infected intradermally with vaccinia F developed a high titer of serum F-specific antibodies, which neutralized infectivity of RSV. This neutralizing antibody response exceeded that induced by infection of the respiratory tract with RSV and was 6-fold higher than that induced by vaccinia G, a recombinant vaccinia virus that expressed the RSV G glycoprotein gene. Immunization with vaccinia F stimulated almost complete resistance to replication of RSV in the lower respiratory tract as well as significant resistance in the upper respiratory tract. The degree of resistance conferred by vaccinia F exceeded that induced by vaccinia G.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynak E. B., Weibel R. E., McLean A. A., Hilleman M. R. Live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1978 Apr;157(4):636–642. doi: 10.3181/00379727-157-40112. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Satake M., Coligan J. E., Norrby E., Camargo E., Venkatesan S. Respiratory syncytial virus fusion glycoprotein: nucleotide sequence of mRNA, identification of cleavage activation site and amino acid sequence of N-terminus of F1 subunit. Nucleic Acids Res. 1985 Mar 11;13(5):1559–1574. doi: 10.1093/nar/13.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983 Apr;64(Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Baric R. S., Sawyer B. A., Johnston R. E. Sindbis virus mutants selected for rapid growth in cell culture display attenuated virulence in animals. Science. 1984 Jul 27;225(4660):424–427. doi: 10.1126/science.6204381. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Chanock R. M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Coligan J. E., Elango N., Norrby E., Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985 Nov 11;13(21):7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Lai C. J. Functional expression in primate cells of cloned DNA coding for the hemagglutinin surface glycoprotein of influenza virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5488–5492. doi: 10.1073/pnas.78.9.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Markoff L. J., Lai C. J. Cell surface expression of the influenza virus hemagglutinin requires the hydrophobic carboxy-terminal sequences. Cell. 1982 Sep;30(2):649–656. doi: 10.1016/0092-8674(82)90261-6. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Brandriss M. W., Schlesinger J. J. Purification and characterization of the respiratory syncytial virus fusion protein. J Gen Virol. 1985 Mar;66(Pt 3):409–415. doi: 10.1099/0022-1317-66-3-409. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Belshe R. B., Kim H. W., Van Voris L. P., Chanock R. M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982 Jul;37(1):397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]