Abstract
Background
The aim of this study was to investigate the protective effects of methylprednisolone (Pn), which is a potent anti-inflammatory agent, and pheniramine maleate (Ph), which is an antihistaminic with some anti-inflammatory effects, on reperfusion injury in brain developing after ischemia of the left lower extremity of rats.
Material/Methods
Twenty-eight randomly selected male Sprague-Dawley rats were divided into 4 groups: Group 1 was the control group, Group 2 was the sham group (I/R), Rats in Group 3 were subjected to I/R and given Ph, and rats in Group 4 were subjected to I/R and given Pn. A tourniquet was applied at the level of left groin region of subjects in the I/R group after induction of anesthesia. One h of ischemia was performed with no drug administration. In the Ph group, half of a total dose of 10 mg/kg Ph was administered intraperitoneally before ischemia and the remaining half before reperfusion. In the Pn group, subjects received a single dose of 50 mg/kg Pn intraperitoneally at the 30th min of ischemia. Brains of all subjects were removed after 24 h for examination.
Results
Malondialdehyde (MDA) levels of the prefrontal cortex were significantly lower in the Ph group than in the I/R group (p<0.05). Superoxide dismutase (SOD) and glutathione peroxidase (GPx) enzyme activities were found to be significantly higher in the Ph group than in the I/R group (p<0.05). Histological examination demonstrated that Ph had protective effects against I/R injury developing in the brain tissue.
Conclusions
Ph has a protective effect against ischemia/reperfusion injury created experimentally in rat brains.
Keywords: ischemia/reperfusion injury, pheniramine maleate, brain
Background
Reperfusion of ischemic tissue may cause systemic injury, leading to multiorgan dysfunction and even to death. Reperfusion causes release of free oxygen radicals (FOR) and inflammatory mediators, contributing to both local and systemic injury [1,2]. This injury may become obvious, particularly in myocardial, renal, pulmonary and brain tissues [2–4].
Glucocorticoids, including methylprednisolone, show their anti-inflammatory effects by influencing prostaglandin synthesis via intracellular receptors and by activating nuclear factor kappa B (NF-kB) [5,6]. It was previously reported that glucocorticoids indirectly reduced release of hydrolytic enzymes, lipid peroxidation, and production of oxygen radicals. Thus, they reduced I/R injury in many organs [5–8].
Histamine basically causes relaxation of vascular smooth muscle cells and contraction of non-vascular smooth muscles. Histamine produces its general effects via H1-receptors. H1-receptors are available in vascular smooth muscles, vascular endothelium, smooth muscles of gastrointestinal tract, and smooth muscles of respiratory tract, atrioventricular (A-V) node, and afferent endings of peripheral nerves.
Pheniramine (Ph) is a first-generation H1-antihistaminic drug. Besides many other effects, H1-antihistaminics also have anti-inflammatory effects. The most important location where they show their anti-inflammatory effects is at the level of transcription factor, specifically NF-kB. Activation of NF-kB is stimulated by histamine and inhibited by antihistaminics [3,9,10]. As with the other H1-antihistaminics, Ph shows its effect on I/R injury by inhibiting release of some specific mediators of inflammation, which is a significant factor in I/R pathophysiology [3,11–13].
Based on the hypothesis that pheniramine maleate may have protective effects in I/R injury, in this study we investigated the efficacy of Ph on brain tissue against I/R injury.
Material and Methods
This study was conducted according to the “Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care)” and we obtained approval from the Local Experimental Animal Ethics Committee of Dokuz Eylul University (protocol no. 40/2010).
During the course of the study, all rats were routinely fed with rat chow and tap water ad libitum. The rats were maintained on a 12:12-h light: dark cycle in an environmentally monitored room with a ventilation system at a temperature of 20±2°C. Twenty-eight male Sprague-Dawley rats weighing 320–370 g were selected. There were 4 groups of animals, each consisting of 7 rats. Group 1 was the control group. Intramuscular injection of the mixture of 50 mg/kg body weight ketamine hydrochloride (Ketalar, Eczacıbaşı, Istanbul-Turkey) and 5 mg/kg body weight xylazine hydrochloride (Rompun, Bayer, Istanbul-Turkey) was performed. Then, their brains were removed without any further intervention and they were sacrificed using 150 mg of thiopental sodium per kg body weight (Pental, Ibrahim Ethem Ulagay, Istanbul-Turkey).
According to the acute ischemia-reperfusion model defined by Hardy et al., a tourniquet was applied at the level of the left groin region of subjects in Group 2 (I/R group) after application of same protocol of anesthesia and loss of flow signal was checked by hand-held Doppler ultrasound [14]. One h of ischemia was performed and no drug was administered. After completion of the ischemia period, the tourniquet was removed and the rat was put into its cage for a reperfusion period of 24 h. Then, its brain was removed under anesthesia and the rat was sacrificed again using 150 mg of thiopental sodium per kg body weight.
The ischemia model was constituted likewise in Group 3 (the Ph group). These rats intraperitoneally received pheniramine maleate at a total dose of 10 mg per kg body weight (a half dose before ischemia and a half dose just before reperfusion) [12]. After completion of the 1-h ischemia period, a reperfusion period of 24 h was started again by removing the tourniquet. At the end of 24 h, brains were removed as detailed above.
The same ischemia model was performed on rats of Group 4 (the Pn group), but these rats received methylprednisolone at a dose of 50 mg per kg body weight intraperitoneally at the end of 30 min of ischemia [8]. At the end of 24 h, brains were removed as detailed above.
Biochemical estimations
Brain samples were taken out for biochemical estimations and for light microscopic assessment. The brains were removed and divided into 2 hemispheres. Prefrontal cortex tissues were dissected on an ice-cold surface. For biochemical estimations, tissue homogenates were prepared as described by Carrillo et al. [15]. Brain samples were washed 3 times with ice-cold 0.9% NaCl solution and weighed. Brain tissue samples were then homogenized in ice-cold 0.1 M phosphate buffer (PBS, pH 7.4) in a volume 10 times the weight of the tissue. The homogenates were sonicated with an ultrasonicator (Bandelin Sonopuls, Germany). The homogenate was centrifuged at 10 000 g for 15 min and aliquots of supernatant separated and used for biochemical estimation. An aliquot of the homogenate and supernatant was stored at −80°C until MDA levels and SOD and GPx enzyme activities were determined. Determination of MDA levels and antioxidant enzyme activities were performed spectrophotometrically (Hach Lange DR5000 UV). MDA in the brain cortex was measured using a standard curve of tetramethoxypropane. The Bioxytech MDA-586 (Oxis International, USA) assay for MDA was performed according to kit protocol. SOD activity was evaluated with a commercially available kit by testing of the rate of auto-oxidation of 5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzo(c)fluorene at 525 nm (Bioxytech SD-525, Oxis International, USA). GPx activity was assayed by following the rate of NADPH oxidation at 340 nm by the coupled reaction with glutathione reductase. The Bioxytech GPx-340 (Oxis International, USA) assay for GPx activity was performed according to kit protocol.
Histological examination
For light microscopic assessment, brain tissue samples were processed by routine histological methods and embedded in paraffin blocks. Paraffin blocks were placed in a Leica RM2255 rotary microtome (Germany) and cut into 5-μm thickness coronal sections through the prefrontal cortex, which corresponded approximately to Plates 9 and 11 in accordance with the rat atlas of Paxinos and Watson [16]. Sections were deparaffinized, hydrated, and stained with cresyl violet and TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling), and caspase-3 immunohistochemistry was performed. The images were analyzed by using a computer-assisted image analyzer system consisting of a microscope (Olympus CX-41, Japan) equipped with a high-resolution video camera (Olympus DP71, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method
Detection of DNA fragmentation in situ was visualized with the use of the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, USA). Deparaffinized tissue sections were incubated with proteinase K (20 μg/ml). Tissue sections were subjected to 3% H2O2 for endogenous peroxidase inhibition and were incubated with 1× equilibration buffer at room temperature for 30 min. The digoxigenin-labelled dNTP tail was incubated with Tdt (terminal deoxynucleotidyl transferase) for 1 h at 37°C, and sections were washed in stop/wash buffer for 10 min at room temperature. Tissue sections were incubated with anti-digoxigenin-peroxidase antibody at room temperature for 30 min and were stained with diaminobenzidine (DAB) as a peroxidase substrate. Staining was evaluated using a light microscope after counterstaining with hematoxylin. Five fields were randomly chosen for each slide and the TUNEL-positive cells per field were counted. The apoptotic index (percentage of apoptotic nuclei) was calculated as apoptotic nuclei/total nuclei counted ×100%. All counting procedures were performed blindly by 1 person with no prior knowledge of the experimental data.
Caspase-3 immunohistochemistry
For visualization of the active caspase-3 expression, immunohistochemistry was performed using an active anti-caspase-3 antibody. The sections were incubated overnight with anti-caspase-3 antibody (1:100; Millipore, AB3623) and then for another 30 min with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with Histostain® plus bulk kit (Invitrogen, 85-9043). The antibody-biotin-avidin-peroxidase complexes were visualized using DAB. The sections were finally mounted onto lysine-coated slides. The percentage of caspase 3-positive cells was determined by counting the positive cells on 5 random fields in each group.
Statistical analysis
Results are presented as means ±SEM. All data were analyzed by one-way analysis of variance (ANOVA) post hoc LSD test. P<0.05 was considered statistically significant.
Results
Biochemical analyses
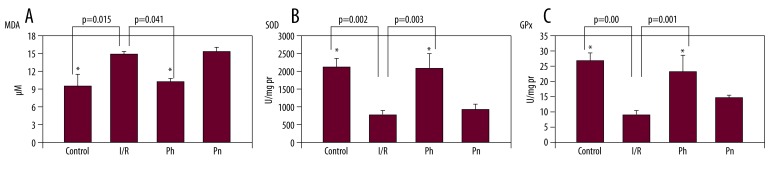
To examine the protective effect of pheniramine, MDA levels of the prefrontal cortex were determined. Figure 1A presents MDA levels in the prefrontal cortex region of rats. In the I/R group, tissue MDA levels were significantly increased compared to the control group (14.54±1.07 and 9.64±2.07, respectively, p=0.015). Pheniramine treatment caused a significant decrease in the prefrontal cortex MDA levels when compared to the I/R group (10.50±0.88, p=0.041). No significant effect of methylprednisolone in lipid peroxidation was found in brains of rats when compared with the I/R group (14.76±0.89, p=0.90).
Figure 1.
(A) Effects of Pheniramine and Methylprednisolone treatment on MDA levels in rat brain cortex. Data are mean ± SEM. * P<0.05 compared with the I/R group. (B) Effects of Pheniramin and Methylprednisolone treatment on SOD activity in rat brain cortex. Data are mean ± SEM. * P<0.05 compared with the I/R group. (C) Effects of Pheniramin and Methylprednisolone treatment on GPx activity in rat brain cortex. Data are mean ± SEM. * P<0.05 compared with the I/R group.
Figure 1B and 1C represent the prefrontal cortex GPx and SOD activities, respectively. I/R caused a decrease in GPx and SOD activities of brain as compared with the control group (P<0.05). Pheniramine-treated rats showed a significant increase in GPx and SOD activities as compared with the I/R group. No significant effect of methylprednisolone in GPx and SOD activities was found in brains when compared with the I/R group (P>0.05).
Histological examination
In the control group, cresyl violet-stained sections showed normal morphology (Figure 2IA). In the I/R group, neurons were found to be shrunken and dark-stained pyknotic neurons dominated (Figure 2IB). In the pheniramine-treated group, most of the neurons were normal and there were few pyknotic neurons (Figure 2IC), but the methylprednisolone group was slightly better than the I/R group regarding the morphology (Figure 2ID).
Figure 2.
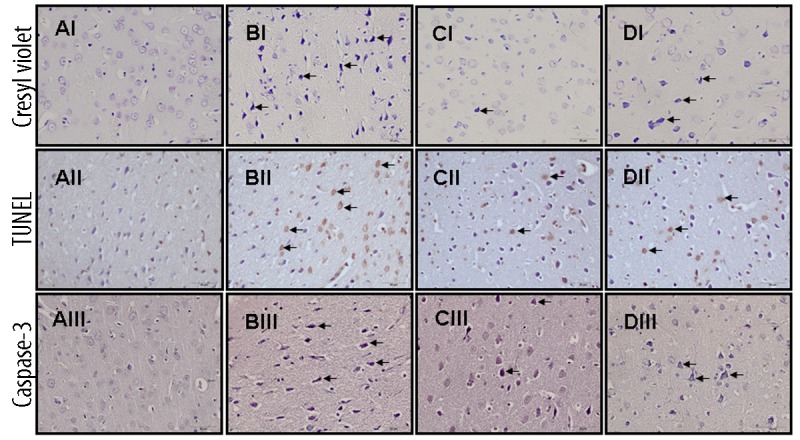
Light microscopic images of rat brain cortex sections. (A) Control, (B) I/R, (C) Pheniramine, (D) Methylprednisolone treated group. Upper (I): Cresyl violet-stained sections of the rat brain cortex. The morphology of neurons in the control group was normal. In I/R group, increased number of cells with dark blue and shrunken morphology can be seen (arrows). Middle (II): TUNEL staining. Representative photomicrographs of TUNEL-positive cells (arrows). Lower (III): Caspase-3 immunoreactivity. Arrows indicate caspase-3 positive cells.
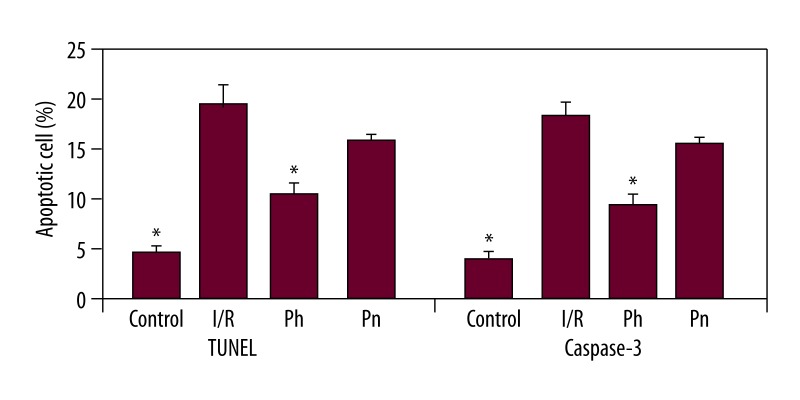
Representative photographs of TUNEL staining in the prefrontal cortex are shown in Figure 2II. The present study shows that I/R enhanced apoptotic cell death in the brain cortex. Control rats showed fewer TUNEL-positive cells (Figure 2IIA). There were more TUNEL-positive cells in the I/R group (Figure 2IIB). Quantification and statistical analysis of the TUNEL staining showed that the number of TUNEL-positive cells increased significantly in the I/R group compared with controls (19.00±1.82 and 4.85±0.51, respectively, p<0.01, Figure 3). It was determined that the number of TUNEL-positive cells was significantly decreased in the pheniramine-treated group (10.14±1.10, Figure 3). The difference in the number of TUNEL-positive cells between the I/R and methylprednisolone groups was statistically insignificant (P<0.05).
Figure 3.
Quantitative analysis of TUNEL-positive cells and caspase 3-positive cells in the rat brain cortex. Data are mean ±SEM. * P<0.05, compared with the I/R group.
The apoptotic feature was further confirmed by caspase-3 immunohistochemistry. Immunohistochemical evaluation based on the intensity of caspase-3 immunoreactivity in the rat brain cortex is shown in Figure 2III. Representative photographs of caspase-3 expression show that caspase-3 reactivity was commonly observed in the rat brain cortex of I/R rats (Figure 2IIIB) compared to sham rats (Figure 2IIIA).The number of caspase-3 positive cells in the I/R group was significantly increased compared to the control group (16.85±1.16 and 3.85±0.40, respectively, p<0.01, Figure 3). It was determined that the number of caspase-3 positive cells was significantly decreased in the pheniramine-treated group (8.42±0.99, Figure 3). The difference in the number of caspase-3-positive cells between the I/R and methylprednisolone groups was not statistically significant (P>0.05).
Discussion
Occlusion of the nutrient artery selectively and reperfusion of the tissue afterwards are frequently used methods to assess the I/R injury to the target tissue [11–13,15–19]. As an alternative, I/R injury to the target tissue after a period of ischemia created away from the target tissue was again investigated in various studies [1,2,7,20]. Ischemia of the lower extremity is inevitably seen in our routine peripheral vascular surgical practice. This causes reperfusion injury in distant crucial organs such as the lungs, kidneys, brain, and heart, although they are not primarily exposed to ischemia. We performed I/R to the lower extremity in this study. We demonstrated that brains of the rats of the study and sham groups were subjected to reperfusion injury when compared with that of the control group. It was previously reported in several studies that glucocorticoids inhibited lipid peroxidation and production of free radicals; thus reducing I/R injury in many organs [3,5–7,15,21,22]. Some authors argued that these effects appeared in high doses [6,15], whereas some authors argued that these effects appeared even in low doses [8,21,22]. Pn was shown to have protective effects against renal reperfusion injury at a relatively lower dose (30 mg/kg) [22]. In another study, Pn was shown to be effective in renal reperfusion injury at a dose of 50 mg/kg after distant ischemia of an extremity [3]. Yeginsu et al. studied the effects of different doses of Pn on reperfusion injury of lungs: 15 mg/kg, 50 mg/kg and 150 mg/kg. They revealed that Pn reduced reperfusion injury of lung tissue at each dose [7]. However, this effect was maximal at a dose of 150 mg/kg and it decreased as the dose declined. In another study demonstrating that Pn was effective at high doses against cerebral reperfusion injury, 105 mg/kg Pn was administered after selective cerebral ischemia [15]. In our study, administration of 50 mg/kg Pn after distant organ ischemia/reperfusion was shown to be ineffective in all of the biochemical parameters and most of the histological parameters in terms of demonstration of cerebral reperfusion injury. This result supports data from the relevant literature showing that Pn was effective against I/R injury at relatively higher doses.
A few studies demonstrated that pheniramine and other H1-antihistaminics were effective in I/R injury, but most of these studies were conducted in the gastrointestinal system [11–13,17]. In one of these, the authors showed that pheniramine reduced production of free radicals by activated neutrophils after occlusion of the superior mesenteric artery followed by reperfusion [11]. Due to their lipophilicity, pheniramines adhere to the cellular membrane and this may inhibit production and/or release of free radicals. They stated that this anti-oxidant effect of H1-antihistaminics may be beneficial in reducing the tissue injury [11]. Neutrophils have a crucial role in formation of reperfusion injury. It was reported that production of reactive oxygen metabolites (ROM) increased during mesenteric I/R injury in rats as shown with increase in chemiluminescence (CL) in whole blood. Based on these data, Nosál et al. created a mesenteric I/R model and showed that Ph suppressed CL intra- and extracellularly in vitro. The same study stated that Pn showed this effect as being a direct radical scavenger or through interaction with regulatory cellular mechanisms [17]. In the only study with use of pheniramine conducted apart from the gastrointestinal system, Bayrak et al. showed that Pn was effective in renal I/R injury after distant organ ischemia [3]. In our study, we observed that Ph was more beneficial in preventing cerebral I/R injury than Mp administered at a relatively smaller dose.
As far as is known, our study is unique in showing the effectiveness of Ph on cerebral reperfusion injury after distant organ ischemia. Nevertheless, it has some limitations. The number of the experimental subjects is too small to draw a definite conclusion. The dose of pheniramine maleate is well above physiological doses for humans. Therefore, there is a need for further randomized controlled studies in which the dose of pheniramine maleate in I/R injury is titered and the number of experimental subjects is larger.
Conclusions
Ph is used in the symptomatic treatment of various hypersensitivity reactions in daily clinical practice, but its anti-inflammatory properties suggest that it might be used for treatment of different disorders. Consequently, Ph may be beneficial in prevention of the cerebral I/R injury by inhibiting release of specific mediators of inflammation formed in reperfusion injury.
Footnotes
Source of support: Departmental sources
References
- 1.Uysal A, Burma O, Akar İ, et al. Protective effect of melatonin on lung injury caused by ischemia-reperfusion of the lower extremities. Turkish J Thorac Cardiovasc Surg. 2006;14(4):308–14. [Google Scholar]
- 2.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–30. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Bayrak S, Yurekli I, Gokalp O, et al. Assessment of Protective Effects of Methylprednisolone and Pheniramine Maleate on Reperfusion Injury in Kidney After Distant Organ Ischemia: A Rat Model. Ann Vasc Surg. 2012;26(4):559–65. doi: 10.1016/j.avsg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Harman F, Hasturk AE, Yaman M, et al. Neuroprotective effects of propofol, thiopental, etomidate, and midazolam in fetal rat brain in ischemia-reperfusion model. Childs Nerv Syst. 2012;28(7):1055–62. doi: 10.1007/s00381-012-1782-0. [DOI] [PubMed] [Google Scholar]
- 5.White BC, Sullivan JM, De Gracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 6.Chimalakonda AP, Mehvar R. Effects of methylprednisolone and its liver-targeted dextran prodrug on ischemia-reperfusion injury in a rat liver transplantation model. Pharm Res. 2007;24:2231–38. doi: 10.1007/s11095-007-9414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enc Y, Karaca P, Ayoglu U, et al. The acute cardioprotective effect of glucocorticoid in myocardial ischemia-reperfusion injury occurring during cardiopulmonary bypass. Heart Vessels. 2006;21:152–56. doi: 10.1007/s00380-005-0887-8. [DOI] [PubMed] [Google Scholar]
- 8.Yeginsu A, Ergin M, Ozyurt H, et al. The effects of methylprednisolone on the lung injury resulted from extremity ischemia reperfusion. Turkish J Thorac Cardiovasc Surg. 2010;18(1):45–51. [Google Scholar]
- 9.Turner S, Derham C, Orsi NM, et al. Randomized clinical trial of the effects of methylprednisolone on renal function after major vascular surgery. Br J Surg. 2008;95:50–56. doi: 10.1002/bjs.5978. [DOI] [PubMed] [Google Scholar]
- 10.Vignola AM, Crampette L, ve ark Mondain M. Inhibitory activity of loratadine and descarboethoxyloratadine on expression of ICAM-1 and HLA-DR by nasal epithelial cells. Allergy. 1995;50:200–3. doi: 10.1111/j.1398-9995.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 11.Church MK. H-1 antihistamines and inflammation. Clin Exp Allergy. 2001;31:1341–43. doi: 10.1046/j.1365-2222.2001.01195.x. [DOI] [PubMed] [Google Scholar]
- 12.Nosálová V, Drábiková K, Jancinová V, et al. Protective effect of pheniramines against mesenteric ischaemia/reperfusion-induced injury. Inflamm Res. 2009;58(1):68–69. doi: 10.1007/s00011-009-2011-5. [DOI] [PubMed] [Google Scholar]
- 13.Nosalova V, Drabikova K, Jancinova V, et al. Effect of H1-antihistamines in the model of mesenteric ischaemia/reperfusion. Inflamm Res. 2008;57:55–56. doi: 10.1007/s00011-007-0627-x. [DOI] [PubMed] [Google Scholar]
- 14.Hardy SC, Homer-Vanniasinkam S, Gough MJ. The triphasic pattern of skeletal muscle blood flow in reperfusion injury: an experimental model with implications for surgery on the acutely ischaemic lower limb. Eur J Vasc Surg. 1990;4:587–90. doi: 10.1016/s0950-821x(05)80812-6. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo MC, Kanai S, Nokubo M, Kitani K. (–) Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 1991;48(6):517–21. doi: 10.1016/0024-3205(91)90466-o. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 17.Slivka AP, Murphy EJ. High-dose methylprednisolone treatment in experimental focal cerebral ischemia. Exp Neurol. 2001;167(1):166–72. doi: 10.1006/exnr.2000.7532. [DOI] [PubMed] [Google Scholar]
- 18.Kim K, Li Y, Jin G, et al. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation. 2012;83(2):243–48. doi: 10.1016/j.resuscitation.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guler A, Sahin MA, Yucel O, et al. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit. 2011;17(11):BR326–31. doi: 10.12659/MSM.882042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosalova V, Navarova J, Mihalova D, Sotnikova R. Mesenteric ischaemia reperfusion – induced intestinal and vascular damage: effect of stobadine. Meth Find Exp Clin Pharmacol. 2007;29:39–45. doi: 10.1358/mf.2007.29.1.1063495. [DOI] [PubMed] [Google Scholar]
- 21.Nosál R, Jancinová V, Nosálová V, et al. Pheniramines and oxidative burst of blood phagocytes during ischaemia/reperfusion. Inflamm Res. 2009;58(1):66–67. doi: 10.1007/s00011-009-2010-6. [DOI] [PubMed] [Google Scholar]
- 22.Saba D, Yavuz H, Senkaya I, et al. The Effects Of Calcium Dobesilate On Skeletal Muscle Ischemia Reperfusion Injury. Turkish J Thorac and Cardiovasc Surg. 2000;8:797–801. [Google Scholar]